Advances in Materials Physics and Chemistry
Vol.05 No.04(2015), Article ID:55858,6 pages
10.4236/ampc.2015.54014
Effect of Degree of ClO− Hypochlorite on the Wet Synthesis of Ferrate (VI)
Abdellatif El Maghraoui, Abdelaziz Zerouale, Mustapha Ijjaali
Laboratory of Chemistry of Condensed Mater (LCMC), Faculty of Sciences and Technology, Sidi Mohammed Ben Abdellah University, Fez, Morocco
Email: elmaghraabdellatif@yahoo.fr
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 13 December 2014; accepted 19 April 2015; published 22 April 2015
ABSTRACT
This work is a result of previously done studies on the synthesis of A2FeVIO4 wet ferrate (VI) formula, using chlorine as an oxidant. The major problem of these ferrates is related to their stability over time. This brings us to identify and optimize the critical parameters influencing the preparation of the Na2FeO4 at room stable phase with acceptable performance. The use of water bleach (hypochlorite ClO−) at a chlorometric degree of 50˚F in the synthesis of the Na2FeO4 ambient stable phase promotes the oxidation of iron (II) iron to (VI) in a concentrated NaOH alkaline medium. The synthesis reaction is in the presence of FeSO4 7H2O hydrated iron sulfate at a temperature of about 55˚C in order to simplify the synthesis process, to enhance the production of the Fe (VI) and to meet the growing demand of ferrates (VI) for their interest in the treatment of water. Monitoring the degradation of synthesized Na2FeO4 shows its stability up to 12 months, which facilitates storage and transportation. The phases obtained were characterized by IR spectroscopy, and RX by UV spectrophotometer, measuring the optical density at 507 nm.
Keywords:
Ferrates, Bactericides, Antioxidant, Flocculant, Coagulant, Wet, Water Treatment

1. Introduction
The ferrate (VI) is a supercharged iron compound in which the iron is in the oxidation state +6. It is known under the name of iron (VI). The ferrate is extremely powerful, can provide multiple treatments from a single application, does not create disinfection by-products, is environmentally friendly, and solves the difficult treatments which represents the challenges of other oxidants can’t touch. The Ferrate treatment option is often the least expensive and most effective.
The synthesis of ferrate (VI) has been studied by many authors [1] -[11] to be simpler and more suitable methods with a higher yield and stable phases. Despite improvements, the results remain limited.
Ockerman et al. [12] and Scheryer et al. [13] show that the precipitation washing and drying protocols are required to achieve a stable and solid outcome.
Publications and patents for K2FeO4 synthesis modes recommend the use of a ferric salt [14] [15] .
In 1950, Hrostowski and Scott [16] proposed a method to prepare ferrate with a purity of 97% of ferric chloride by oxidation with sodium hypochlorite in a concentrated sodium hydroxide solution at temperatures ranging between 50˚C and 55˚C. Now, for the environment in which operate Hrostowski et al. [16] is highly NaOH concentrated. Na2FeO4 is assumed very soluble, whereas NaCl has precipitated in the solution [17] , which then makes a separation by filtration possible.
El Maghraoui et al. [18] achieved the synthesis of ambient stable Na2FeO4 by the oxidation of iron (II) to iron (VI) by electrochemical means.
The wet method is considered the most practical but remains very expensive.
The aim of this work is to synthesize compounds based on stable Iron (VI), particularly Na2FeO4, at room temperature, to determine the effect of the degree bleach on the synthesis and monitoring of the degradation of Iron (VI) over time.
2. Material and Method
First, the hydrated iron sulfate FeSO4, 7H2O and ClO− bleach (50˚F) are mixed in a NaOH alkaline medium. The mixture is stirred for one hour at a temperature of 55˚C until the mixture becomes red purple characterizing the presence of iron (VI).
