Advances in Materials Physics and Chemistry
Vol.3 No.1A(2013), Article ID:30317,7 pages DOI:10.4236/ampc.2013.31A001
Low-Temperature Synthesis of Nanocrystalline Mn0.2Ni0.8Fe2O4 by Oxalate Precursor Route
1Department of Chemistry, Faculty of Science, Taif University, Taif, KSA
2Advanced Materials Department, Central Metallurgical Research and Development Institute (CMRDI), Cairo, Egypt
Email: *hessienmahmoud@yahoo.com
Copyright © 2013 Mahmoud M. Hessien et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 16, 2013; revised February 20, 2013; accepted March 25, 2013
Keywords: Ni-Mn Ferrite; Crystal Structure; Soft Magnets; Oxalate Precursor Route; Magnetic Properties
ABSTRACT
Manganese nickel ferrite (Mn0.2Ni0.8Fe2O4) powder was synthesized through oxalate precursor route. The effect of annealing temperature (400˚C - 1100˚C) on the formation, crystalline size, morphology and magnetic properties was systematically studied. The resultant powders were investigated by thermal analyzer (TG-DTG-DSC), X-ray diffractometer (XRD), scanning electron microscopy (SEM) and vibrating sample magnetometer (VSM). Based on thermal analysis results, the oxalate mixture decomposed thermally in multisteps weight loss up to about 680˚C. XRD indicated that Mn0.2Ni0.8Fe2O4 formed at much lower annealing temperature (≤400˚C) but contained α-Fe2O3 impurity. The hematite phase decreased by increasing the annealing temperature. The lattice parameters were increased with increasing annealing temperature up to 1000˚C. The average crystalline size increased by increasing the annealing temperature. Single well crystalline ferrite was obtained at 800˚C with crystallite size about 109 nm. The saturation magnetization of the ferrites powders continuously increased with the increase in annealing temperature. Maximum saturation magnetization 48.2 emu/g was achieved for the formed Mn0.2Ni0.8Fe2O4 phase at annealing temperature 1100˚C.
1. Introduction
Ferrites, both soft and hard, are used in many technological applications because of their excellent magnetic and electrical properties. Spinel ferrite (soft ferrite) with the general formula MFe2O4, where M = Mn, Co, Ni, Cu or Zn or a mixture of these ions, posses unique electronic or physical structure and chemical stability [1,2]. It is an ideal material for high-frequency passive components because of its high permeability, resistivity and permitivity. These materials have been used technologically in magnetic recording media and magnetic fluids for the storage and/or retrieval of information, magnetic resonance imaging (MRI) enhancement, catalyses, magnetically guided drug deliveries, sensors and pigments [3,4]. The magnetic properties of these ferrites can be changed by the substitutions of various kinds of M2+ among divalent cations (Zn2+, Mn2+, Ni2+, Mg2+, Co2+, Cu2+, etc.) or by introducing a relatively small amount of rare-earth ions [5]. However, the properties of these materials are determined by their chemical composition, microstructure and process mechanism. So, the interesting features are that their properties can be enhanced by controlling the chemical composition and the preparation methodology.
Spinel ferrites accommodate cations among two possible available interblaner sublattices namely tetrahedral (A) sites and octahedral (B) sites. Spinel ferrites can be either normal spinel (M2+)A[Fe3+ Fe3+]BO4 or inverse spinel with half of the trivalent ions in the A-sites and the other half together with divalent ions in the B-sites [6-8]. Ferrites of similar composition differ in their physical and chemical properties depending on the distribution of cations, having different oxidation states, among the available tetrahedral and octahedral sites. Moreover, substitution of magnetic and non-magnetic cations in ferrite materials changes their magnetic and electrical properties [9,10]. The change in physical and chemical properties of ion substituted spinel ferrites arises from the ability of these compounds to distribute the cations amongst the available tetrahedral and octahedral sites [11].
Nanocrystalline Ni-Mn ferrite system is one of the most substituted ferrites due to its excellent properties which is mainly applied in electrical devices and in catalyses. The Ni-Mn ferrite (MnxNi1−xFe2O4) crystallizes in an inverse spinel structure and can be represented by (MnxFe1−x)A (Ni1−xFe1+x)BO4 where x is defined as the inversion parameter and depends upon preparation conditions and heat treatment [1,11-18]. Spinel nanocrystalline ferrite displays enhanced magnetic and electronic properties by virtue of their unique electronic or physical structure which may be harnessed for technological applications [2,4,19,20]. Moreover, some phenomena like modified saturation magnetization, superparamagnetism, metastable cation distributions, etc., have been observed in nanoparticles of various ferrites [18,21,22].
