Open Journal of Safety Science and Technology
Vol.07 No.01(2017), Article ID:74184,10 pages
10.4236/ojsst.2017.71001
The Measurement and Prediction of Flash Point for Binary Mixtures {C1 ~ C3 Alcohols + p-Xylene} at 101.3 kPa
Kyu Jin Han1, Se Jin In2
1Department of Ammunitions, Daeduk College, Daejeon, Republic of Korea
2Department of Fire and Disaster Protection Engineering, Woosong University, Daejeon, Republic of Korea

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: December 20, 2016; Accepted: February 13, 2017; Published: February 16, 2017
ABSTRACT
The flash point of flammable liquid mixture is very important parameter to characterize the ignition and explosion hazards. Flash points at 101.3 kPa were measured for several binary systems containing p-xylene, including {methanol + p-xylene}, {ethanol + p-xylene}, and {2-propanol and p-xylene}. Experimental measurements were performed using a SETA closed cup flash point tester. The measured flash points were compared with the predicted values calculated using the following activity coefficient models: Wilson, Non- Random Two Liquid (NRTL), and UNIversal QUAsi-Chemical (UNIQUAC). The results from the NRTL model provided the best comparison to the experimentally determined values.
Keywords:
Flash Point, SETA Closed Cup Flash Point Tester, Binary Mixtures, Activity Coefficient Models

