Advances in Materials Physics and Chemistry
Vol.04 No.10(2014), Article ID:50650,7 pages
10.4236/ampc.2014.410021
Chemical Durability and Structural Proprieties of the Vitreous Part of the System xCaO-(40-x)ZnO-15Na2O-45P2O5
Zineb Chabbou1, Said Aqdim1,2*
1Laboratoire de Physique de haute Energie et de l’Etat Condensé, Faculty of Science, University Hassan II Ain Chock, Casablanca, Morocco
2Laboratoire de Chimie Minérale, Département de Chimie, Faculty of Science, Université Hassan II Ain Chock, Casablanca, Morocco
Email: *said_aq@yahoo.fr, chabbou.zineb@hotmail.fr
Academic Editor: Gustavo Platt, Rio de Janeiro State University, Brazil
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 8 August 2014; revised 23 September 2014; accepted 8 October 2014
ABSTRACT
The influence of CaO on the glass forming characteristics and properties of Na2O-CaO-ZnO-P2O5 glasses has been investigated. According to the studies that we performed on phosphate based glass within system xCaO-(40-x)ZnO-15Na2O-45P2O5 (10 ≤ x ≤ 30; mol%), it was found that the in- crease of CaO and substitution of ZnO can give a good chemical durability. Both Cristallographies X-ray and IR spectroscopy have confirmed the structure change when the CaO content increases in the glass. This change results in the formation of metaphosphate and/or rings of metaphosphate groups at the expense of pyrophosphate. So it indicates the formation of Ca-O-P bonds in the network glass that replaces hydrated P-O-Na and P-O-P bands. The phosphate chains units can be bonded together in rings forming meta-phosphate groups. These rings likely lead to the formation of agglomerates of crystalline phases, which is the main cause of the increase in the chemical du- rability of the glasses when the CaO content increases. The latter may lead to wider use of these materials, especially in the biomedical field.
Keywords:
Phosphate Glasses, Glass Formation, IR Spectroscopy, X-Ray Diffraction, SEM

1. Introduction
Glass is used before long in aesthetic field. Therefore, this material has never ceased to evolve and diversify as required. Across all continents and most civilizations, glass has always been appreciated for its aesthetic qualities and its physical properties [1] - [6] . These properties, such as low melting point, high thermal expansion coefficient, and optical properties make these glasses potential candidates for many technological applications in the medical field (biomaterials), as solid electrolytes, sealing materials and as alternative methods for the vitrification of nuclear waste, etc. [4] [7] - [10] . The aim of the present study is to investigate the structural change and modification of chemical durability versus composition as the proportion of CaO is varied along the series of phosphate glasses xCaO-(40-x)ZnO-15Na2O-45P2O5 with 10 ≤ x ≤ 30, mol%. So we have explored that the increase of CaO content in the glass network leads to an improvement of chemical durability. The structural change enhanced the formation of metaphosphate and/or rings of metaphosphate groups, with some pyrophosphate groups, that were confirmed by I.R spectroscopy and X-ray diffraction.
2. Experimental Procedures
The synthesis of phosphate-based glasses is carried out by the direct fusion of mixtures of (NH4)2HPO4, CaCO3, Na2CO3 and ZnO in suitable proportions. The reactants are finely ground and then fed into a porcelain crucible. They are heated in a first stage at 300˚C for 2 hours and then at 500˚C for 1 hour to complete their decomposition. The reaction mixture is then heated to 1060˚C - 1100˚C for 30 min. The resulting liquid is homogeneous. It is then poured into an aluminium plate previously heated to 200˚C to prevent thermal shocks. In our case, pellets about 1 cm in diameter and 2 - 3 mm thick were obtained. The chemical durability of these glasses has been evaluated from the weight loss of sample. The samples were then polished with silica carbon sandpaper (CSI adequate standard), cleaned with acetone and immersed in Pyrex beakers containing 100 ml of distilled water and brought to 90˚C. The surface of the sample must be constantly immersed in distilled water for 20 days. Density measurements were made by the method of Archimedes. The glass is immersed in a diétyl orthophthalate solution whose density, depending on the temperature, is known. The precision was 0.05 g/cm3. The infrared spectra of the phosphate glasses studied were determined in the region between 1600 and 400 cm−1 with a resolution of 2 cm−1. The samples were finely ground and mixed with KBr (potassium bromide), which is transparent in the infrared, and whose role was to serve as a matrix. The ratio of material/KBr in the pellets was 10% against 90% by weight. The infrared spectroscopic analysis of our materials was performed on a Fourier transform spectrometer Vertex 70 and saved on a DTGS detector (Tri glucine deuterium sulphate). The glassy state was highlighted by its gloss and transparency, and confirmed by XRD. S1 and S4 annealed glasses were made at 550˚C and 650˚C, respectively, for 48 hours. The first structural approach was made using X-ray diffraction, which allowed us to follow the structural evolution. The samples were analysed by a X’Pert Pro MPD Panalyti diffractometer. The chemical composition and microstructure of the sample glasses was characterized using a scanning electron microscope (SEM) equipped with a full system of micro-analysers (EDX-EDAX).