The conventional way to synthesize ferrites, involves the use of high-temperature solid-state reaction between the oxides/carbonates and then annealing at high temperatures (>1200˚C), has some inherent disadvantages. These include chemical inhomogeneity, coarser particle size and introduction of impurities during ball milling. Various soft chemical methods [23-29] like mechanical milling, inert gas condensation, hydrothermal reaction, oxidative precipitation, sol-gel synthesis and reverse micelle technique are employed for the preparation of nanoferrites. Most of these methods are economically unfeasible for large scale production. The oxalate precursor technique was used for synthesis of ferrites where a single phase of nanocrystalline ferrite powders with homogeneous microstructure, narrow size distribution and uniform shape at low processing temperature can be produced. The oxalate precursor technique uses the carboxylic groups of oxalic acid to chelate the required cations in a proper solution conditions. Chelating metal ions in solution using oxalic acid allows the formation, after drying, of a solid oxalate precursor with uniformly distributed cations in the required ratio. Relatively low annealing temperatures were required to form the corresponding oxides since all cations are mixed at molecular scale.
In the present investigation, we synthesized a pure Mn0.2Ni0.8Fe2O4 ferrite using the oxalate precursor method. X-ray diffraction (XRD), scanning electron microscope (SEM) and a vibrating sample magnetometer (VSM) were utilized in this study. The effect of annealing temperature (400˚C - 1100˚C) on the formation, microstructure and magnetic properties of MnFe2O4 ferrites was also investigated.
2. Experimental
The oxalate precursor method was applied for the preparation of Mn-Ni ferrite (Mn0.2Ni0.8Fe2O4). Chemically grade ferric chloride (FeCl3∙6H2O), manganese chloride (MnCl2∙2H2O), nickel chloride (NiCl2∙H2O) and oxalic acid anhydrous (C2H2O4) as source of organic were used as starting materials. Pre-calculated stoichiometric ratios of ferric chloride, manganese chloride and nickel chloride solutions and containing equivalent amounts of oxalic acid were prepared. The mixture of ferric chloride, manganese chloride and nickel chloride solution was firstly prepared and then stirred for 15 minutes on hot plate magnetic stirrer, followed with addition of an aqueous solution of oxalic acid to the mixture with stirring. The solution was evaporated at 80˚C with constant stirring rate until dryness and then completely dried in a dryer at 100˚C overnight. The dried powder then obtained as precursors. The block flow sheet for synthesis of Mn0.2Ni0.8Fe2O4 ferrite materials by oxalic acid method is shown in Figure 1.
Thermal analysis of uncalcined precursor was carried out. The rate of heating was kept at 10˚C/min between room temperature and 1200˚C. The measurements were carried out in a current of nitrogen atmosphere. For the formation of the Mn-Ni ferrites phase, the dry precursors were calcined at a rate of 10˚C/min in static air atmosphere up to required annealing temperatures (400˚C - 1100˚C) and maintained at the temperature for annealing time 2 h.
The crystalline phases present in the different annealed samples were identified by X-Ray diffraction (XRD) on a Brucker axis D8 diffractometer using Cu-Kα (λ = 1.5406) radiation and secondary monochromator in the range 2θ from 10˚ to 80˚. The ferrites particles morphologies were observed by scanning electron microscope (SEM, JSM-5400).
The magnetic properties of the ferrites were measured at room temperature using a vibrating sample magnetometer (VSM; 9600-1 LDJ, USA) in a maximum applied field of 10 kOe. From the obtained hysteresis loops, the saturation magnetization (Ms) was determined
3. Results and Discussion
3.1. Thermal Analysis
Figure 2 illustrates the thermal analysis profiles (TG-
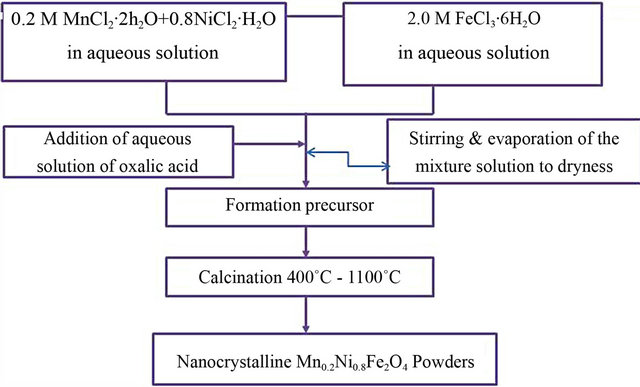
Figure 1. Flow chart for synthesis of Mn0.2Ni0.8Fe2O4 ferrite by oxalate precursor route.
