Computational Chemistry
Vol. 1 No. 1 (2013) , Article ID: 38696 , 4 pages DOI:10.4236/cc.2013.11001
Theoretical Study of 5-HTP. Potential New Drug Resulting from the Complexation of 5-HTP with ATP
![]()
1“Ilie Murgulescu” Physical Chemistry Institute of the Romanian Academy, Bucharest, Romania; 2Institut Charles Gerhardt Montpellier, UMR 5253 CNRS-UM2-ENSCM-UM1, Matériaux Avancés pour la Catalyse et la Santé, ENSCM, Montpellier, France.
Email: josettecarline@yahoo.com
Copyright © 2013 Josette Weinberg, Dan A. Lerner. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received August 16th, 2013; revised September 17th, 2013; accepted September 25th, 2013
Keywords: ATP; 5-HTP; Density Functional Theory (DFT); NBO
ABSTRACT
5-HTP (5-Hydroxytryptophan), is the precursor of the neurotransmitter serotonin. Molecular parameters (interatomic distances and angles, total atomic charge, dipole moments) of 5-HTP (5-Hydroxytryptophan) and ATP (Adenosine triphosphate), and of their possible complex, including its heat of formation, have been computed in an ab initio study involving DFT calculations. The 6-31G* basis set and the B3LYP functional were employed. The aim of this study is to emphasize by DFT calculation the possible existence of a complex between ATP and 5-HTP that may have the properties of a new drug. A Natural Bond Orbital analysis description offers supplementary details for the structure of the molecular units and their interaction.
1. Introduction
5-HTP (5-Hydroxytryptophan), is itself the precursor of the neurotransmitter serotonin. In one study, when normal, healthy humans were deprived of L-tryptophan, a rapid lowering of mood occurred, similar to that of depressed individuals [1]. In another study, depression occurred when supplies of serotonin were depleted [2]. Also, when normal, healthy humans were given 5-HTP, mood-elevation characteristics developed similar to those that occur when antidepressants are taken [3-5]. Indeed, 5-HTP has become very popular with the public for just this reason: people feel better when they use it.
The special carrier of energy in human organism is the molecule adenosine triphosphate, or ATP.
ATP consists of adenosine composed of an adenine ring and a ribose sugar and three phosphate groups (triphosphate).
