International Journal of Analytical Mass Spectrometry and Chromatography
Vol.03 No.01(2015), Article ID:54931,12 pages
10.4236/ijamsc.2015.31001
GC/MS Analyses of Thiosemicarbazones Synthesized from Acetophenones: Thermal Decay and Mass Spectra Features
Belén Gastaca1, Gastón Galletti1, Hernán Rubén Sánchez2, Reinaldo Pis Diez2, María de las Mercedes Schiavoni1, Jorge Javier Pedro Furlong1*
1Laboratorio de Estudio de Compuestos Orgánicos (LADECOR), Departamento de Química, Facultad de Ciencias Exactas, Universidad Nacional de La Plata, La Plata, Argentina
2CEQUINOR, Centro de Química Inorgánica (CONICET, UNLP), Facultad de Ciencias Exactas, UNLP, La Plata, Argentina
Email: *furlong@quimica.unlp.edu.ar
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 20 February 2015; accepted 23 March 2015; published 24 March 2015
ABSTRACT
The mass spectral fragmentation of thiosemicarbazones synthesized from acetophenones has been studied by CG/MS. These carbonyl compounds exhibit chromatographic peaks which are not observed in aliphatic analogues or those synthesized from aldehydes. The analysis of the corresponding spectra has allowed structural assignment to the dimerization of gas phase neutral fragments. Theoretical calculations (DFT level) also provide evidence to support the experimental observations.
Keywords:
Thiosemicarbazones, Mass Spectrometry Analyses, Dimerization of Thiosemicarbazones

1. Introduction
Thiosemicarbazones constitute an interesting family of organic compounds due to their bioactivity as well as their structural properties.
Previous studies have demonstrated that these substances exhibit a wide variety of biological actions. It is known that they have antitumor, antibacterial, antiviral, antiprotozoal and cytotoxic effects [1] .
It has been reported that this family of compounds represents a pharmacologic alternative for the treatment of Chagas-Mazza disease. Chagas’ disease afflicts more than 24 million individuals in South and Central America producing a debilitating life-long disease. Thiosemicarbazones can inhibit cruzain, the major cysteine protease present in Trypanosome cruzi that is expressed in every step of the life of the parasite that causes the mentioned disease [2] .
Considering their structural properties, these molecules exhibit tautomeric equilibria. This is relevant because the occurrence of a certain tautomer determines the reactivity of the compound. Thiosemicarbazones can exhibit several tautomers [3] with open and ring structures. Open thioketo forms are certainly the most stable ones under the GC injection conditions.
The occurrence of the ring structures has been investigated by NMR [4] and it is known that the solvent polarity modifies the equilibria position. It has been reported that the 4-methyl and the 2-methyl-thiosemicarba- zones of acetone convert into the corresponding 1,2,4-triazolidine-3-thiones in deuteratedtrifluoroacetic acid, while they are present exclusively as the open chain forms in d6-dimethylsulfoxide.
Thiosemicarbazones are also used as models in bioinorganic processes [5] [6] . One of the important aspects of semicarbazones and thiosemicarbazones is that they can form complexes with metals such as Ni(II), Co(III), Zn(II) and Fe(III) [7] . The ligands may exhibit tautomerism and can be complexed with metals allowing them to act with an unusual coordination number, such as six or seven. The complexes in turn, are biologically important, since they have antimicrobial and antitumor activity. The thiosemicarbazones are also complexed with Cu(II), and can make it through the sulfur and imine nitrogen. Compounds that contain a hydroxyl group ortho to the imino group are also studied; they can form hydrogen bonds of type OH ... N or O ... HN in enol, keto-amine and imine tautomeric forms [8] .