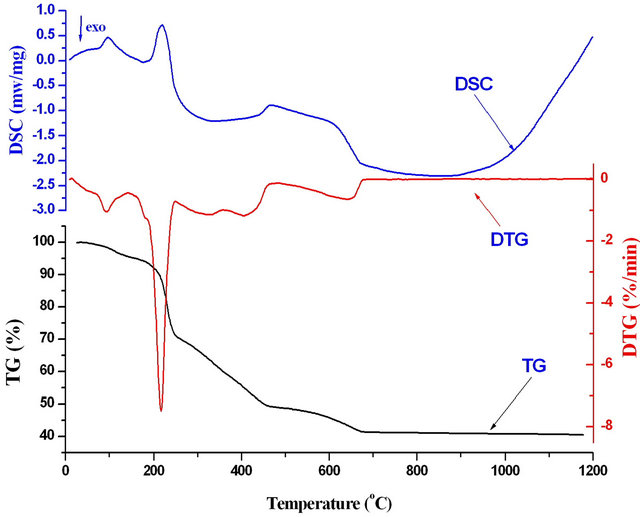
Figure 2. Thermal profiles (TG-DTG-DSC) of mixtures of Ni-Mn-Fe oxalates precursors at Fe3+/(Mn2+ + Ni2+) mole ratios 2.
DTG-DSC) obtained during the decomposition of the manganese-nickel-iron oxalates precursors at a heating rate of 10˚C∙min−1 in nitrogen atmosphere. The thermal decomposition of the precursor begins as soon as the heating starts. The TG and DTG curves show about 5.2% mass loss upon heating up to 145˚C. This mass loss was accompanied by one endothermic peak in the DSC curve at 95˚C that could be assigned to the elimination of water from the precursor (dehydration). Major weight loss of 25% mass loss was observed between 150˚C and 250˚C that can be attributed to metal coordinated water molecules [30]. This weight loss appeared as endothermic peak in DSC curve at 219˚C. By further heating, the decomposition of anhydrous oxalate precursor takes place in multistep weight loss. The sample undergoes exothermic multistep weight loss between 250˚C and 675˚C due to the decomposition of carbonaceous mass of the oxalate precursor. This weight loss was reflected as broad exothermic peaks in DSC curve at 340˚C and 670˚C. However, the total mass loss was about 59.3% at 6750˚C which agrees well with a calculated loss of 60.6% for the decomposition of Mn0.2Zn0.8Fe2(C2O4)4∙4H2O to Mn0.2Ni0.8Fe2O4. Beyond 675˚C, there was no significant weight loss until 1200˚C.
3.2. XRD and Microstructure Characterization
Figure 3 shows the XRD patterns of the calcined powder of Mn0.2Ni0.8Fe2O4 obtained from manganese-nickel-iron oxalate precursor thermally treated at different temperature (400˚C - 1100˚C) for 2 h. The annealing temperature has considerable effects on the formation of Mn-Ni ferrite. On the start of the annealing process at 400˚C, the formation of single-phase Mn0.2Ni0.8Fe2O4 could not be obtained at this annealing temperature and, in addition, it

Figure 3. XRD patterns of Mn0.2Ni0.8Fe2O4 from nickelmanganese-iron oxalate precursor treated at different temperatures (400˚C - 1100˚C) for 2 h.
contains impurity peaks of α-Fe2O3 phase. The patterns consisted of a crystalline cubic spinel ferrite phase (JCPDS#74-2082) and a small amount of α-Fe2O3 (JCPDS # 85-0599). In this case, the Mn0.2Ni0.8Fe2O4 is formed at much lower annealing temperature (≤400˚C). The formation of Mn0.2Ni0.8Fe2O4 ferrite increased as the calcination temperature increased. This is clearly seen from the decrease peak intensities of the free oxides and the increase of the peak intensities of the formed Mn0.2Ni0.8Fe2O4 ferrite. At calcination temperature 800˚C and higher, a well-crystallized pure single Mn0.2Ni0.8Fe2O4 phase evidently was formed. However, no dissociation for the formed ferrite was observed in all annealing temperatures and, on contrast, the formation of Mn0.2Ni0.8Fe2O4 ferrite increased with rising of annealing temperature. Moreover, a well-crystallized pure single Ni0.6Mn0.4Fe2O4 phase was formed at low annealing temperature (800˚C). It is reported that at elevated temperatures, MnFe2O4 is unstable in air and Mn2+ ions on the surface of MnFe2O4 firstly oxidize to form Mn3+ and resulting in the dissociation of the formed MnFe2O4 [31]. In this work, no dissociation for the formed ferrite in all annealing temperature and instead the formation of Mn0.2Ni0.8Fe2O4 ferrite increase. This suggests that the substation of Mn ion in the manganese ferrite with Ni ion stabilizes the MnFe2O4 phase and decreases the oxidation of Mn2+ to Mn3+ in the surface of ferrite. The stability of ferrite increases with the increase of Ni ion content. This is in agreement with that observed by Yang et al. [5]. They found that a single phase of Ni-Mn ferrite was obtained when the Mn2+ was smaller or equal to 0.3 mole content.