Adenosine 5’-triphosphate (ATP) is a multifunctional nucleotide, discovered in 1929 by Lohmann [4] and proposed as the main energy carrier in the cell by Lipmann in 1941 [5] nowadays being notoriously known as the “molecular currency” of the intracellular energy transfer [6]. It is produced during the processes of photosynthesis and cellular respiration, being subsequently used as energy source in a multitude of cellular processes including biosynthetic reactions, motility and cell division.
The discussed molecules are presented in Scheme 1.
The advances made in various areas of chemistry with the help of the supramolecular paradigm emphasize the importance of a theoretical analysis of intermolecular interactions in relevant couples of weakly bound bio-
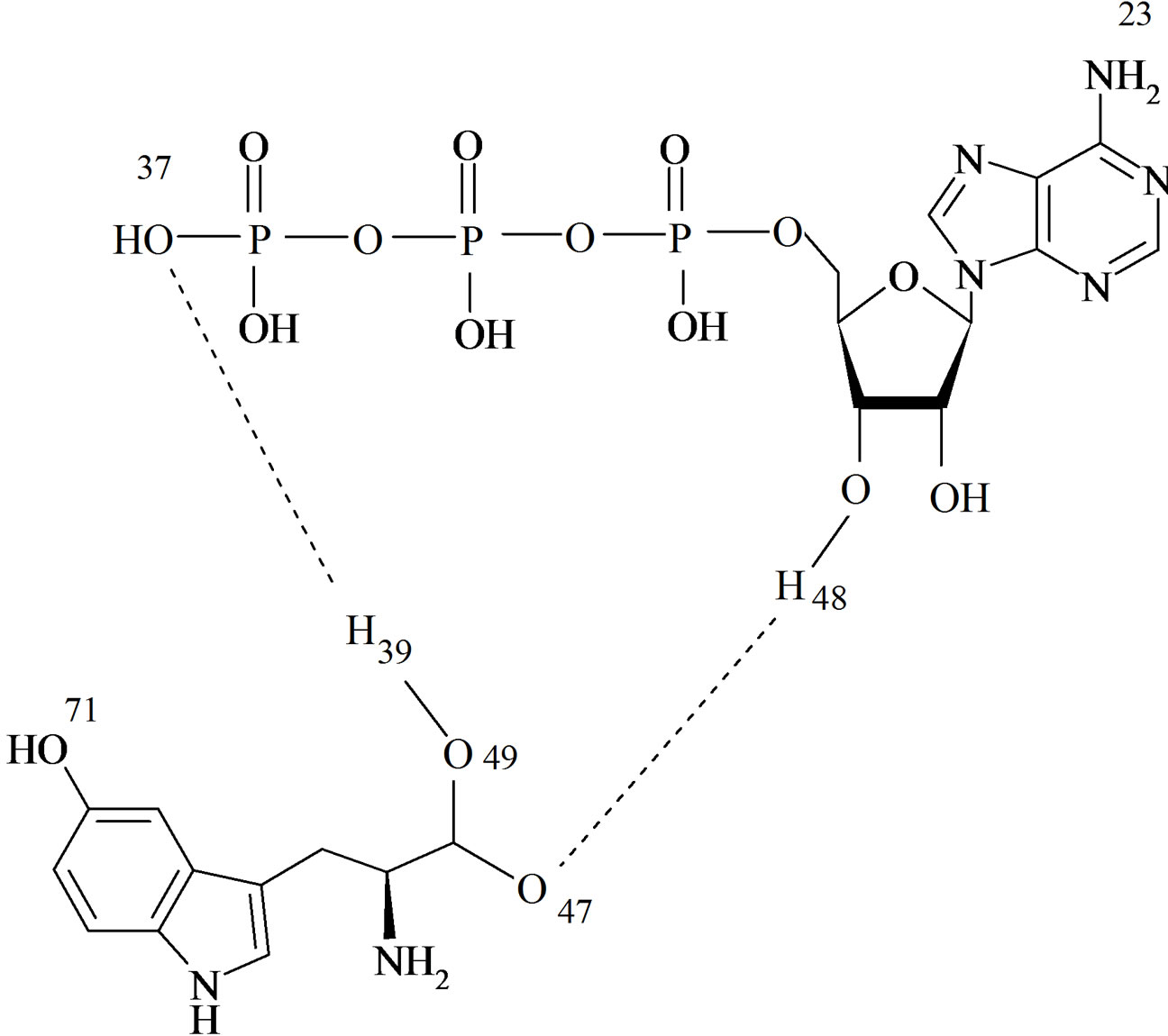
Scheme 1. Structure and the numbering of selected atoms in the ATP-5-HTP complex.
logically active molecules. The present paper targets such a goal by characterizing 5-HTP and the nucleotide ATP, as well as their association in a complex.
The electronic structure of a possible complex DHEA with serotonine and a complex DHEA with ATP has been described by the authors [7-9].
2. Methods
The initial input for 5-HTP and for ATP were obtained from a molecular mechanics calculation (MM+ force field) [10]. The molecular geometries were optimized without any constraints.
The ab initio calculations were carried out using the Gaussian 09 program [11]. The geometries of ATP, 5-HTP and of their complex were optimized at the 3-21G* level, starting from an INDO guess. A stationary point was found. At this point, a refinement was carried out by a single point at the B3LYP/6-31G* level [12].
In the last part of this paper, an analysis of the molecular wave function performed in terms of localized electron-pair bonding units using the NBO program is given [13,14]. This analysis is deemed very important to understand the various interactions involving each component of the complex under study.
Computed HOMO and LUMO orbitals were drawn with the Gview-09 program [15].
The structure and the numbering of selected atoms in the ATP-5-HTP complex are shown in Scheme 1.