1. Introduction
Flammable substances, such as organic solvents, are commonly used in laboratories and industrial processes. The flash point (FP) is one of the most important parameters used to characterize the ignition and explosion hazards of these liquids [1] . The lower flammable limit (LFL) provides information on the fundamental physical and chemical processes of combustion. The FP of a given liquid can be experimentally determined, and the resulting temperature may then be adjusted to the standard sea level atmospheric pressure of 101.3 kPa, the pressure at which a substance emits sufficient vapor to form a combustible mixture with air [2] .
As the temperature increases, there is a concomitant increase in both the vapor pressure and the amount of evaporated, flammable liquid in equilibrium with the air. When the temperature is reached at FP, a simple ignition source is able to combust the vapor mixture [3] . Experimental FP data for the multi- component mixtures have become important in ensuring safe storage of flammable materials and, for this reason, studies for predicting the FP of pure substances and mixtures are increasingly important.
Benzene, toluene and xylene are the most used solvents in the rubber products such as tire manufacturing, rubber bands, rubber gloves and appliance moldings. The several alcohols and their mixtures are very useful in the rubber industry [4] [5] . The most common of these solvents is xylene which is used as a solvent in the manufacturing of chemicals, tires, agricultural sprays, adhesives and coatings, as an ingredient in aviation fuel and gasoline, and as a feedstock in manufacturing various polymers, including phthalic anhydride, isophthalic acid, terephthalic acid and dimethyl terephthalate [6] . The purpose of this study is to determine the FPs for flammable binary mixtures commonly used as industrial solvents such as alcohols and xylene.
In the present work, the FPs at 101.3 kPa were determined using a SETA closed cup flash point tester on the following solvent mixtures: {methanol (1) + p-xylene (2)}, {ethanol (1) + p-xylene (2)} and {2-propanol (1) + p-xylene (2)}. The measured FP data for these binary systems were compared with predicted values from a variety of local composition activity coefficient models, including the Wilson, Non-Random Two-Liquid (NRTL) and UNIversalQUAsiChemical (UNIQUAC)models [7] [8] [9] .
2. Material and Methods
2.1. Materials
Commercial, analytical-grade chemicals were used in this investigation. p-Xylene (C8H10, M = 106.17 g·mol−1, CAS-RN 106-42-3, 99.9 %) was obtained from Fluka Co. Methanol (CH4O, M = 32.04 gmol−1, CAS-RN 67-56-1, 99.9%), Ethanol (C2H6O, M = 46.07 gmol−1, CAS-RN 64-17-5, 99.9%) and 2-propanol (C3H8O, M = 60.10 g·mol−1, CAS-RN 67-63-0, 99.9%) were supplied by J. T. Baker Chemical Co. All of the chemicals were dried using molecular sieves with a pore diameter of 0.4 nm. The water contents of the chemicals were determined by Karl-Fischer titration (using a Metrohm 684 KF-Coulometer) and were found to be less than 6 ´ 10−5 g/g. The purities of the chemicals were assessed by gas chromatography. The reported values for the purities, FPs and UNIQUAC parameters [10] [11] are listed in Table 1.
2.2. Procedure
A SETA closed cup flash point tester (Series 8 SETAFLASH, model 82000-0, Surrey, UK) was used to measure the FPs for the miscible mixture samples. The SETA closed cup flash point tester was operated according to the standard test method, ASTM D 3278 [12] . Detailed descriptions for the measuring system and
Table 1. The purities and UNIQUAC parameters of chemicals used in this work.
aRef [10] ; bRef [11] .
procedure can be found in the previous work [13] [14] . Mixture samples for the experiments were weighed using a microbalance (Ohaus DV215CD) with a precision of 1 ´ 10−5 g. Further details are also appeared in elsewhere [13] [14] .
3. Results and Discussion
Le Chatelier’s rule [15] for a mixture of flammable vapor and air may be expressed as follows:
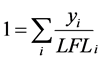 (1)
(1)
where yi is the vapor phase composition of a flammable substance i and LFLi is the lower flammable limit of the pure component i. The LFLi is expressed in relation to the pure component i vapor pressure at its FP, 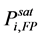 , as
, as
 (2)
(2)
where P represents the ambient pressure. The FP of a pure substance is typically measured at standard atmospheric pressure. Under this condition, the vapor phase can be assumed with behaving ideally. With the non-ideal liquid phase containing flammable substances in the presence of the non-condensable components of air, the vapor?liquid equilibrium of component i is described by the modified Raoult’s law:
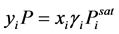 (3)
(3)
where γi is activity coefficient for the liquid phase.
As proposed by Liaw et al. [16] , one can substitute Equation (2) and Equation (3) into Equation (1), resulting in Equation (4), which allows evaluation of FPs for flammable liquid mixtures:
 (4)
(4)
The saturated vapor pressure for a pure substance i can be obtained by the Antoine Equation [17] :
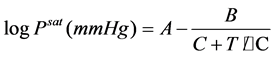 (5)
(5)
Antoine constants (A, B and C in Equation (5)) were adapted from the literature [11] and are given in Table 2.
Table 2. The Antoine constants of the pure components.
aRef [11] .
Assuming the solution behave ideally, the activity coefficients of the liquid phase are equal to unity. Therefore, Equation (4) was reduced according to Raoult’s law and expressed as [14] :
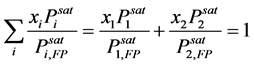 (6)
(6)
The temperature that satisfies Equation (6) is obtained to be the flash point of the ideal solution.
For non-ideal liquid mixtures, activity coefficients (γi) were estimated with the optimum binary interaction parameters of the Wilson, NRTL and UNIQUAC equations, described below [6] [7] [8] .
Wilson Equation:
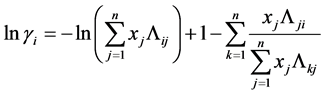 (7)
(7)
with

NRTL Equation:
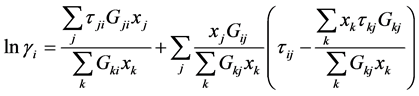 (8)
(8)
with
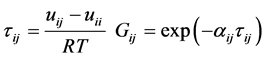
UNIQUAC Equation:
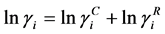 (9)
(9)
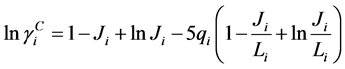
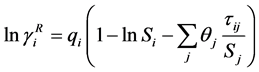
with
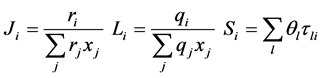

The binary parameters used to calculate the activity coefficients were taken from the references and are provided in Table 3 [18] [19] .
The experimental binary FP data for the three systems tested as part of this work, {methanol + p-xylene}, {ethanol + p-xylene} and {2-propanol + p-xylene}, are given in Table 4. The experimentally determined binary data were compared with the predicted values from the Wilson, NRTL and UNIQUAC models. The binary parameters of each model equation were used to calculate the activity coefficients of liquid mixture under the same conditions employed in the experiments, and the initial temperature for calculation was assigned the numerical average FP of the pure components. Then, the FP was obtained from adjustment of initial temperature by satisfying the Le Chatelier’s rule (Equation 4). The objective function (OF) used was