The lattice parameter for the cubic Mn0.2Ni0.8Fe2O4 samples annealed at 400˚C, 900˚C, 1000˚C and 1100˚C for 2 h as a function of annealing temperature was calculated for the (311) plane (311) plane using the formula:

Figure 4 shows the effect of annealing temperature on the lattice parameter of Mn0.2Ni0.8Fe2O4 powder. The results indicate that the annealing temperature strongly affects the lattice parameter. The value of lattice parameter increases with the increasing of annealing temperature up to 1000˚C. Beyond this annealing temperature (1000˚C), a decrease in the lattice parameters was found. These results can be due to some structural changes accompanied the formation of manganese ferrite. These changes may be due to the rearrangement of the ions in the ferrite lattice (tetrahedral or octahedral sites) [32]. This change of lattice parameter with the change of annealing temperature can be directly influence the magnetic performance of the ferrite. The effect of annealing temperature on the crystalline size of the produced Mn0.2Ni0.8Fe2O4 ferrite phase for the most intense peak (311) was determined from X-ray diffraction data using the Debye-Scherrer (Figure 5). As expected, the crystalline size of Mn0.2Ni0.8Fe2O4 increased by increasing the annealing temperature. The crystalline size for the first single phase obtained at 800˚C was about 109 nm. However, the crystalline size of the prepared ferrites increased from about 79 nm for the ferrite powders produced at annealing temperature 400˚C to about 190 nm for the powders produced at 1100˚C.
Further investigation was carried out by SEM to show the morphology of the particles as synthesized, as well as the development of grain structure. Figure 6 displays SEM micrographs of the effect of annealing temperature (600˚C, 800˚C, 900˚C, 1000˚C and 1100˚C) on the SEM microstructure of Mn0.2Ni0.8Fe2O4. At 600˚C (Figure 6(a)), the powders possessed irregular microstructures with spherical small particles in addition to a relatively large.
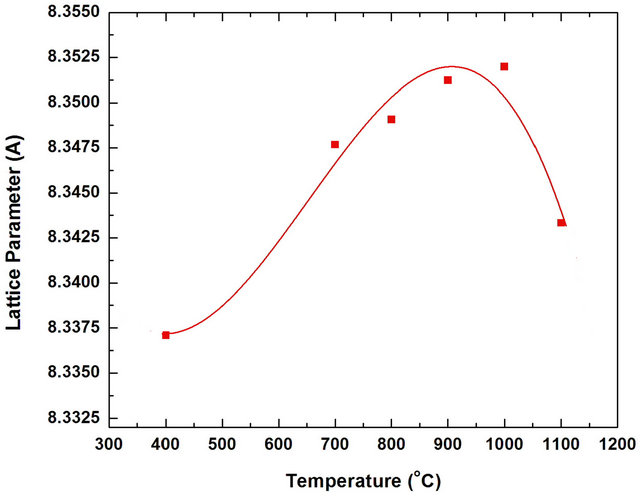
Figure 4. Effect of annealing temperature on the lattice parameters of Mn0.2Ni0.8Fe2O4 powders annealed for 2 h.

Figure 5. Effect of annealing temperature on the crystalline size of Mn0.2Ni0.8Fe2O4 powders annealed for 2 h.