Recovering Na2FeO4 is performed by vacuum filtration in order to dry the product at a temperature of 120˚C for 12 hours. Then, the product is dried in a desiccator for at least one hour before grinding to prevent moisture problem [12] .
The obtained final product is analyzed and stored at room temperature in order to monitor its degradation over time.
The synthesis reaction is as follows:

3. Results
The results obtained are shown in Figure 1 and Figure 2. These show that the yield of the oxidation of iron (II) to iron (VI) varies, depending on the degree of ClO− bleach and the drying time.
According to these results (Figure 1 and Figure 2), we noticed that the increase in the chlorometric degree of ClO− bleach water led to higher yields of the reactions but with a maximum at 50˚F, this shows the significant
Figure 1. iron oxidation yield (II) to iron (VI) according to the degree of ClO− bleach to a drying time of 12 hours and at a temperature of 120˚C.
effect of the degree of bleach water on the oxidation of iron (II) to iron (VI). The optimal drying time to achieve a yield of 70% iron (VI) is stable at ambient for 12 hours.
4. Characterization
4.1. Infrared Spectroscopy
The appearance of an infrared spectrum is related to the symmetry of the molecule or group studied. It is expected to 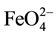 tetrahedral structure to find:
tetrahedral structure to find:
Fundamental characteristic bands of symmetry τd: either υ3, υ4 bands and from the two degenerate modes of vibration: the symmetrical angular elongations and deformations within the tetrahedron resulting in inactive modes in infrared absorption, bands and the υ1, υ2 must be absent from the spectra [19] . Similarity between infrared spectrum isomorphic series [20] .
The presence of the υ1 band and a triplet for υ3 (elongation of the tetrahedron) led Griffith (1966) to consider a lower symmetry τd, very close to τS for  anion [21] . IR spectroscopy is a quantitative method for the determination of Iron (VI) compounds in ferrates. The shape of the spectra is due to the symmetry of the molecule or
anion [21] . IR spectroscopy is a quantitative method for the determination of Iron (VI) compounds in ferrates. The shape of the spectra is due to the symmetry of the molecule or 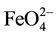 groups (tetrahedral structure). The IR spectrum of the obtained Na2FeO4 (user 820 and 770 cm−1) (Figure 3) showed an identical appearance to that obtained in the high frequency domain [22] .
groups (tetrahedral structure). The IR spectrum of the obtained Na2FeO4 (user 820 and 770 cm−1) (Figure 3) showed an identical appearance to that obtained in the high frequency domain [22] .
Comparing the outgoing strip 820 cm−1 and 770 cm−1 IR spectrum (Figure 3) of the phase with that of Na2FeO4, Weichun et al. [5] observed a similarity of these spectra with light bands of travel Na2FeO4 which may be due to the conditions of preparation and crystallization.
Figure 2. iron oxidation yield (II) to iron (VI) according to the product of the drying time at a temperature of 120˚C and the degree of ClO− bleach 50˚F.
Figure 3. Spectrum infra-red prepared Na2FeO4.
The bands 1140 cm−1 and 620 cm−1 are characteristic of 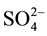 group, while those at 950 cm−1, 930 cm−1 and 860 cm−1 can be assigned to an intermediate compound between Na2SO4 and Na2FeO4 form of a solid solution of the formula Na2FexS1−xO4: sulfate-ferrate [23] .
group, while those at 950 cm−1, 930 cm−1 and 860 cm−1 can be assigned to an intermediate compound between Na2SO4 and Na2FeO4 form of a solid solution of the formula Na2FexS1−xO4: sulfate-ferrate [23] .
4.2. X-Ray Diffraction
The XRD spectrum obtained for Na2FeO4 powdered compound (Figure 4) to verify the crystal structure of this phase [24] [25] and demonstrate the existence of an isomorphism with K2FeO4 and BaFeO4 found by Licht et al. [3] . Dropoff window Diffraction RX is one of the means used to verify the presence of ferrate (VI).