3. Results and Discussion
3.1. Structure and Bonding in the Molecular Units and Their Intermolecular Complex
The formation of the ATP-5-HTP complex is analyzed in terms of geometry, charge and energy parameters. Finding the absolute minimum for a complex is a nontrivial question, given the subtle balance of the intraand intermolecular factors. The different nature of the overall molecular constitution of the two biomolecules, ATP and 5-HTP which possesses essentially a planar p-conjugated core, practically precludes a significant association of the p-p stacking type. The strongest association involves hydrogen bonds. There are several possible patterns for hydrogen bonding (O-H...O, O-H...N, involving the various heteroatom combinations). The supramolecular association presented here is the optimal one due to the supplementary stabilization resulting from the alignment of the dipoles on the molecular constituents. The bonding associations O(37)...H(39) and O(47)…H(48) have a regular length for the given type, 1.38415 Å and 1.51651 Å respectively. The geometry of association is further characterized by O37….H39-O49 angle and O47…H48O15 angle values (168.192 and 149.386 respectively). It should be noted that the atoms O37…H39-O49 of hydrogen bond are almost collinear, which suggests that this interaction is the dominant one in the association.
DFT results can be credited with a higher confidence in the quantitative respects because of their treatment of correlation effects. On the other hand, it is acknowledged that the regular DFT functionals face intrinsic problems in the long-range regime [10].
Table 1 shows that atom O47 from 5-HTP acquires the largest negative charge in B3LYP calculations. A larger positive increase of the charge is noted for the bridge hydrogen, H39. It is also interesting to note that the inductive effect produces a positive charge increase on atom H48 from the outer O-H bond of the intermolecular association region.
The effect of the association with ATP is then an activation of 5-HTP induced by this electronic distribution change. The resulting activation mechanism of 5-HTP would then be due to a potentially significant structural rearrangement as shown by the data in Table 1. In 5-HTP, the HOMO and LUMO orbitals are seen as pure π orbitals. The frontier orbitals from 5-HTP lose their almost pure π-nature, and acquire a hybrid character in the complex. This reveals a subtle influence of the electronegativity factors involved in the donor-acceptor interactions that is accounted for in the frame of the DFT approach.
3.2. Frontier Molecular Orbitals
The highest molecular orbitals (HOMO) and the lowestlying unoccupied molecular orbitals (LUMO) are named as frontier molecular orbitals FMO) [16-18].
The FMOs play an important role in spectra and chemical reactions [18]. The HOMO-1, HOMO, LUMO and LUMO + 1 orbitals distributions computed at the B3LYP/6-31G (d. p) level for the title molecule are illustrated in Figure 1. The HOMO and LUMO are localized on almost the whole molecule of 5-HTP (Table 2 and Figure 1). HOMO-1 and LUMO + 1 orbitals are localized on the adenine part of adenosine molecule from ATP.
Since the HOMO-LUMO energy separation has been used as a simple indicator of kinetic stability it can be said that the molecules complex which has:
mHOMO − mLUMO = 7.5638 eV it seems to be implies in a high kinetic stability and low chemical reactivity [19].
The dipole moment appearing as a result of the formation of the complex (Table 2).
3.3. NBO Analysis of the Complex by B3LYP Calculations
The natural bond orbital (NBO) method [13,14] offers supplementary structural information. The NBO analysis automatically identifies two molecular units correspond

Table 1. Total atomic charge on selected atoms in the molecular components and in the association complex, from DFT Mulliken population (B3LYP) analysis.

Table 2. Reactivity parameters calculated at B3LYP/6-31G* level.
 (a)
(a) (b)
(b)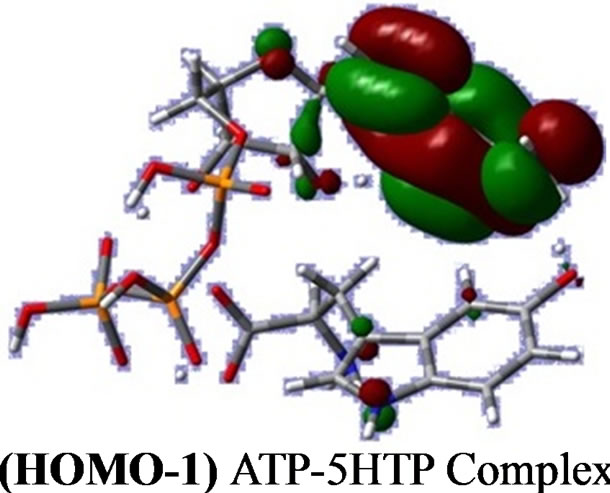 (c)
(c) (d)
(d)
Figure 1. Frontier orbitals in the complex, from DFT calculations.