3.3. Magnetic Properties
The magnetization of the produced Mn0.2Ni0.4Fe2O4 ferrite powders was performed at room temperature under an applied field of 10 kOe and the hysteresis loops of the ferrite powders were obtained. Figure 7 Plotted magnetization (M) as a function of applied field (H) for the effect of annealing temperature while Figure 8 plotted the saturation magnetization of the Mn0.2Ni0.4Fe2O4 ferrites powders as a function of annealing temperatures (400˚C - 1100˚C). In general, the produced ferrite was a soft magnetic material due to the deviation from cubic particles, indicating that the annealing temperature was insufficient for the complete formation of clear crystalline microstructure. The presence of small grains can be attributed to no coarsened α-Fe2O3 existing in the annealed samples obtained at this annealing temperature. Similar finding were previously obtained during the microstructure investigation of different ferrites [6,33-35]. For the powder thermally treated at 800˚C (increase of annealing temperature), the ferrite powders possessed a uniform cubic structure with a crystalline microstructure as shown in Figure 6(b). It can be also observed that the grain size was larger than that observed at lower temperature (Figure 6(a)). The average grain size for the powders annealed at 800˚C were about 0.5 - 1 μm. The clear and homogeneous microstructure becomes more pronounced for the sample annealed at 900˚C, 1000˚C and 1100˚C, respectively, as shown in Figures 6(c)-(e). The average grain sizes for the samples annealed at ≥900˚C were in the range of 1 to 1.5 μm. rectangular form and their very low coercivity. There was a large magnetization at 400˚C (≈24 emu/g) indicating that the Mn0.2Ni0.8Fe2O4 ferrite was formed at much lower annealing temperature (<400˚C). The magnetization of the ferrite was steadily increased with increasing treatment
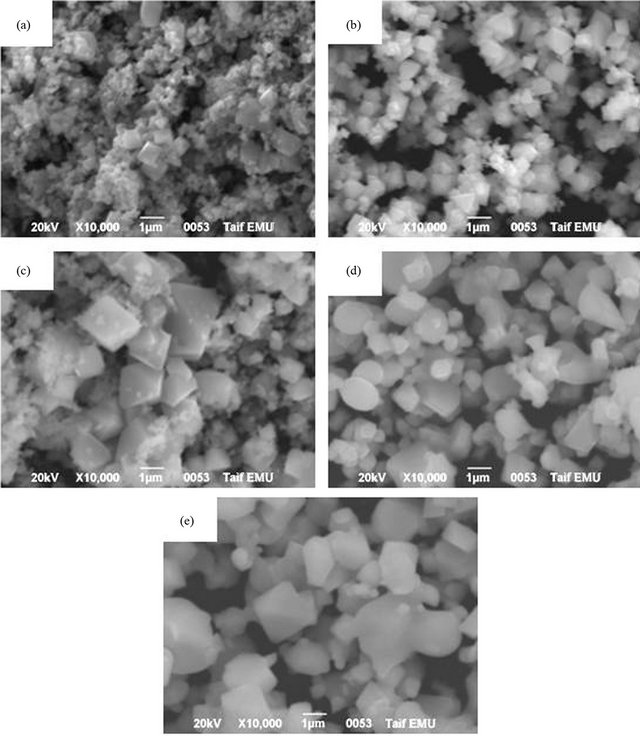
Figure 6. Effect of annealing temperatures on the microstructure of Mn0.2Ni0.8Fe2O4 annealed for 2 h. (a) 600˚C; (b) 800˚C; (c) 900˚C; (d) 1000˚C; (e) 1100˚C.
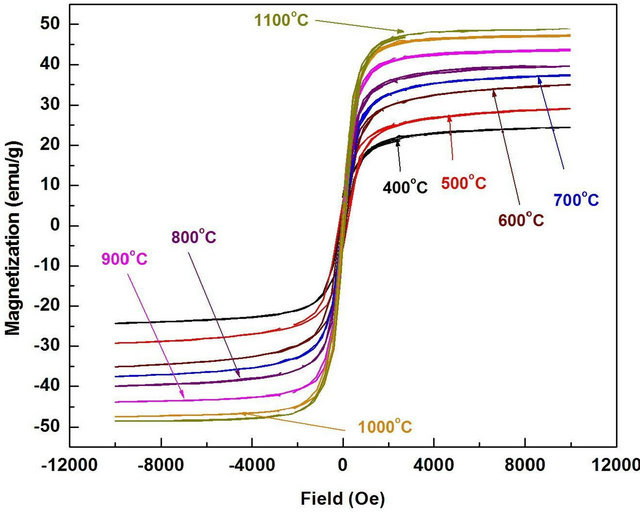
Figure 7. Effect of annealing temperature on the M-H hysteresis loop of synthesized Mn0.2Ni0.8Fe2O4 annealed for 2 h.
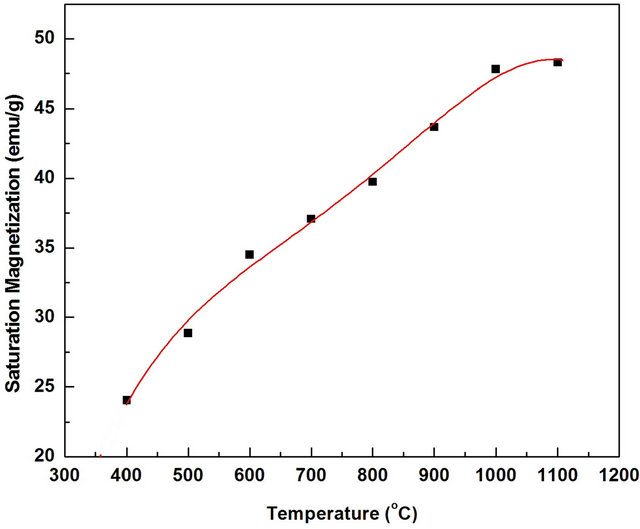
Figure 8. Effect of annealing temperature on the saturation magnetization of Mn0.2Ni0.8Fe2O4 powders annealed for 2 h.
