Computational Chemistry
Vol.04 No.04(2016), Article ID:71297,6 pages
10.4236/cc.2016.44009
Omega and Cluj-Ilmenau Indices of Hydrocarbon Molecules “Polycyclic Aromatic Hydrocarbons PAHk”
M. R. Rajesh Kanna1, R. Pradeep Kumar2, Muhammad Kamran Jamil3, Mohammad Reza Farahani4
1Department of Mathematics, Maharani’s Science College for Women, Mysore, India
2Department of Mathematics, The National Institute of Engineering, Mysuru, India
3Department of Mathematics, Riphah Institute of Computing and Applied Sciences (RICAS), Riphah International University, Lahore, Pakistan
4Department of Applied Mathematics, Iran University of Science and Technology (IUST), Tehran, Iran

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: August 11, 2016; Accepted: October 16, 2016; Published: October 19, 2016
ABSTRACT
A topological index is a numerical value associated with chemical constitution for correlation of chemical structure with various physical properties, chemical reactivity or biological activity. In this paper, we computed the Omega and Cluj-Ilumenau indices of a very famous hydrocarbon named as Polycyclic Aromatic Hydrocarbons  for all integer number k.
for all integer number k.
Keywords:
Molecular Graph, Hydrocarbons, Topological Indices

1. Introduction
Let 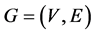 be a simple finite connected graph, where V and E are the sets of vertices and edges, respectively. The distance between two vertices u and v in a graph G is the length of the shortest path connecting them, it is denoted by
be a simple finite connected graph, where V and E are the sets of vertices and edges, respectively. The distance between two vertices u and v in a graph G is the length of the shortest path connecting them, it is denoted by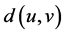 . Two edges
. Two edges 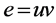 and
and 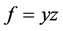 in graph G are said to be codistant if they satisfy the following condition [1]
in graph G are said to be codistant if they satisfy the following condition [1]
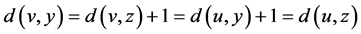 .
.
If the edges e and f are codistant we write it as e co f. Relation co is reflexive and symmetric but generally not transitive. If co relation is transitive then it is an equiva- lence relation. A graph G in which co is an equivalence relation is called co-graph, and the subset of edges 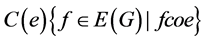 is called an orthogonal cut (oc) of G, also the edge set
is called an orthogonal cut (oc) of G, also the edge set  can be written as the union of disjoint orthogonal cuts, i.e.
can be written as the union of disjoint orthogonal cuts, i.e.
 .
.
Let  be two edges of G which are opposite or topologically parallel and denote this relation by e op f. A set of opposite edges, within the same ring eventually forming a strip of adjacent rings, is called an opposite edge strip ops, which is a quasi orthogonal cut (qoc). The length of ops is maximal irrespective of the starting edge. Let
be two edges of G which are opposite or topologically parallel and denote this relation by e op f. A set of opposite edges, within the same ring eventually forming a strip of adjacent rings, is called an opposite edge strip ops, which is a quasi orthogonal cut (qoc). The length of ops is maximal irrespective of the starting edge. Let 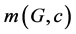 be the number of ops strips of length c.
be the number of ops strips of length c.
The physico-chemical properties of chemical compounds are often modeled by means of molecular graph based structure descriptors, known as topological indices [2] , [3] . The Wiener index is the first distance based topological index [4] . The Wiener index of a graph G is defined as
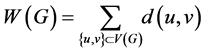 .
.
M. V. Diudea introduced the Omega Polynomial 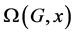 for counting ops strips in graph G [5]
for counting ops strips in graph G [5]
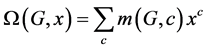 .
.
First derivative of Omega polynomial at 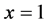 equals the size of the graph G, i.e.
equals the size of the graph G, i.e.
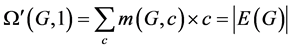 .
.
The Cluj-Ilumenau index [6] is defined with the help of first and second derivative of Omega polynomial at 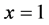 as
as
 .
.
The Omega index is defined as

2. Discussion and Main Result
Polycylic Aromatic Hydorcarbons (



Theorem 1. Consider the graph of Polycyclic aromatic hydrocarbons

Proof Consider the general representation of the Polycyclic aromatic hydrocarbons 



To obtain the required result, we used the Cut Method [23] - [25] . We calculated the 






Figure 1. General representation of polycyclic aromatic hydro- carbons
Figure 2. A quasi orthogonal cuts strips on polycyclic aro- matic hydrocarbons