3. Results
3.1. Chemical Durability
The chemical durability of the series of glasses xCaO-(40-x)ZnO-15Na2O-45P2O5 with 10 ≤ x ≤ 30, mol% was approached by measuring the dissolution (DR) rate, which was defined as the weight loss of the glass expressed as g∙cm−2∙mn−1. The values of DR reported in Table 1 show dissolution decreased versus the CaO content of our samples after their immersion in 100 ml of distilled water heated at 90˚C for 20 consecutive days (Figure 1) [11] .
Table 1. Glass composition in mol% and some characteristics of the quaternary glasses (xCaO-(40-x)ZnO-15Na2O-45P2O5).
Figure 1. Curve representing the dependency of the chemical durability of the phosphate glasses on the CaO (mol%) level.
3.2. Density and Molar Volume
The density of the glass was measured at room temperature. As can be deduced from the plots in Figure 2, density (more precisely the specific mass) decreased with increasing CaO content [11] . This behaviour can be explained by the decrease in the glass weight as the Ca replaces Zn, which has a smaller atomic weight. The oxygen molar volume and the oxygen anion radius in the glass were determined from Equations (1) and (2), respectively:
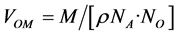 (1)
(1)
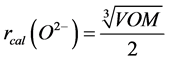 (2)
(2)
with M = molar mass; ρ = density; NA = Avogadro number; No = number of oxygen atoms in the molar formula.
A detailed analysis of the data in Table 2 shows that the molar volume remained almost constant while the CaO content increased. The value of the relative oxygen radius rcal (O2−), calculated from the molar volume Equation (2) [12] and recapitulated for each composition in Table 2, also did not change.
3.3. X-Ray Diffraction
The X-ray crystallography confirmed the single glassy character of all investigated samples. Indeed, the records of the X-ray diffraction (XRD) patterns were found to be typical of amorphous substances [12] . As expected, it is also worth noting that the annealing of the samples led to their crystallization as evidenced by their new XRD patterns given in Figure 3. Annealed glasses in the series with composition xCaO-(30-x)ZnO-25Na2O-45P2O5 were prepared at temperatures between 550˚C and 650˚C for 48 hours. Counting the RX spectra obtained shows (Figure 3) that the S4 composition 30CaO∙10ZnO∙15Na2O∙45P2O5 tended to crystallize as a mixture of metaphosphate and pyrophosphate, with metaphosphate and/or rings metaphosphate chains majorities, while S1 belonging to oligophosphate field samples is expected to contain a mixture of meta-and pyrophosphate networks related to those of calcium and zinc [13] .
3.4. Infrared Spectra
The infrared spectra of glasses in the series xCaO-(40-x)ZnO-15Na2O-45P2O5 are shown in Figure 4. All vibration bands in the phosphate-treated samples are presented in the field between frequencies of 1400 and 599 cm−1. The band at 1300 - 1200 cm−1 is assigned to asymmetric vibration modes νas (PO2) [12] [14] , comprising two non-bridging oxygen atoms of phosphorus in a Q2 phosphate tetrahedron. The vibration bands at around 1100 and 1000 cm−1 are characteristic of terminal PO3− groups [12] . In addition, the band at 884 - 890 cm−1 shifts to a low wavenumber due to the asymmetric vibration νas (P-O-P) while the band at 753 cm−1 is attributed to symmetric vibration νs (P-O-P) groups bridging oxygen atoms bonded to a phosphorus atom in a Q2 phosphate tetetrahedron [12] [14] [15] . The spectrum of C4P6O19 shows a strong vibration in the region around 696 - 733 cm−1 [5] [10] with a CaO content £ 15 mol%. This vibration shifts to low frequencies when the CaO content increases, whereas the same band disappears from the spectrum when the CaO content is ³25 mol%. The band
Figure 2. Variation of the Density (ρ) versus CaO mol% along the Glass series xCaO-(40-x)ZnO-15Na2O-45P2O5.
Figure 3. XRD patterns for glass samples S1 and S4 after heat treatment for 48 hrs under an air atmosphere at 550˚C and 650˚C, respectively.