temperature from 400 (≈24 emu/g) to 1000˚C (47.82 emu/g). At 1100˚C (48.2 emu/g), a slight increase in the magnetization than at 1000˚C was observed. Such high saturation magnetization for the synthesized ferrite annealed at ≤1000˚C can be attributed to the high phase purity and well-defined crystallinity of Mn0.2Ni0.4Fe2O4 as clearly evidenced from XRD (Figure 3) and SEM (Figure 6). The crystallite size increased from ≈79 nm for the samples annealed at 400˚C to ≈190 nm for the samples annealed at 1100˚C. This indicates that the oxalate precursor technique can be used for synthesis of single phase of soft nanocrystalline ferrite powders with homogeneous microstructure, narrow size distribution, uniform shape and a large magnetization at low processing temperature.
4. Conclusions
Nanocrystalline Mn0.2Ni0.8Fe2O4 has been synthesized via oxalate precursor route. The effect of annealing temperature on the formation, crystalline size, morphology and magnetic properties were systematically studied. The results showed that:
1) The oxalate precursor of Mn-Ni-Fe mixture decomposed thermally in multistep weight loss up to about 680˚C.
2) Mn0.2Ni0.8Fe2O4 was started to form at much lower annealing temperature (≤400˚C).
3) The lattice parameters were increased with increasing annealing temperature up to 1000˚C. The average crystalline size increased by increasing the annealing temperature.
4) Single well crystalline ferrite was obtained at 800˚C with crystallite size about 109 nm.
5) The saturation magnetization of the ferrite powders continuously increased with the increase in annealing temperature.
6) The formed Mn0.2Ni0.8Fe2O4 ferrite powders had good soft magnetic properties with maximum saturation magnetization (48.2 emu/g) was achieved at annealing temperature 1100˚C.
REFERENCES
- M. Popa, P. Bruna, D. Crespo and J. M. C. Moreno, “Single Phase MnFe2O4 Powders Obtained by the Polymerized Complex Method,” Journal of the American Ceramic Society, Vol. 91, No. 8, 2008, pp. 2488-2494. doi:10.1111/j.1551-2916.2008.02501.x
- S. Son, M. Taheri, E. Carpenter, V. G. Harris and M. E. McHenry, “Synthesis of Ferrite and Nickel Ferrite Nanoparticles Using Radio-Frequency Thermal Plasma Torch,” Journal of Applied Physics, Vol. 91, No. 10, 2002, pp. 7589-7591. doi:10.1063/1.1452705
- S. Maensiri, C. Masingboon, B. Boonchom and S. Seraphin, “A Simple Route to Synthesize Nickel Ferrite (NiFe2O4) Nanoparticles Using Egg White,” Scripta Materialia, Vol. 56, No. 9, 2007, pp. 797-800. doi:10.1016/j.scriptamat.2006.09.033
- M. Sugimoto, “The Past, Present and Future of Ferrites,” Journal of the American Ceramic Society, Vol. 82, No. 2, 1999, pp. 269-280. doi:10.1111/j.1551-2916.1999.tb20058.x
- H. Yang, L. Zhao, X. Yang, L. Shen, L. Yu, W. Sun, Y. Yan, W. Wang and S. Feng, “The Synthesis and the Magnetic Properties of Nd2O3-Doped Ni-Mn Ferrites Nanoparticles,” Journal of Magnetism and Magnetic Materials, Vol. 271, No. 2-3, 2004, pp. 230-236. doi:10.1016/j.jmmm.2003.09.030
- M. M. Hessien, M. M. Rashad, K. El-Barawy and I. A. Ibrahim, “Influence of Manganese Substitution and Annealing Temperature on the Formation, Microstructure and Magnetic Properties of Mn-Zn Ferrites,” Journal of Magnetism and Magnetic Materials, Vol. 320, No. 9, 2008, pp. 1615-1621. doi:10.1016/j.jmmm.2008.01.025
- J. A. Toledo, M. A. Valenzuela, P. Bosch, H. Armendariz, A. Montoya, N. Nava and A. Vazquez, “Effect of AI3+ Introduction into Hydrothermally Prepared ZnFe2O4,” Applied Catalysis A: General, Vol. 198, No. 1-2, 2000, pp. 235-245. doi:10.1016/S0926-860X(99)00514-1
- N. M. Deraz and M. M. Hessien, “Structural and Magnetic Properties of Pure and Doped Nanocrystalline Cadmium Ferrite,” Journal of Alloys and Compounds, Vol. 475, No. 1-2, 2009, pp. 832-839. doi:10.1016/j.jallcom.2008.08.034