The average absolute deviations (A.A.D) between the measured and calculated values are included in Table 4.
A.A.D is defined as:

where 

The data of each binary system at 101.3 kPa pressure are plotted in Figures 1- 3. The parameters for the activity coefficient models are given in Table 3, along with the A.A.D between the experimental and predicted values. All FP data agreed very well, as illustrated in the figures. Minimum flash point behavior was
Table 3. The optimized binary parameters of the Wilson, NRTL and UNIQUAC equa- tions for each binary system.
aRef [18] ; bRef [19] .
Table 4. The experimental and predicted FPs for each binary system at 101.3 kPa.
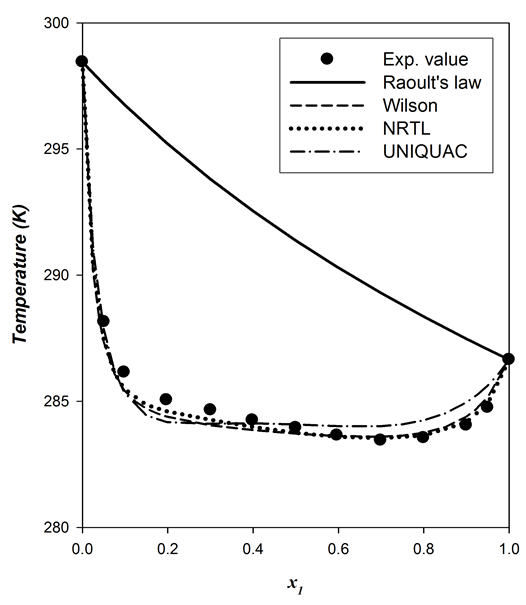
Figure 1. The comparison of the flash point prediction curves with the experimental data for the binary system {methanol (1) + p-xylene (2)} at 101.3 kPa.
Figure 2. The comparison of the flash point prediction curves with the experimental data for the binary system {ethanol (1) + p-xylene (2)} at 101.3 kPa.
Figure 3. The comparison of the flash point prediction curves with the experimental data for the binary system {2-propanol (1) + p-xylene (2)} at 101.3 kPa.
observed in all the binaries. Minimum flash point is caused from minimum boiling azeotrope in vapor-liquid equilibrium of the mixture. Moreover, the minimum flash point behavior values of each binary system were estimated using the best fitted model, NRTL. They are x1 = 0.745, T = 280.03 K for the system {methanol + p-xylene}, x1 = 0.693, T = 283.54 K for the system {ethanol + p-xylene} and x1 = 0.683, T = 284.14 K for the system {2-propanol + p-xylene}.
For the investigated systems, the A.A.D between the predicted and measured FP values were less than 0.85 K, except when calculated by Raoult’s law. Among the models, the NRTL model yielded results closest to the experimentally determined values. The minimum values of A.A.D by NRTL are 0.59, 0.29 and 0.52 K for {methanol + p-xylene}, {ethanol + p-xylene} and {2-propanol + p-xylene}, respectively.
4. Conclusion
Flash point data for {methanol + p-xylene}, {ethanol + p-xylene} and {2-pro- panol + p-xylene} binary systems were determined at atmospheric pressure. Minimum flash point behavior was observed in all three binaries. Moreover, the minimum flash point behavior values of each binary system were estimated using the best fitted model. The measured FP data agreed well with the predicted values derived from the Wilson, NRTL and UNIQUAC models, with the NRTL model providing the most comparable results. The average absolute deviations between the measured and predicted FPs were less than 0.85K.
Cite this paper
Han, K.J. and In, S.J. (2017) The Measurement and Prediction of Flash Point for Binary Mixtures {C1 ~ C3 Alcohols + p-Xylene} at 101.3 kPa. Open Journal of Safety Science and Technology, 7, 1-10. https://doi.org/10.4236/ojsst.2017.71001
References
- 1. Crowl, D.A. and Louvar, J.F. (1990) Chemical Process Safety: Fundamentals with Applications. Prentice Hall, Englewood Cliffs, NY.