In the present work, a study of thiosemicarbazones synthetized from acetophenone and substituted acetophenones is carried out by means of gas chromatography-mass spectrometry. It should be mentioned that the GCMS spectra revealed not only the dissociation of the thiosemicarbazones in the injection port, but also the formation of compounds of higher molecular weight at longer retention times. Additionally, they exhibit unusual fragmentation routes (homolytic ruptures of even electron ions). Theoretical calculations (DFT level) were used to evaluate the feasibility of such fragmentation mechanisms. It is well known that computational tools are of great aid to gain insights that could help to understand and predict the structure, stability, and reactivity of organic compounds. Among them, methods based on the Density Functional Theory (DFT) have proven to be very effective.
2. Materials and Methodology
The compounds under study, thiosemicarbazone of acetophenone (I), MW 193 Dalton; thiosemicarbazone of 4-chloroacetophenone (II), MW 227 Dalton; thiosemicarbazone of 4-nitroacetophenone (III), MW 238 Dalton; thiosemicarbazone of 4-methylacetophenone (IV), MW 207 Dalton, were synthesized adapting procedures from literature [9] [10] . Table 1 depicts the corresponding mass spectral data.
2.1. Reagents
All the reagents have been commercially acquired and have been used without further treatment:
・ Thiosemicarbazide. Sigma-Aldrich, St. Louis, MO, USA, 99%;
・ Acetophenone. Sigma-Aldrich, St. Louis, MO, USA, 99%;
・ 4-chloroacetophenone. Sigma-Aldrich, St. Louis, MO, USA, 97%;
・ 4-nitroacetophenone. Sigma-Aldrich, St. Louis, MO, USA, 98%;
・ 4-methylacetophenone. Sigma-Aldrich, St. Louis, MO, USA, ≥95%.
2.2. Structural Determinations
2.2.1. Gas Chromatography-Mass Spectrometry-Single Quadrupole
These determinations were performed by injection of dimethyl sulfoxide solutions (0.5 - 1 μL, 15 mg∙mL−1) in an HP 5890 Chromatograph connected to an HP 5972 A mass selective detector. An HP5-MS (Hewlett-Packard) capillary column ( 30 m × 0.25 mm × 5 μm) was used with helium (99.999%) as the carrier gas (0.6 mL∙min −1 in column, split ratio 1:30). The temperatures set points were: 250˚C in the split injector, 300˚C in the interface, 280˚C in the ion source and the oven ramp started at 100˚C (5 min) and ended at 350˚C with a heat rate of 10˚C∙min−1. The electron energy was 70 eV and the pressure in the mass spectrometer was about 10−5 torr, thus
Table 1. Mass spectrometric data of the studied compounds.
precluding ion-molecule reactions. These determinations were carried out to examine the mass spectrometrical behavior of these compounds which could exhibit tautomeric equilibria and are thermolable.
2.2.2. Gas Chromatography-Mass Spectrometry-Ion Trap
These determinations were performed by injection of methanol solutions (1 μL) in a Thermo Quest Trace 2000 (Thermo Electron) gas chromatograph connected to a Finnigan Polaris ion trap detector (Thermo Electron) under the same experimental conditions already mentioned for the single quadrupole GC/MS system. This instrumentation was utilized to confirm proposed fragmentation pathways by CID (collision induced dissociation) using helium as the damping gas, a CID voltage of 4 - 7 eV and excitation energy of 0.30 - 0.45. These experiments were done by selecting a precursor ion from the full-scan spectrum and carrying out the corresponding MS/MS product ion scan. All 2D spectra were recorded with the same spectrometer.
2.2.3. Computational Procedures
Bond dissociation energies for some homolytic fragmentations were calculated within the context of the DFT [11] - [13] . Geometry optimization of the molecules involved in the processes were accomplished using the Becke’s three parameters hybrid density functional [14] with the gradient-corrected correlation functional due to Lee, Yang and Parr [15] , a combination that gives rise to the well-known B3LYP method [16] . The double- hybrid RI-PWPB95 functional [17] , including the D3 dispersion correction due to Grimme [18] and the Becke- Johnson damping function [19] , were used to obtain a better estimation of total electronic energies from single point calculations on previously optimized geometries. The def2-SVP and the def2-QZVPP basis sets [20] were utilized for geometry optimizations and for single point calculations, respectively. All the calculations were carried out with the ORCA package [21] .