ing to the ATP and 5-HTP molecules. The perturbation donor-acceptor analysis of the NBO method offers information about intermolecular interactions. A look at the corresponding data shows that the most important intermolecular donor-acceptor contact occurs between an antibonding NBO function (NBO no. 176. LP (3) O37/ 456. BD* (1) O49-H39 of the O-H group and the lone pair of O(37), corresponding to an energy of 70.99 Kcal/ mol.
Though B3LYP in this study is the following: in the NLMO calculations there are a contribution to 174. (2.00000) 95.8636% LP (1) O37 and 182. (2.00000) 93.0824% LP (2) O47 that contain 0.945% H39 s(100.00%) and 2.798% H48 s(100.00%), respectively.
The simplest analysis consists in checking the composition of the natural hybrid orbitals (NHO), which may reveal details about differential hybridization, i.e. sometimes rather important deviations from the usual sp2 (s:p = 33%:67%) or sp3 (s:p = 25%:75%) compositions.
Particularly interesting are the hybrid orbitals associated with the intermolecular hydrogen bond formed by the sequence of atoms O(37).......H(39)-O(49). The hybrids along BD (1) P32-O37 bond has the following compositions: (28.54%) P 32 and (71.46%) O37 but exists also a contribution of H39 of 0.083% (the hydrogen from the bond) in NLMO analysis. The NBO analysis shows that the hybrid composition is not so standard, and is in fact better characterized by an sp2 differential hybridization.
The lone pair devoted to the H(39)...O(37) hydrogen bond has the nonstandard composition s(17.02%) p 4.87 (82.98%) which practically suggests an sp3 character.
The hybrids along the H39-O49 and H48-O15 bonds have the following compositions s(39.47%) p 1.53 (60.53%) and s(28.60%) p 2.50 (71.40%) respectively.
The first one has an sp character and the last one has an sp3 character.
The heterogeneous nature of the bond is measured by the 71.46% participation of the oxygen hybrid orbitals in the P-O bond. Similarly in the O-H bond described, the oxygen hybrid orbital percentage is 81.63%.
This one emphasizes the presences of the H bonds in the complex.
4. Conclusions
From these calculations, it appears the possibility of forming an association complex between ATP and 5-HTP, emphasized by the B3LYP calculations.
The NBO analysis reveals several nonstandard hybrid compositions and the associated donor-acceptor perturbative schemes support the idea of a moderate strength hydrogen bond, cumulated with electrostatic effects, leading to a firmly bound molecular complex.
The present theoretical study on the electronic changes brought about by complexation leads to the hypothesis that an enhancement in the biological action of 5-HTP and/or ATP could result from their interaction. This hypothesis should now be reinforced by the experimental observation of an interaction between those two molecules.
The role of ATP (adenosine triphosphate acid) that is the major as a source of energy in the human organism. associated to 5-HTP, a precursor in the synthesis of serotonine, may lead to the design of a new very useful drug.
REFERENCES
- H. Weinstein and R. Osman, “On the Structural and Mechanistic Basis of Function, Classification, and Ligand Design for 5-HT Receptors,” Neuropsychopharmacology, Vol. 3, No. 5-6, 1990, pp. 397-409.
- K. A. Smith, C. G. Fairburn and P. J. Cowen, “Relapse of Depression after Rapid Depletion of Tryptophan,” Lancet, Vol. 349, No. 9056, 1997, pp. 915-919. http://dx.doi.org/10.1016/S0140-6736(96)07044-4
- M. Kaneko, N. Oshima, Y. Numata, K. Honda, R. Tachibana, A. Watanabe, Y. Takahashi and H. Kumashiro, “Psychopharmacological and Biochemical Characteristics of 5-Hydroxytryptophan Responder in Depression,” Neuroscience, Vol. 17, No. 3, 1991, pp. 349-358.
