Open Journal of Stomatology
Vol.07 No.08(2017), Article ID:77899,9 pages
10.4236/ojst.2017.78027
An Experimental Study on the Effects of Platelet Rich Plasma on the Wound Healing of Tooth Extraction-Related Bone Defects
Kanae Niimi1*, Michiko Yoshizawa1,2, Takahiro Koyama3, Akinori Funayama1, Toshihiko Mikami1, Tadaharu Kobayashi1
1Division of Reconstructive Surgery for Oral and Maxillofacial Region, Department of Tissue Regeneration and Reconstruction, Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan
2Department of Oral and Maxillofacial Surgery, School of Dentistry, Matsumoto Dental University, Shiojiri, Japan
3Division of Oral and Maxillofacial Surgery, Department of Oral Health, Course of Science, Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: June 27, 2017; Accepted: July 22, 2017; Published: July 25, 2017
ABSTRACT
Keywords:
Platelet Rich Plasma, Wound Healing, Bone Defect

1. Introduction
Platelet-rich plasma (PRP) is an autologous preparation of platelets in concentrated plasma. It has been reported that a number of growth factors, have been sequestered from platelets [1] [2] . These growth factors include platelet-derived growth factors (PDGF), transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), epithelial growth factor (EGF), etc. As these growth factors promote wound healing [3] , PRP has been used as a therapeutic tool, although the mechanisms underlying these wound healing effects remain unclear. Mariano et al. describes that Autologous PRP accelerates alveolar bone generation after tooth extraction [4] , while Mooren et al. describe that early and late bone healing was not enhanced when PRP was used [5] . At the point of oral mucosa, Powell et al. told that PRP did not enhance gingival wound healing as measured by flap strength, nor did it alter the histologic appearance of the tissue compared to sites not treated with PRP [6] . The purpose of this study is to evaluate the effectiveness of applying PRP in the management of tooth extraction sockets determined by histological and immune histochemical examination.
2. Materials and Methods
10-week-old male Fisher rats were used for this study (200 - 220 g in weight), and the animals were breed following a protocol approved by the Center of Bioresources, Niigata University. The experiment protocol was inspected by the Ethics Committee of the Center of Bioresources, and obtained permission.
2.1. Preparation of PRP
The animals for preparing PRP were anesthetized using sevoflurane inhalation anesthesia and the intraperitoneal administration of chloral hydrate. Heart puncture with an 18-G needle and exsanguination were performed, and blood was collected into tubes containing ACDA. The tubes were centrifuged at 450 G for 10 minutes. After the first spin, the serum, buffy coat and red cells located 2 mm under the buffy coat were aspirated, collected into another tube and centrifuged at 850 G for 15 minutes at room temperature. The PRP was collected at the bottom of the second tube, and blood count was done before activation. The activator liquid was made in another tube with 5000 U bovine thrombin and 0.5 cc of 1 mEq/ml calcium chloride mixture. The activator liquid was added to collected PRP. The ratio of PRP to activator liquid was 10:1. This protocol was in accordance with Marx et al. [1] .
2.2. Preparation of Bone Defect and Application of PRP
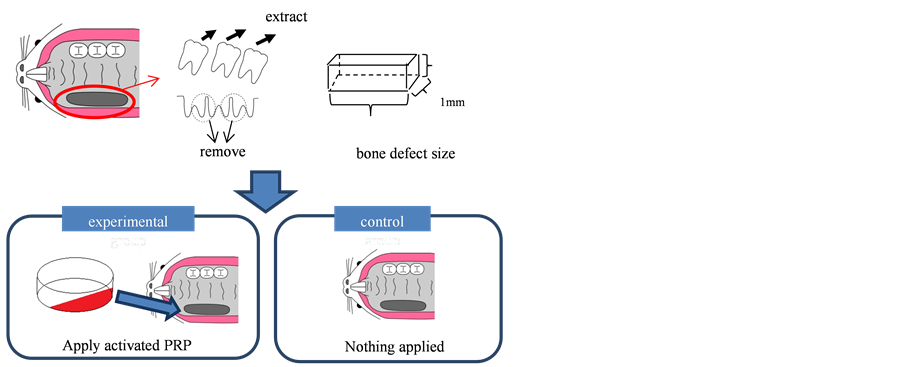
All three upper molars of the other rats were extracted, and the bone between the molars was removed with a round bur 1 mm in diameter, to create a bone defect measuring approximately 1 × 1 × 3 mm in size. 0.8 ml of activated PRP was applied to the each bone defects in the experimental group, while nothing was applied to the bone defects in the control group (Figure 1).
Figure 1. Scheme of study model. All three upper molars of the rats were extracted, and the bone between the molars was removed with a round bur to create a bone defect measuring approximately 1 × 1 × 3 mm in size. Activated PRP was applied to the bone defects in the experimental group, nothing was applied to the bone defects in the control group.
2.3. Histological and Immunohistochemical Analysis
The rats were euthanized on days 1, 3, 5 and 7. The specimens were fixed with 20% buffered formalin and decalcified with EDTA. Serial sections measuring 7 µm in diameter were obtained and stained with hematoxylin and eosin. The sections were also studied immunohistochemically using type IV collagen in order to visualize the small vessels. Elastica van Gieson stain was also performed to investigate elastic fibers.
3. Results
The blood counts of the whole blood and PRP is shown in Table 1. Platelet count of whole blood was 35.6 × 104/mm3, which was 230.0 × 104/mm3 in PRP. These results showed that the platelets were concentrated approximately 6.5 times compared to the whole blood in the PRP.
On macroscopic findings, no significant differences were observed between the experimental and control groups on days 1 and 3. On day 5, granulation tissues were observed in the control group within a wider area than that seen in the experimental group (Figure 1). On day 7, large areas of excavation were detected in the bone defects in the control group, while only small areas of excavation were noted in the experimental group (Figure 2).
On microscopic findings, inflammatory cells and a fibrin network were observed under the PRP layer in the bone defects in the experimental group on day 1, whereas inflammatory cells and a fibrin network were rarely seen in the control group (Figure 3).
On day 3, conglomerates of inflammatory cells and a fibrin network that was abundant in blood capillaries were seen under the PRP layer in the experimental
Table 1. The average of blood count of whole blood and PRP (n = 2).