Na2FeO4 the spectrum obtained shows similarity with that of isomorphous compounds including K2FeO4 [8] .
There is a duplication of lines corresponding to the planes (102), (202), (013), (200), (002), (004,) (105), (226), (114), (205), (412), (006), (026), (008), (301) [3] [5] [6] [8] [26] - [29] .
We note the existence of the lines in the X-ray diffractogram of Na2FeO4 not observed in that of K2FeO4. These lines can be assigned to an intermediate between Na2FeO4 and Na2SO4 formula Na2FexS1−xO4 and the most intense peak at 2θ = 27˚ (Figure 4), corresponds to the XRD spectrum of Na2SO4.
5. Monitoring the Degradation of the Ferrate over Time
Spectrophotometry is a quantitative analytical method of measuring the absorbance or optical density of a given chemical substance, generally in solution. The more concentrated the sample is, the more it absorbs light in the proportionality limits set by the Beer-Lambert law.
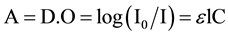
The optical density of samples was determined by a spectrophotometer previously calibrated on the absorption wavelength of the test substance.
According to Sapin et al. [28] , measuring the optical density of the solution of ferrate (VI) is at a wavelength of 507 nm with a pH greater than 10.
The iron of the characteristic peak (VI) exits this wavelength.
The results of calculating the rate of degradation between the month and the state of the production ferrate VI and the different months of storage from the measured optical density is given by the following table.
The relation used to calculate the percentage of degradation of Iron (VI) is given by the following formula:
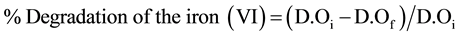
D.Oi: Optical densities of the iron (VI) respectively in the initial state;
D.Of: Optical densities of iron (VI) in the final state.
Figure 4. RX diffractogram prepared Na2FeO4.
Table 1. Optical density of the solution of ferrate (VI) Phase Na2FeO4 synthesized according to the degradation rate between the initial state of the production and storage of ferrate VI during different months (%) and based on the degradation rates between months storage of ferrate VI (%).
According to our results (Table 1), the synthesized iron (VI) may be ambient stable during up to 12 months of storage at room temperature, and the iron degradation rate (VI) in the first six months does not exceed 9.64%.
Note that the rate of degradation of iron (VI) remains variable in function of time and varies differently from one month to the other during storage. Climate change plays a very important role in the degradation rate of ferrate (VI) due to changes in humidity.
6. Discussion
The optimal degree of ClO− bleach or hypochlorite used for the synthesis of ambient stable Na2FeO4 is of the order of 50˚F. This rate plays an important role in iron (VI) synthesis yield.
This is comparable to the studies already made by Hrostowski and Scott [18] Thus, to obtain a strong and stable product requires a drying time of about 12 hours at a temperature of 120˚C [12] [18] .
According to the results, we found out that the duration of 12 months of storage is an important progress in the field of synthetic ambient stable iron (VI) to meet the growing global demand for it to get an industrial plant for the manufacture of this superoxydant and disinfectant [29] .
7. Conclusions
This manuscript reviews the effect of the degree of bleach water (ClO− hypochlorite) on the yield of the synthesis of iron (VI) and its stability over time. This level is of the order of 50˚F with a drying time of 12 hours at a temperature of 120˚C.
Comparing stable Na2FeO4 synthesis results with the bibliography, we note that we have obtained for the first time wet ambient stable ferrates VI with quite a yield of 70% for a period of 12 months. This result represents a significant advance in the field of synthetic iron (VI) at a laboratory scale. This result is very encouraging for mass production of ferrate (VI) on an industrial scale.
References
- Hoy, G. and Corson, M. (1980) Critical Slowing Down of Spin Fluctuations in K2FeO4. Journal of Magnetism and Magnetic Materials, 15, 627.