3. Results and Discussion
3.1. Gas Chromatography-Mass Spectrometry
Figure 1 shows the total ion chromatogram (TIC) after injection of Compound IV.
As it can be seen, there are two substances. Peak purity allowed discarding co-elution in any of the observed peaks.


Figure 1. Chromatographic peaks observed after GC/MS analysis of Compound IV at (a) 250˚C, (b) 300˚C in the injection port.
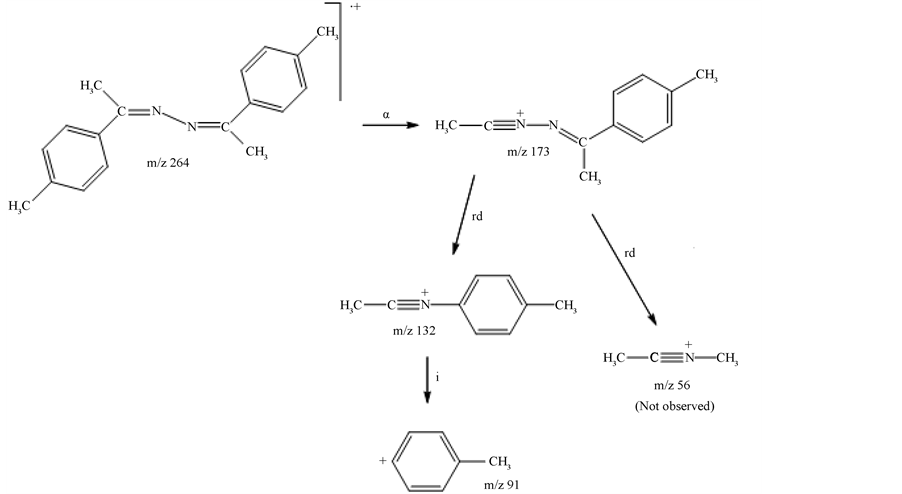
The mass spectrum in Figure 2(a) corresponds to the chromatographic peak at 5.2 minutes. According to the fragmentation routes analysis (Scheme 1), the mentioned spectrum is due to the thiosemicarbazone of 4-methy- lacetophenone (ammonia loss has very low energy requirement thus precluding the observation of the molecular ion in the spectrum). Schemes notations for fragmentation mechanisms are: α (alpha rupture), i (inductive cleavage), rH (Hydrogen rearrangement), rd (displacement reaction) and Hom. (homolityc cleavage). This last fragmentation pathway is somehow unusual and not favored for even electron ions. This work gives support to these mechanisms not only from the mass spectrometric data but also from theoretical calculations discussed later.
The inductive cleavage forms an unstable ion (low abundance) that easily decomposes inductively to form the tropylium ion. A rather unusual fragmentation pathway is observed to form the radical ion at m/z 133 from the rearranged molecular ion. The occurrence of the homolytic cleavage is a consequence of the relatively low energy requirement to break the N-N bond. The fragmentation pathways have been confirmed by GC-MS2 (Ion trap, see experimental part), with the exception of those coming from the molecular ion (m/z 207), that has not been observed.
For thiosemicarbazones I and III the easy loss of ammonia is also the reason for the molecular ion absence in their spectra. For the thiosemicarbazone of 4-chloroacetophenone (II), the observation of the ion at m/z 168 in the highest mass region of the mass spectrum would be due to the easy loss of S=C=NH from the corresponding molecular ion.
The mass spectrum in Figure 2(b) corresponds to the chromatographic peak at retention time 10.2 minutes. It does match neither the predicted product spectrum nor any of the reactants spectra. Considering the fact that the substance is pure, it is clear that a reaction takes place in the injection port of the chromatograph.