- 2. CCPS/AIChE (1993) Guidelines for Safe Automation of Chemical Processes. American Institute of Chemical Engineering, New York.
- 3. Lees, F.P. (1996) Loss Prevention in the Process Industries. 2nd Edition, Butterworth-Heinemann, Oxford, UK.
- 4. Lawrence, F. (1990) Chemicals Used in the Rubber Industry. Springer, Berlin Heidelberg, 45-95.
- 5. Manuel, H.J. and Dierkes, W. (1997) Recycling of Rubber. Rapra Review Reports, 9, Report 99.
- 6. US Environmental Protection Agency (1994) Locating and Estimating Air Emissions from Sources of Xylene. EPA-454-R-93-048.
- 7. Wilson, G.M. and Deal, C.H. (1962) Activity Coefficients and Molecular Structure. Activity Coefficients in Changing Environments-Solutions of Groups. Industrial & Engineering Chemistry Fundamentals, 1, 20-23.
https://doi.org/10.1021/i160001a003 - 8. Renon, H. and Prausnitz, J.M. (1968) Local Compositions in Thermodynamic Excess Functions for Liquid Mixtures. AIChE Journal, 14, 135-144.
https://doi.org/10.1002/aic.690140124 - 9. Abrams, D.S. and Prausnitz, J.M. (1975) Statistical Thermodynamics of Liquid Mixtures: A New Expression for the Excess Gibbs Energy of Partly or Completely Miscible Systems. AIChE Journal, 21, 116-128.
https://doi.org/10.1002/aic.690210115 - 10. National Fire Protection Association (1985) Fire Investigations. Batterymarch Park, Quincy, MA., National Fire Codes, 7.
- 11. Dortmund Data Bank Software Package (DDBSP) Version 2006 Professional, Software and Separation Technology GmbH.
http://www.ddbst.de - 12. American Society for Testing Materials (1999) Annual Book of ASTM Standards. 6.
- 13. In, S.J. (2015) Flash Point for Binary MIXTURES of methylcyclohexane, n-Heptane and p-Xylene. Journal of Industrial and Engineering Chemistry, 32, 327-331.
https://doi.org/10.1016/j.jiec.2015.09.013 - 14. Oh, I.S. and In, S.J. (2015) The Measurement and Prediction of Flash Point for Binary Mixtures of Methanol, Ethanol, 2-Propanol and 1-Butanol at 101.3 kPa. Fire Science and Engineering, 29, 1-6.
https://doi.org/10.7731/KIFSE.2015.29.5.001 - 15. Le Chatelier, H. (1891) Estimation of Firedamp by Flammability Limits. Annales des Mines, 19, 388-395.
- 16. Liaw, H.J., Tang, C.L. and Lai, J.S. (2004) A Model for Predicting the Flash Point of Ternary Flammable Solution of Liquid. Combust Flame, 138, 308-319.
https://doi.org/10.1016/j.combustflame.2004.06.002 - 17. Poling, B.E., Prausnitz, J.M. and O’connell, J.P. (2001) The Properties of Gases and Liquids. 5th Edition, McGraw-Hill, New York.
- 18. Oracz, P. and Kolasinska, G. (1987) Vapour-Liquid Equilibria-III. Total Vapour Pressure Measurements for Binary Mixtures of Methanol, Ethanol, 1-Propanol and 1-Butanol with Benzene, Toluene and p-Xylene at 313.15 K. Fluid Phase Equilibria, 35, 253-278.
https://doi.org/10.1016/0378-3812(87)80016-X - 19. Gupta, V., Maken, S., Kalra, K.C. and Singh K.C. (1996) Molar Excdess Gibbs Free Energy of 1-Propanol or 2-Propanol + Aromatic Hydrocarbons at 298.15 K in terms of an Association Model with a Flory Contribution Term. Thermochimica Acta, 277, 187-198.
https://doi.org/10.1016/0040-6031(95)02745-9