- Menil, F. (1985) Systematic Trends of the 57Fe Mössbauer Isomer Shifts in (FeOn) and (FeFn) Polyhedra. Evidence of a New Correlation between the Isomer Shift and the Inductive Effect of the Competing Bond T-X (→Fe) (Where X Is O or F and T Any Element with a Formal Positive Charge. Journal of Physics and Chemistry of Solids, 46, 763-789. http://dx.doi.org/10.1016/0022-3697(85)90001-0
- Licht, S., Naschitz, V., Halperin, L., Halperin, N., Lin, L., Chen, J., Ghosh, S. and Liu, B. (2001) Analysis of Ferrate(VI) Compounds and Super-Iron Fe(VI) Battery Cathodes: FTIR, ICP, Titrimetric, XRD, UV/VIS, and Electrochemical Characterization. Journal of Power Sources, 101, 167-176. http://dx.doi.org/10.1016/S0378-7753(01)00786-8
- Licht, S., Tel-Vered, R. and Halperin, L. (2002) Direct Electrochemical Preparation of Solid Fe(VI) Ferrate, and Super- Iron Battery Compounds. Electrochemistry Communications, 4, 933-937. http://dx.doi.org/10.1016/S1388-2481(02)00493-9
- He, W.C., Wang, J.M., Shao, H.B., Zhang, J.Q. and Cao, C.-N. (2005) Novel KOH Electrolyte for One-Step Electrochemical Synthesis of High Purity Solid K2FeO4: Comparison with NaOH. Electrochemistry Communications, 7, 607- 611. http://dx.doi.org/10.1016/j.elecom.2005.04.011
- Xu, Z.H., Wang, J.M., Shao, H.B., Tang, Z. and Zhang, J.Q. (2007) Preliminary Investigation on the Physicochemical Properties of Calcium Ferrate(VI). Electrochemistry Communications, 9, 371-377. http://dx.doi.org/10.1016/j.elecom.2006.09.015
- Híveša, J., Benová, M., Bouzek, K., Sitek, J. and Sharma, V.K. (2008) The Cyclic Voltammetric Study of Ferrate(VI) Formation in a Molten Na/K Hydroxide Mixture. Electrochimica Acta, 54, 203-208. http://dx.doi.org/10.1016/j.electacta.2008.08.009
- Wang, Y.L., Ye, S.H., Wang, Y.Y., Cao, J.S. and Wu, F. (2009) Structural and Electrochemical Properties of a K2FeO4 Cathode for Rechargeable Li Ion Batteries. Electrochimica Acta, 54, 4131-4135.
- Zuzana, M., Bouzek, K., Híveš, J., Sharma, V.K., Raymond, J.T. and Baum, J.C. (2009) Research Progress in the Electrochemical Synthesis of Ferrate(VI). Electrochimica Acta, 54, 2673-2683. http://dx.doi.org/10.1016/j.electacta.2008.11.034
- Jain, A., Sharma, V.K. and Mbuya, M.S. (2009) Removal of Arsenite by Fe(VI), Fe(VI)/Fe(III), and Fe(VI)/Al(III) Salts: Effect of pH and Anions. Journal of Hazardous Materials, 169, 339-344. http://dx.doi.org/10.1016/j.jhazmat.2009.03.101
- Lee, Y.H., Cho, M., Kim, J.Y. and Yoon, J.Y. (2004) Chemistry of Ferrate (Fe(VI)) in Aqueous Solution and Its Application as a Green Chemical. Journal of Industrial and Engineering Chemistry, 10, 161-171.
- Ockerman, L.T., Schreyer, J.M. and Thompson, G.W. (1951) Preparation and Purification of Potassuim Ferrate. VI. Journal of the American Chemical Society, 73, 1379-1381. http://dx.doi.org/10.1021/ja01147a536
- Schreyer J.M., Thompson, G.W. and Ockerman, L.T. (1953) Potasuim Ferrate (VI). Inorganic Synthesis, 4, 164-169.