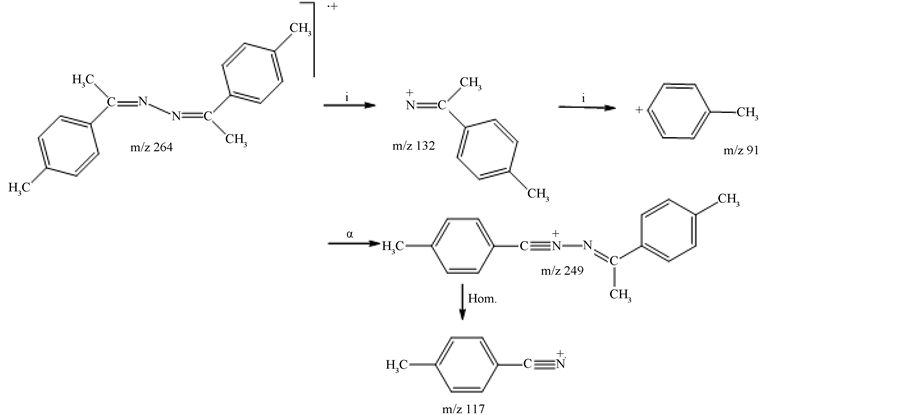
An exhaustive analysis of the ions present in the spectrum and of the fragmentation routes, made possible to propose the “dimer” structure shown in Figure 3, which is consistent with experimental data (Scheme 2). The second largest peak at m/z 249 is assigned to the methyl loss and the base peak (m/z 91) is the tropylium ion.
The considerations already commented for the thiosemicarbazone spectrum also apply to the dimer spectrum although the homolytic cleavage takes place in an even electron ion at m/z 249 to render a stable 4-methylben-
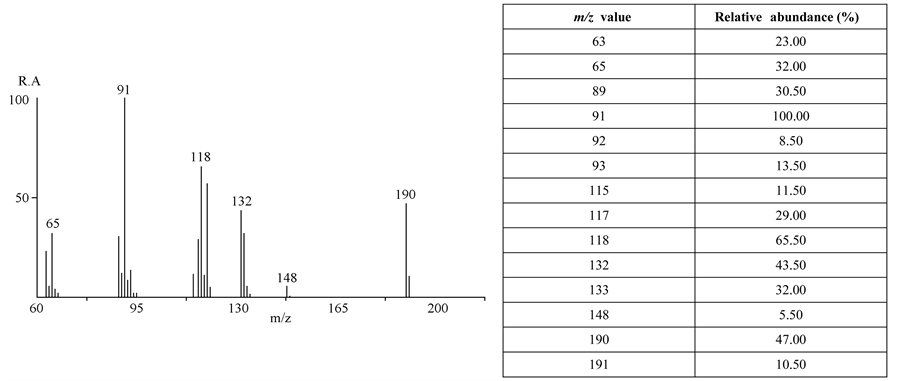

Figure 2. Mass spectra of (a) peak (1) at 5.2 minutes and (b) peak (2) at 10.2 minutes from Figure 1.
Figure 3. Proposed structure for compound (2) in the chromatogram shown in Figure 1.
zonitrile radical ion (m/z 117). Additionally, transposition-like reactions are observed with formation of nitriles (4-methylbenzonitrile and acetonitrile, to form m/z 132 in both cases). A four centers transition state should be considered to explain these displacement reactions. The fragmentation pathways have been confirmed by GC- MS2 (Ion trap, see experimental part).
This mass spectral behavior is observed for every compound under study. The fragments shown in Scheme 2 are present in all spectra. In addition, the ratio [Fragment Dimer]/[Thiosem] is always much higher than 1 and it increases while injection temperature increases. Direct injection to the mass spectrometer resulted in mass spectra superposition with similar injection temperature dependence.
On the other hand, aliphatic semicarbazones and thiosemicarbazones, that have been previously prepared and studied, have not shown a similar behavior.