Table 2. Density and related molar data of the xCaO-(40-x)ZnO-15Na2O-45P2O5 system.
that appears between 615 and 630 cm−1 is attributed to asymmetric vibration modes of the P-O-P skeleton [16] . All characteristic phosphate vibrations show that the bands in phosphate glasses with compositions xCaO-(40-x) ZnO-15Na2O-45P2O5 may have chains and/or rings of metaphosphate structure with some groups of pyrophosphate structure, which is confirmed by the crystalline phases identified by X-ray diffraction. Furthermore, the structure deduced from the vibrational spectroscopy is compatible with the localizations of the analysed compounds (S1, S2, S3, S4) inside the ternary diagram given in Figure 5 and in Table 3.
Figure 4. IR spectra of phosphate glasses of composition xCaO-(40-x)ZnO-15Na2O-45P2O5.
Figure 5. Localization of the investigated glass compositions S1→S4 within the ternary diagram (CaO∙P2O5)- (ZnO∙P2O5)-(3Na2O∙P2O5). The table gives the corresponding compositions within the quaternary system (P2O5-CaO-Na2O-ZnO).
Table 3. Glass compositions expressed in terms of quaternary systems.
3.5. SEM Micrographs
The SEM micrograph shows the existence of two phases, one crystalline and the other glass (Figure 6). It also indicates the formation of agglomerates of crystalline phases amounting to a few tens of micrometres. The presence of the crystalline phase seems to explain the increase in chemical durability [13] . Comparing the SEM results for sample S4 (Figure 7) before and after being attacked by water at 90˚C for 20 days, it was found that the percentage of calcium in the glass composition increased while the percentages of sodium and oxygen decreased.
4. Discussion
The influence of CaO on glass forming characteristics and properties of of xCaO-(40-x)ZnO-15Na2O-45P2O5 (10 ≤ x ≤ 30; mol%) has been investigated. The density measurement shows that the value of relative radius of oxygen anion rcal (O2−), calculated from the molar volume remain almost constant for all the glasses, whereas, the glass composition exhibits a tendency to move from field pyrophosphate to metaphosphate units when the CaO content increases. This structural change enhances the formation of metaphosphate and/or rings of metaphosphate groups, with some pyrophosphate groups, confirmed by X-ray diffraction and I.R spectroscopy [15] . The X-ray diffraction patterns of S1 and S4, heated to 550˚C and 650˚C, respectively, illustrate the formation of crystalline phases Zn(PO3)2, Zn2P2O7, CaZnP2O7, Ca(PO3)2 and the crystalline phase Ca4P6O19. The IR spectra indicate that the vibration bands 696 - 733 cm−1 disappear from the spectrum when the CaO content ≥ 25 mol%. These bands are assigned to vibrations of olygophosphate groups (mixture of Q2 and Q1 with majority of Q2) P6O194− [5] . This also explains that the increase of CaO content, led to the decrease of the phase Ca4P6O19 and promotes the formation of metaphosphate and/or rings metaphosphate chains at the expense of olygophosphate groups. Hence, the substitution of zinc oxide by calcium oxide in the glass structure can reduce the non-bridging oxygens and induce an increase in the bonding strength of the glass phosphate. On the other hand, 45% P2O5 is supposed to give a mixture of metaphosphate and pyrophosphate as indicated in Figure 3. When the CaO content increases in the glass network, we see the disappear of the phase Ca4P6O19 (mixture of 4(PO3−) + P2O74−) for x ≥ 25 mol%, which indicates the change of olygophosphate units in the form, presumably, of cyclic metaphosphate chains that result in agglomerates of crystallites amounting to a few tens of micrometres. These agglomerates lead to a clear improvement in chemical durability [14] .
5. Conclusion
The structure and chemical durability of the phosphate glass series xCaO-(40-x)ZnO-15Na2O-45P2O5, (10 ≤ x ≤ 30; mol%) have been investigated using various techniques such as IR, XRD, SEM, etc. The structural characteristics of these glasses by I.R spectroscopy show a structural change when the CaO content increases. This change leads to the formation of mostly metaphosphates and/or rings of metaphosphate groups with pyrophosphate chains in low concentration. SEM micrographs indicate the formation of agglomerated crystalline phases, which are units of phosphate chains bonded together in rings (cyclic structures) forming meta-phosphate groups, the main cause of the increase in resistant Ca-O-P bands in the glass network. This change led to an important change in chemical durability. The dissolution ratio obtained in these glasses is in the order of 10−6 (g/cm−2∙min−1). This result is promising and can be further improved to lead to wider use of these glasses, particularly in the medical field. The outlook will therefore move in the direction of improving the performance of these glasses for possible technological applications.
Figure 6. (a) SEM optical micrograph showing the structure of sample S4 before aqueous attack; (b) SEM optical micrograph showing the structure of sample S4 after aqueous attack.
Figure 7. EDS spectra of glass sample S4 before and after aqueous attack.