- K. Lohmann, “Über die Pyrophosphatfraktion im Muskel,” Naturwissenschaften, Vol. 17, No. 31, 1929, pp. 624-625. http://dx.doi.org/10.1007/BF01506215
- F. Lipman, “Metabolic Generation Andutilization of Phosphate Bond Energy,” Advances in Enzymology and Related Subjects of Biochemistry, Vol. 1, 1941, pp. 99-162.
- W. A. Bridger and J. F. Henderson, “Cell ATP,” Wiley, New York, 1983, pp. 9-19.
- D. A. Lerner, J. Weinberg and C. Balaceanu-Stolnici, “Ab Initio and Semiempirical Molecular Orbital Calculations on DHEA I. The Electronic Structure,” Revue Roumaine de Chimie, Vol. 47, 2002, pp. 893-899.
- D. A. Lerner, J. Weinberg, F. Cimpoesu and C. Balaceanu-Stolnici, “Theoretical Study of DHEA: Comparative HF and DFT Calculations of the Electronic Properties of a Complex between DHEA and Serotonin,” Journal of Molecular Modeling, Vol. 12, No. 2, 2006, pp. 146-151. http://dx.doi.org/10.1007/s00894-005-0007-9
- J. Weinberg, F. Cimpoesu and D. A. Lerner, “The Association of Dehydro-Epiandrosterone and Adenosine Triphosphate Acid: A DFT Study of Interactions between Prototypic Biologically Active Molecules,” Journal of Molecular Structure: THEOCHEM, Vol. 912, No. 1-3, 2009, pp. 32-37. http://dx.doi.org/10.1016/j.theochem.2009.04.040
- N. L. Allinger, “Conformational Analysis. 130. MM2. A Hydrocarbon Force Field Utilizing V1 and V2 Torsional Terms,” Journal of the American Chemical Society, Vol. 99, No. 25, 1977, pp. 8127-8134. http://dx.doi.org/10.1021/ja00467a001
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, “Gaussian 09,” Revision A.1, Gaussian, Inc., Wallingford, 2009.
- C. J. Cramer, “Essentials of Computational Chemistry. Theories and Models,” Wiley, New York, 2002.
- A. E. Reed, L. A. Curtiss and F. Weinhold, “Intermolecular Interactions from a Natural Bond Orbital, DonorAcceptor Viewpoint,” Chemical Review, Vol. 88, No. 6, 1988, pp. 899-926. http://dx.doi.org/10.1021/cr00088a005
- E. D. Glendening, A. E. Reed, J. E. Carpenter and F. Weinhold, “The NBO3.0 Program,” University of Wisconsin, Copyright 1996-2001.
- E Frisch, D. R. Dennington II, T. A. Keith, A. B. Nielsen and A. J. Holder, “The ‘GaussView 09W’ Version 7.0,” Copyright 1995-2009.
- K. Fukui, T. Yonezawa and C. Nagata, “An Investigation into the Reactivity of Isotetralin,” Bulletin of the Chemical Society of Japan, Vol. 27, No. 7, 1954, pp. 423-427. http://dx.doi.org/10.1246/bcsj.27.423
- K. Fukui, T. Yonezawa and C. Nagata, “Interrelations of Quantum-Mechanical Quantities Concerning Chemical Reactivity of Conjugated Molecules,” Journal of Chemical Physics, Vol. 26, No. 4, 1957, p. 831. http://dx.doi.org/10.1063/1.1743416
- I. Fleming, “Frontier Orbitals and Organic Chemical Reactions,” Wiley, London, 1976.
- S. Yazici, C. Albayrak, I. E. Gümrükçüoğlu, I. Senel and O. Büyükgüngör, “Experimental and Density Functional Theory (DFT) Studies on (E)-Acetyl-4-(4-Nitrophenyldiazenyl) Phenol,” Journal of Molecular Structure, Vol. 985, No. 2-3, 2011, pp. 292-298. http://dx.doi.org/10.1016/j.molstruc.2010.11.009