These observations have allowed postulating that the considered substances react in the injection port, breaking out and undergoing dimerization of one of the resultant fragments, as it is shown in Scheme 3.

Scheme 1. Fragmentation routes of the thiosemicarbazone of 4-methylacetophenone.
Small volatile molecules could be formed by radical reactions in the injection port of the GC (HN=C=S, H2NNH2), not detected under the experimental conditions (in very early eluates).
In order to get additional information that may support or reject this hypothesis, a series of combined substrates (binary samples) have been injected in the chromatograph (“cross experiment”).
These solutions have been prepared using equimolar quantities of each compound (2.2 × 10−5 moles in 2 mL of DMSO).
Table 2 shows the composition of the mentioned mixtures.

Scheme 2. Fragmentation routes of the proposed structure.
Table 2. Combined solutions of the studied thiosemicarbazones.

Scheme 3. Dimer structure formation.
Figure 4(a) shows the chromatogram of mixture E (4-chloroacetophenone thiosemicarbazone and 4-methy- lacetophenone thiosemicarbazone).
As it can be seen, there are three neutral species eluting from the chromatographic column.
A comparative analysis with the chromatograms of the individual solutions (see Figure 1 and Figure 2(a)), reveals that peak at 10 min corresponds to dimer species of neutral fragment from II and the other one at 10.6 min is assigned to the analogous fragment dimer from IV. Retention times and spectra are independent of injection temperature although this parameter modifies the relative intensities of the signals.
The mass spectrum of the chromatographic peak at 11.2 min is shown in Figure 4(b).
The molecular ion is at m/z 284. The ion at m/z 269 is the methyl loss and that one at m/z 91 should be assigned to the tropylium ion. The base peak, at m/z 117, can be assigned to the ionic structure of p-methyl-benzo- nitrile.
In the mass spectral analysis of the Compound II and its neutral fragment dimer, the signal at m/z 111 has been assigned to the ion C6H4Cl]+, and the peak at m/z 137 has been assigned to the structure Cl(C6H4)CN]+.
The experimental data and their analysis have allowed proposing the formation of a crossed dimer species (“heterodimers”) in the injection port, whose molecular weight is an intermediate between the molecular weights of the respective “homodimers”, as it is shown in Figure 5.
The structure shown above is consistent with all the spectral assignments.
The fragmentation routes and the respective ions are shown in Scheme 4, for this particular case.
Mass spectral considerations are similar to those from previous comments for the thiosemicarbazone and its corresponding homodimer although the presence of the chlorine atom would be the explanation for the very low abundances observed for m/z 152 and 111.
Additionally, displacement reactions that render ion products with aliphatic moieties attached to the charge nitrogen, have lower abundances (e.g. m/z 152 and 132), compared with ions having aromatic moieties instead (e.g. m/z 228).
The behavior previously described is observed for every mixture and the tendency involving the intensities ratios of the chromatographic peaks is followed in all cases. The fact that the highest intensity is shown by the heterodimer in each mixture, is caused by a statistical effect: in these experimental conditions, it is more likely the bonding between two fragments produced by the thermolysis of molecules of different nature than the formation of the homodimers.
Table 3 shows m/z values of the analogous ions to those presented in Scheme 4 for each pair of compounds. These data not only support the dimer formation in the injection port of the gas chromatograph but also the proposed fragmentation pathways which have been additionally proved by GC/MS in an ion trap mass spectrometer (see experimental part).


Figure 4. Chromatogram of mixture E (a) and mass spectrum of peak at 11.2 min (b).
Figure 5. Heterodimer of Compounds II and IV.