- S. Kumar, R. Kumar, S. K. Sharma, V. R. Reddy, A. Banerjee and Alimuddin, “Temperature-Dependent Mössbauer and Dielectric Studies of Mg0.95Mn0.05Fe1.0Ti1.0O4,” Solid State Communications, Vol. 142, No. 12, 2007, pp. 706-709. doi:10.1016/j.ssc.2007.04.019
- C. B. Kolekar, A. Y. Lipare, B. P. Ladganokar, P. N. Vasambekar and A. S. Vaingankar, “The Effect of Gd3+ and Cd2+ Substitution on Magnetization of Copper Ferrite,” Journal of Magnetism and Magnetic Materials, Vol. 247, No. 2, 2002, pp. 142-146. doi:10.1016/S0304-8853(01)00974-X
- X. Qi, J. Zhou, Z. Yue, Z. Gui and L. Li, “Effect of Mn Substitution on the Magnetic Properties of MgCuZn Ferrites,” Journal of Magnetism and Magnetic Materials, Vol. 251, No. 3, 2002, pp. 316-322. doi:10.1016/S0304-8853(02)00854-5
- M. Naeem, N. Abbas Shah, I. H. Gul and A. Maqsood, “Structural, Electrical and Magnetic Characterization of Ni-Mg Spinel Ferrites,” Journal of Alloys and Compounds, Vol. 487, No. 1-2, 2009, pp. 739-743. doi:10.1016/j.jallcom.2009.08.057
- X. Hou, J. Feng, X. Xu, and M. Zhang, “Synthesis and Characterizations of Spinel MnFe2O4 Nanorod by SeedHydrothermal Route,” Journal of Alloys and Compounds, Vol. 491, No. 1-2, 2010, pp. 258-263. doi:10.1016/j.jallcom.2009.10.029
- L. Zhen, K. He, C. Y. Xu and W. Z. Shao, “Synthesis and Characterization of Single-Crystalline MnFe2O4 Nanorods via a Surfactant-Free Hydrothermal Route,” Journal of Magnetism and Magnetic Materials, Vol. 320, No. 21, 2008, pp. 2672-2675. doi:10.1016/j.jmmm.2008.05.034
- Y. W. Ju, J. H. Park, H. R. Jung, S. J. Cho and W. J. Lee, “Electrospun MnFe2O4 Nanofibers: Preparation and Morphology,” Composites Science and Technology, Vol. 68, No. 7-8, 2008, pp. 1704-1709. doi:10.1016/j.compscitech.2008.02.015
- S. Gubbala, H. Nathani, K. Koizol and R. D. K. Misra, “Magnetic Properties of Nanocrystalline Ni-Zn, Zn-Mn, and Ni-Mn Ferrites Synthesized by Reverse Micelle Technique,” Physica B: Condensed Matter, Vol. 348, No. 1-4, 2004, pp. 317-328. doi:10.1016/j.physb.2003.12.017
- L. Zhao, W. Xu, H. Yang and L. Yu, “Effect of Nd Ion on the Magnetic Properties of Ni-Mn Ferrite Nanocrystal,” Current Applied Physics, Vol. 8, No. 1, 2008, pp. 36-41. doi:10.1016/j.cap.2007.04.011
- A. S. Albuquerque, J. D. Ardisson and W. A. A. Macedo, “Nanosized Powders of NiZn Ferrite: Synthesis, Structure, and Magnetism,” Journal of Applied Physics, Vol. 87, No. 9, 2000, pp. 4352-4357. doi:10.1063/1.373077
- P. C. Fannin, A. Slawska-Waniewska, P. Didukh, A. Giannitoics and S. W. Charles, “Dynamic Properties of a System of Cobalt Nanoparticles,” The European Physical Journal Applied Physics, Vol. 17, No. 1, 2002, pp. 3-9. doi:10.1051/epjap:2001012
- M. H. Sousa, F. A. Tourinho, J. Depeyrot, G. J. Da Silva and M. C. F. Lara, “New Electric Double-Layered Magnetic Fluids Based on Copper, Nickel, and Zinc Ferrite Nanostructures,” The Journal of Physical Chemistry B, Vol. 105, No. 7, 2001, pp. 1169-1175. doi:10.1021/jp0028195
- P. J. van der Zaag, P. J. van der Valk and M. T. Rekveldt, “A Domain Size Effect in Magnetic Hystersis of NiZnFerrite,” Applied Physics Letters, Vol. 69, No. 19, 1996, p. 2927. doi:10.1063/1.117326
- P. C. Fannin, S. W. Charles and J. L. Dormann, “Field Dependence of the Dynamic Properties of Colloidal Suspensions of Mn0.66Zn0.34Fe2O4 and Ni0.5Zn0.5Fe2O4 Particles,” Journal of Magnetism and Magnetic Materials, Vol. 201, No. 1-3, 1999, pp. 98-101. doi:10.1016/S0304-8853(99)00067-0
- M. Radwan, M. M. Rashad and M. M. Hessien, “Synthesis and Characterization of Barium Hexaferrite Nanoparticles,” Journal of Materials Processing Technology, Vol. 181, No. 1-3, 2007, pp. 106-109. doi:10.1016/j.jmatprotec.2006.03.015
- H. Sato and T. Umeda, “Grain Growth of Strontium Ferrite Crystallized from Amorphous Phases,” Journal of Materials Transactions, Vol. 34, No. 1, 1993, pp. 76-81.