・ For

・ For all

・ For

From this, we obtain that

This gives that the Omega polynomial of the Polycyclic aromatic hydrocarbons 

Now with the help of above polynomial we will investigate the Cluj-Ilmenau and Omega indices of Polycyclic aromatic hydrocarbons
As
Cite this paper
Kanna, M.R.R., Kumar, R.P., Jamil, M.K. and Farahani, M.R. (2016) Omega and Cluj-Ilmenau Indices of Hydrocarbon Molecules “Polycyclic Aromatic Hydrocarbons PAHk”. Computational Chemistry, 4, 91-96. http://dx.doi.org/10.4236/cc.2016.44009
References
- 1. John, P.E., Vizitiu, A.E., Cigher, S. and Diudea, M.V. (2007) CI Index in Tubular Nanostructures. MATCH Communications in Mathematical and in Computer Chemistry, 57, 479.
- 2. Diudea, M.V. (2001) Wiener Index of Dendrimers. NOVA, New York.
- 3. Trinjastic, N. (1992) Chemical Graph Theory. CRC Press, Boca Raton.
- 4. Wiener, H. (1947) Structural Determination of Paraffin Boiling Points. Journal of the American Chemical Society, 69, 17-20. http://dx.doi.org/10.1021/ja01193a005
- 5. Diudea, M.V. (2006) Omega Polynomial. Carpathian Journal of Mathematics, 22, 43-47. http://carpathian.ubm.ro/?m=past_issues&issueno=Vol
- 6. Diudea, M.V. (2010) Counting Polynomials and Related Indices by Edge Cutting Procedures. MATCH Communications in Mathematical and in Computer Chemistry, 64, 569.
- 7. Alaeiyan, M., Farahani, M.R. and Jamil, M.K. (2016) Computation of the Fifth Geometric Aithmetic Index for Polycyclic Aromatic Hydrocarbons . Applied Mathematics and Nonlinear Sciences, 1, 283-290. http://dx.doi.org/10.21042/AMNS.2016.1.00023
- 8. Farahani, M.R., Jamil, M.K., Kanna, M.R.R. and Kumar, R.P. (2016) Computation on the Fourth Zagreb Index of Polycyclic Aromatic Hydrocarbons . Journal of Chemical and Pharmaceutical Research, 8, 41-45.
- 9. Farahani, M.R., Jamil, M.K. and Kanna, M.R.R. (2016) The Multiplicative Zagreb eccentricity Index of Polycyclic Aromatic Hydrocarbons . International Journal of Scientific and Engineering Research, 7, 1132-1135.
- 10. Farahani, M.R., Rehman, H.M., Jamil, M.K. and Lee, D.W. (2016) Vertex Version of PI Index of Polycyclic Aromatic Hydrocarbons . The Pharmaceutical and Chemical, 3, 138-141.
- 11. Farahani, M.R., Rajesh Kanna, M.R., Pradeep Kumar, R. and Wang, S. (2016) The Vertex Szeged Index of Titania Carbon Nanotubes TiO2(m,n). International Journal of Pharmaceutical Sciences and Research, 7, 1000-08.
- 12. Jamil, M.K., Farahani, M.R. and Rajesh Kanna, M.R. (2016) Fourth Geometric Arithmetic Index of Polycyclic Aromatic Hydrocarbons . The Pharmaceutical and Chemical Journal, 3, 94-99.
- 13. Jamil, M.K., Farahani, M.R., Imran, M. and Malik, M.A. (2016) Computing Eccentric Version of Second Zagreb Index of Polycyclic Aromatic Hydrocarbons . Applied Mathematics and Nonlinear Sciences, 1, 247-251. http://dx.doi.org/10.21042/AMNS.2016.1.00019
- 14. Liu, J.B., Wang, C., Wang, S. and Wei, B. (Submitted) Zagreb Indices and Multiplicative Zagreb Indices of Eulerian Graphs.
- 15. Wang, C., Wang, S. and Wei, B. (2016) Cacti with Extremal PI Index. Transactions on Combinatorics, 5, 1-8.
- 16. Wang, S. and Wei, B. Padmakar-Ivan Indices of K-Trees. (Submitted Paper).
- 17. Wang, S.H., Farahani, M.R., Kanna, M.R.R., Kumar, R.P. (2016) Schultz Polynomials and Their Topological Indices of Jahangir Graphs J2,m. Applied Mathematics (Scientific Research Publishing), 7, 1632-1637. http://dx.doi.org/10.4236/am.2016.714140
- 18. Wang, S. and Wei, B. (2016) Multiplicative Zagreb Indices of Cacti. Discrete Mathematics, Algorithms and Applications, 8, Article ID: 1650040. http://dx.doi.org/10.1142/s1793830916500403
- 19. Wang, S., Farahani, M.R., Rajesh Kanna, M.R. and Pradeep Kumar, R. (2016) The Wiener Index and the Hosoya Polynomial of the Jahangir Graphs. Applied and Computational Mathematics, 5, 138-141. http://dx.doi.org/10.11648/j.acm.20160503.17
- 20. Wang, S., Farahani, M.R., Baig, A.Q. and Sajja, W. (2016) The Sadhana Polynomial and the Sadhana Index of Polycyclic Aromatic Hydrocarbons PAHk. Journal of Chemical and Pharmaceutical Research, 8, 526-531.
- 21. Wang, S. and Wei, B. (2015) Multiplicative Zagreb Indices of K-Trees. Discrete Applied Mathematics, 180, 168-175. http://dx.doi.org/10.1016/j.dam.2014.08.017
- 22. Yan, L., Li, Y., Farahani, M.R., Jamil, M.K. and Zafar, S. (2016) Vertex Version of Co-PI Index of the Polycyclic Armatic Hydrocarbon Systems PAHk. International Journal of Biology, Pharmacy and Allied Sciences, 5, 1244-1253.
- 23. Farahani, M.R. (2013) Using the Cut Method to Computing Edge Version of Co-PI Index of Circumcoronene Series of Benzenoid . Pacific Journal of Applied Mathematics, 5, 65-72.
- 24. John, P.E., Khadikar, P.V. and Singh, J. (2007) A Method of Computing the PI Index of Benzenoid Hydrocarbons Using Orthogonal Cuts. Journal of Mathematical Chemistry, 42, 27-45. http://dx.doi.org/10.1007/s10910-006-9100-2
- 25. Klavzar, S. (2008) A Bird’s Eye View of the Cut Method and a Survey of Its Applications in Chemical Graph Theory. MATCH Communications in Mathematical and in Computer C, 60, 255-274.