3.2. Theoretical Calculations
The bond dissociation energy for the homolytic fragmentation of the N-N bond of the ion shown in Scheme 5 was calculated to be 3.55 eV. For comparison purposes, the bond dissociation energy for the homolysis of an even electron ion in which the nitrogen atom is bound to an sp2 carbon atom (see the ion produced by displacement reaction in Scheme 5), was also calculated. In this case, the bond dissociation energy is considerably higher,
Scheme 4. Fragmentation routes of the heterodimer of Compounds II and IV.
Table 3. m/z values of ions produced by fragmentation of the “heterodimers” in every mixture of compounds compared to those from “homodimer” of Compound IV (H)a.
aEach column corresponds to a specific fragmentation pathway. bIon particularly unstable (low abundance) due to the electro-withdraw- ing nitro group.
namely 5.36 eV, rendering also the m/z 117 ion. So that, this ion would be mainly formed by the first homolytic route. Respect to the inductive cleavage, the calculation renders an energy value of 1.35 eV (driven by the loss of the stable 4-methylbenzonitrile molecule) which is even lower than the first homolytic rupture. This pathway leads to the production of a highly unstable ion (m/z 132) which rapidly forms the very stable ion at m/z 91 (tropylium ion).
4. Conclusion
The GC/MS spectra together with the chromatographic behavior of thiosemicarbazones synthesized from acetophenones has been analyzed. Unexpected chromatographic peaks were present which were not observed for
Scheme 5. Fragmentation pathways for the heterodimer of the thiosemicarbazone IV and corresponding energies from theoretical calculations (DFT).
those synthesized from aldehydes. The analysis of the corresponding spectra has allowed structural assignment to the dimerization of gas phase neutral fragments. Crossed experiments with mixtures of substituted thiosemicarbazones confirmed that this transformation was taking place. Rather unusual ion fragmentations (radical ions as product ions of even electron precursor ions) had to be considered for mass spectral interpretation of the mass spectra of the selected compounds. This fragmentation route has been additionally supported by theoretical calculations at DFT level.
Acknowledgements
Financial support is highly acknowledged to Facultad de Ciencias Exactas, UNLP, ANPCYT (National Agency for the Promotion of Sciences and Technology), and UNLP (La Plata National University), Buenos Aires, Argentina. RPD is member of CONICET, Argentina. HRS acknowledges CONICET for a PhD scholarship.
References
- Tenório, R.P., et al. (2005) Tiossemicarbazonas: Métodos de obtenção, aplicações sintéticas e importância biológica. Química Nova, 28, 1030. http://dx.doi.org/10.1590/S0100-40422005000600018
- Du, X., et al. (2002) Synthesis and Structure-Activity Relationship Study of Potent Trypanocidalthiosemicarbazone Inhibitors of the Trypanosomal Cysteine Protease Cruzain. Journal of Medicinal Chemistry, 45, 2695-2707. http://dx.doi.org/10.1021/jm010459j
- Ershov, A.Yu., Koshmina, A.N.V., Mokeev, M.V. and Gribanov, A.V. (2003) The Isoxazolidine-1,2,4-Triazolidine-3- Thione Tautomeric System. Chemistry of Heterocyclic Compounds, 39, 1257-1258. http://dx.doi.org/10.1023/B:COHC.0000008277.45834.c5
- Zelenin, K., Kuznetsova, O. and Alekseyev, V. (1993) Ring-Chain Tautomerism of N-Substituted Thiosemicarbazones. Tetrahedron, 49, 1257-1270. http://dx.doi.org/10.1016/S0040-4020(01)85816-6