- Scholder, R., Bunsen, H., Kin, F., Zeiss, W. and Anorg, Z. (1955) Zur Kenntnis der Ferrate(VI). Zeitschrift für anorganische und allgemeine Chemie, 282, 268-279. http://dx.doi.org/10.1002/zaac.19552820129
- El Maghraoui, A., Zerouale, A. and Ijjaali, M. (2015) Process for the Synthesis of Ferrate(VI) Alkali Metal Dry. Advances in Materials Physics and Chemistry, 5, 10-15.
- Hrostowski, H.J. and Scott, A.B. (1950) The Magnetic Susceptibility of Potassium Ferrate. Journal of Chemical Physics, 18, 105-107. http://dx.doi.org/10.1063/1.1747423
- Hooker, A. (1920) Hipocloritos. Chem. and Met. eng., 23, 961.
- El Maghraoui, A., Zerouale, A., Ijjaali, M. and Sajieddine, M. (2013) Synthesis and Characterization of Ferrate(VI) Alkali Metal by Electrochemical Method. Advances in Materials Physics and Chemistry, 3, 83-87. http://dx.doi.org/10.4236/ampc.2013.31013
- Becarud, N. and Dural, C. (1963) Reactions and Properties of Potassium, Barium, and Strontium Ferrates. Comptes Rendus, 257, 1930-1933.
- Gonzales-Vilchez, F. and Griffith, W. (1972) Transition-Metal Tetra-oxo-Complexes and Their Vibrational Spectra. Journal of the Chemical Society, Dalton Transactions, 13, 1416-1421. http://dx.doi.org/10.1039/dt9720001416
- Griffith, W. (1966) Infrared Spectra of Tetrahedral Oxyanions of the Transition Metals. Journal of the Chemical Society A: Inorganic, Physical, Theoretical, 1467-1468. http://dx.doi.org/10.1039/j19660001467
- Tarte, P. and Nizet, G. (1964) Etude infrarouge de quelque composés du type K2FeO4 et BaSO4. Spectrochimica Acta, 20, 503-513. http://dx.doi.org/10.1016/0371-1951(64)80045-X
- Neveux, N. (1993) Voie de synthése originale de ferrates(VI) alcalins stabilisès et leurs applications potentielles dans le traitement des eaux. Thése de doctorat, Universitè de nancy I, Nancy.
- Krebs, V.H. (1950) The Structure of the Potassium Ferrate and Barium Ferrats with 2 Figures. Zeitschrift für anor- ganische und allgemeine Chemie, 263, 175-176. http://dx.doi.org/10.1002/zaac.19502630405
- Helfrich, B. and Lang, K. (1950) Uber salze der eisensaure. Zeitschrift für anorganische und allgemeine Chemie, 263, 169-174.
- Audette, R.J. and Quail, J.W. (1972) Potassium, Rubidium, Césium, and Barium Ferrates VI: Préparations, Infrared Spectra, and Magnetic Susceptibilities. Inorganic Chemistry, 11, 1904-1908. http://dx.doi.org/10.1021/ic50114a034
- Lia, C., Lia, X.Z. and Grahamb, N. (2005) A Study of the Preparation and Reactivity of Potassium Ferrate. Chemosphere, 61, 537-543. http://dx.doi.org/10.1016/j.chemosphere.2005.02.027
- Tsapin, A.I., Goldfeld, M.G., Mcdonald, G.D., Nealson, K.H., Moskovitz, B., Solheid, P., Klemner, W., Kelly, S.D. and Orlandini, K.A. (2000) Iron(VI): Hypothetical Candidate for the Martian Oxidant. Icarus, 147, 68-78.
- El Maghraoui, A., Zerouale, A., Ijjaali, M. and Fikri Benbrahim, K. (2013) The Role of Ferrates(VI) as a Disinfectant: Quantitative and Qualitative Evaluation for the Inactivation of Pathogenic Bacteria. African Journal of Microbiology Research, 7, 3690-3697.