- P. Hu, H. Yang, D. Pan, H. Wang, J. Tian, S. Zhang, X. Wang and A. A. Volinsky, “Heat Treatment Effects on Microstructure and Magnetic Properties of Mn-Zn Ferrite Powders,” Journal of Magnetism and Magnetic Materials, Vol. 322, No. 1, 2010, pp. 173-177. |doi:10.1016/j.jmmm.2009.09.002
- J. Huo and M. Wei, “Characterization and Magnetic Properties of Nanocrystalline Nickel Ferrite Synthesized by Hydrothermal Method,” Materials Letters, Vol. 63, No. 13-14, 2009, pp. 1183-1184. doi:10.1016/j.matlet.2009.02.024
- L. Wang, J. Ren, Y. Wang, X. Liu and Y. Wang, “Controlled Synthesis of Magnetic Spinel-Type Nickel Ferrite Nanoparticles by the Interface Reaction and Hydrothermal Crystallization,” Journal of Alloys and Compounds, Vol. 490, No. 1-2, 2010, pp. 656-660. doi:10.1016/j.jallcom.2009.10.131
- J. Ding, W. F. Miao, R. Street, P. G. McCormick and R. Street, “High-Coercivity Ferrite Magnets Prepared by Mechanical Alloying,” Journal of Alloys and Compounds, Vol. 281, No. 1, 1998, pp. 32-36. doi:10.1016/S0925-8388(98)00766-X
- N. Y. Mostafa, M. M. Hessien and A. A. Shaltout, “Hydrothermal Synthesis and Characterizations of Ti Substituted Mn-Ferrites,” Journal of Alloys and Compounds, Vol. 529, 2012, pp. 29-33. doi:10.1016/j.jallcom.2012.03.060
- P. P. Sarangi, S. R. Vadera, M. K. Patra and N. N. Ghosh, “Synthesis and Characterization of Pure Single Phase NiZn Ferrite Nanopowders by Oxalate Based Precursor Method,” Powder Technology, Vol. 203, No. 2, 2010, pp. 348-353. doi:10.1016/j.powtec.2010.05.027
- H.-F. Yu and S.-W. Yang, “Formation of Crystalline MnFe2O4 Powder by Flame-Combusting Freeze-Dried Citrate Precursors,” Journal of Alloys and Compounds, Vol. 394, No. 1-2, 2005, pp. 286-291. doi:10.1016/j.jallcom.2004.11.011
- N. S. Gajbhiye and G. Balaji, “Synthesis, Reactivity, and Cations Inversion Studies of Nanocrystalline MnFe2O4 Particles,” Thermochemica Acta, Vol. 385, No. 1-2, 2002, pp. 143-151. doi:10.1016/S0040-6031(01)00714-6
- A. C. F. M. Costa, E. Tortella, M. R. Morelli and R. H. G. A. Kiminami, “Synthesis, Microstructure and Magnetic Properties of Ni-Zn Ferrites,” Journal of Magnetism and Magnetic Materials, Vol. 256, No. 1-3, 2003, pp. 174-182. doi:10.1016/S0304-8853(02)00449-3
- A. K. M. Akther Hossain, S. T. Mahmud, M. Seki, T. Kawai and H. Tabat, “Structural, Electrical Transport, and Magnetic Properties of Ni1−xZnxFe2O4,” Journal of Magnetism and Magnetic Materials, Vol. 312, No. 1, 2007, pp. 210-219. doi:10.1016/j.jmmm.2006.09.030
- M. M. Hessien, “Synthesis and Characterization of Lithium Ferrite by Oxalate Precursor Route,” Journal of Magnetism and Magnetic Materials, Vol. 320, No. 21, 2008, pp. 2800-2807. doi:10.1016/j.jmmm.2008.06.018
NOTES
*Corresponding author.