Acknowledgements
The authors wish to thank National Center for Scientific and Technical Research [Division of Technical Support Unit for Scientific Research (TSUSR) Rabat, Morocco] for their assistance to the realization of this work. We also thank Ms S. KRIMI (Laboratory physic and chemistry of inorganic materials) for the support that has brought us.
References
- Tournié, A. (2009) Analyse raman sur site de verres et vitraux anciens: Modélisation, procedure, lixiviation et cara- ctérisation. Doctoral Thesis, University Pierre et Marie Curie, Pairs.
- Vast, P. and Semmoud, A. (1994) Comportement thermique de difluorodioxophosphate ferreux. Journal of Thermal Analysis, 41, 1489-1493. http://dx.doi.org/10.1007/BF02549945
- Moss, R.M., Abou Neel, E.A., Pickup, D.M., Twyman, H.L., Martin, R.A., Henson, M.D., Barney, E.R., Hannon, A.C., Knowles, J.C. and Newport, R.J. (2010) The Effect of Zinc and Titanium on the Structure of Calcium-Sodium Phosphate Based Glass. Journal of Non-Crystalline Solids, 356, 1319-1324. http://dx.doi.org/10.1016/j.jnoncrysol.2010.03.006
- Lao, J. (2007) Caractérisation par micro-faisceau d’ions des relations physic-chimique induite in Vitro par des verres biosctifs nanostructures élaborés par la methode sol-gel. Doctoral Thesis, University Blaise Pascal, Clermont-Ferrand.
- Brow, R.K. (2000) The Structure of Simple Glass. Journal of Non-Crystalline Solids, 263 & 264, 1.
- Sales, B.C. and Batner, L.A. (1984) Lead-Iron Phosphate Glass: A Stable Storage Medium for High-Level Nucleaire Wastes. Science, 226, 45-48. http://dx.doi.org/10.1126/science.226.4670.45
- Thonglem, S., Eitssayaem, S., Rujijanagul, G., Tunkasiri, T., Pengpat, K. and Munpakdee, A. (2012) Fabrication of P2O5-CaO-Na2O Glasses Doped with Zinc Oxide for Artificial Bone Application. Advance Material Research, 506, 509-512. http://dx.doi.org/10.4028/www.scientific.net/AMR.506.509
- Dietrich, E. (2008) Synthèse et étude physoco-chimique des verres bioactifs denses et poreux: Application ne tant que biomatériaux en site osseux. Doctoral Thesis, University Rennes 1, Rennes.
- Vast, P. (1993) Bonding between Metals and Multi-Component Phosphate Based Ceramic Glass: Application to Enamelling of Nickel Titanium. Journal Physique IV France, 3, C7-1383-C7-1388.
- Soulié, J. (2011) Synthèse par voie sol-gel et reactivité in vitro de verre bioactifs dopes, mésostructurés, Caractérisation par micro-faisceaux, d’ions. Doctoral Thesis, Univesity Clermont II Blaise Pascal, Clermont-Ferrand.
- Ahmed, I., Lewis, M., Olsen, I. and Knowles, J.C. (2004) Phosphate Glasses for Tissue Engineering: Part 2. Processing and Characterisation of a Ternary-Based P2O5-CaO-Na2O Glass Fibre System. Biomaterials, 25, 501-507. http://dx.doi.org/10.1016/S0142-9612(03)00547-7
- Aqdim, S., Sayouty, E.H. and Elouadi, B. (2008) Structural and Durability Investigation of the Vitreous Part of the System (35-z)Na2O-zFe2O3-5Al2O3-60P2O5. Eurasian Chemico-Technological Journal, 10, 9-17.
- Cai, S., Zhang, W.J., Xu, G.H., Li, J.Y., Wang, D.M. and Jiang, W. (2009) Microstructural Characteristics and Crystallisation of CaO-P2O5-Na2O-ZnO Glass Ceramics Prepared by Sol-Gel Method. Journal of Non-Crystalline Solids, 355, 273-279. http://dx.doi.org/10.1016/j.jnoncrysol.2008.11.008
- Aqdim, S. and Ouchetto, M. (2013) Elaboration and Structural Investigation of Iron (III) Phosphate Glasses. Advances in Materials Physics and Chemistry, 3, 332-339. http://dx.doi.org/10.4236/ampc.2013.38046
- Aqdim, S., Sayouty, E.H., Elouadi, B. and Greneche, J.M. (2012) IOP Conference Series: Materials Science and Engineering. 27, 012003.
- Ahmed, I., Lewis, M., Olsen, I. and Knowles, J.C. (2004) Phosphate Glasses for Tissue Engineering: Part 1. Processing and Characterisation of a Ternary-Based P2O5-CaO-Na2O Glass System. Biomaterials, 25, 491-499. http://dx.doi.org/10.1016/S0142-9612(03)00546-5
NOTES

*Corresponding author.