- Singh, N.K., et al. (2001) Spectral, Magnetic and Biological Studies of 1,4-Dibenzoyl-3-Thiosemicarbazide Complexes with Some First Row Transition Metal Ions. Proceedings of the Indian Academy of Sciences: Chemical Sciences, 113, 257-273. http://dx.doi.org/10.1007/BF02708645
- Offiong, O.E. and Martelli, S. (1997) Stereochemistry and Antitumor Activity of Platinum Metal Complexes of 2-Ace- tylpyridine Thiosemicarbazones. Transition Metal Chemistry, 22, 263-269. http://dx.doi.org/10.1023/A:1018416624951
- Samanta, B., Chakraborty, J., Shit, S., Batten, S.R., Jensen, P., Masuda, J.D. and Mitra, S. (2007) Synthesis, Characte- rization and Crystal Structures of a Few Coordination Complexes of Nickel(II), Cobalt(III) and Zinc(II) with N'-[(2- Pyridyl)Methylene]Salicyloylhydrazone Schiff Base. Inorganica Chimica Acta, 360, 2471-2484. http://dx.doi.org/10.1016/j.ica.2006.12.019
- Yildiz, M., Ünver, H., Erdener, D., Kiraz, A. and Ocakİskeleli, N. (2009) Synthesis, Spectroscopic Studies and Crystal Structure of (E)-2-(2,4 Dihydroxybenzylidene) Thiosemicarbazone and (E)-2-[(1H-indol-3-yl)Methylene]Thiosemicar- bazone. Journal of Molecular Structure, 919, 227-234. http://dx.doi.org/10.1016/j.molstruc.2008.09.008
- Wheeler, A.S. and Bost, R. (1924) 4-Para-Tolylsemicarbazide and Certain Derivatives. Journal of the American Chemical Society, 46, 2813-2816. http://dx.doi.org/10.1021/ja01677a032
- Cheronis, N.D.I., Hodnett, E.M.A. and Entrikin, J.B.E. (1968) Semimicro Qualitative Organic Analysis: The Systematic Identification of Organic Compounds.
- Hohenberg, P. and Kohn, W. (1964) Inhomogeneous Electron Gas. Physical Review, 136, B864-B871. http://dx.doi.org/10.1103/PhysRev.136.B864
- Kohn, W. and Sham, J. (1965) Self-Consistent Equations including Exchange and Correlation Effects. Physical Review, 140, A1133-A1138. http://dx.doi.org/10.1103/PhysRev.140.A1133
- Parr, R.G. and Yang, W. (1989) Density-Functional Theory of Atoms and Molecules. Oxford University Press, Oxford.
- Becke, A.D. (1993) Density-Functional Thermochemistry. III. The Role of Exact Exchange. The Journal of Chemical Physics, 98, 5648-5652. http://dx.doi.org/10.1063/1.464913
- Lee, C., Yang, W. and Parr, R.G. (1988) Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Physical Review, B37, 785-789. http://dx.doi.org/10.1103/PhysRevB.37.785
- Stephens, P.J., Devlin, F.J., Chabalowski, C.F. and Frisch, M.J. (1994) Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. The Journal of Physical Chemistry, 98, 11623- 11627. http://dx.doi.org/10.1021/j100096a001
- Goerigk, L. and Grimme, S. (2011) Efficient and Accurate Double-Hybrid-Meta-GGA Density Functionals―Evaluation with the Extended GMTKN30 Database for General Main Group Thermochemistry, Kinetics, and Noncovalent Interactions. Journal of Chemical Theory and Computation, 7, 291-309. http://dx.doi.org/10.1021/ct100466k
- Grimme, S., Antony, J., Ehrlich, S. and Krieg, J. (2010) A Consistent and Accurate ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. The Journal of Chemical Physics, 132, Article ID: 154104. http://dx.doi.org/10.1063/1.3382344
- Grimme, S., Ehrlich, S. and Goerigk, L. (2011) Effect of the Damping Function in Dispersion Corrected Density Functional Theory. Journal of Computational Chemistry, 32, 1456-1465. http://dx.doi.org/10.1002/jcc.21759
- Weigend, F. and Ahlrichs, R. (2005) Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. The Journal of Physical Chemistry, 7, 3297-3305.
- Neese, F. (2012) The ORCA Program System. WIREs Computational Molecular Science, 2, 73-78. http://dx.doi.org/10.1002/wcms.81
NOTES
*Corresponding author.