Figure 2. Macroscopic findings of the fixed specimens of experimental group (a), (b), (c) and control group (d), (e), (f). Bone defects are shown in yellow double- arrows. There were no significant differences were observed between the experimental (a) and control groups (d) on day 3. On day 5, granulation tissue was observed within a wider area than that seen in the experimental group (b) than in the control group (e). On day 7, only small areas of excavation were noted in the experimental group (c), while large areas of excavation were detected in the bone defects in the control group (f).
group. In contrast, inflammatory cells and a fibrin network with blood capillaries were observed in the bone defects in the control group in immunohistochemical findings of Type 4 collagen (Figure 4).
On day 5, in H-E stain and Elastica van Gieson stain, granulation tissue was detected in the middle part of the bone defects in the control group. The


Figure 3. Histological findings of experimental group (a), (b) and control group (c), (d) of day 1. Inflammatory cells (*) and a fibrin network (**) were observed in the experimental group. On the other hand, few inflammatory cells are seen, but fibrin network were rarely seen under the PRP layer in the bone defects (double-head arrows) in the control group. Original magnification ×20 (a), (c) and ×40 (b), (d). Scale bar; 500 μm.


Figure 4. Histological (a), (c) and immunohistological findings of Type 4 collagen (b), (d) of day 3. Conglomerates of inflammatory cells and a fibrin network that abundant in blood capillaries (***) were seen under the PRP layer in the experimental group (b). In contrast, inflammatory cells and a fibrin network with blood capillaries were rarely observed in the bone defects in the control group (c), (d). Original magnification ×20 (a), (c) and ×40 (b), (d). Scale bar; 500 μm.
granulation tissue over the bone defects in the experimental group was thicker than that observed in the control group (Figure 5).
On day 7, granulation tissue was seen in the bone defects in both the control group and experimental group, although excavation of granulation tissue was noted in the control group. The basement membrane underlining epithelium is regenerated in wider area in control groups, than in the experimental groups in immunohistochemical findings of Type 4 collagen (Figure 6).
4. Discussion
Much has been studied about the mechanisms about regeneration of tissues in recent years. To accelerate tissue repair, growth factors play a contributory role [7] .
PRP is an autologous concentration of platelets in a small volume of plasma, and contains many kinds of growth factors, such as PDGF, EGF, VEGF, and TGF-β etc. In 1998, Marx et al. reported that bone grafting with PRP stimulates ossification in patients with mandibular defects 1. Some reports described bone formation is stimulated [1] [2] [4] [8] , while other reports described there is no significant difference with use of PRP [5] [9] .
At the point of soft tissue, PRP is applied to dermatitis combustion, fat grafting, scar revision, and repair of tendon [3] [10] [11] . Rajabi et al. reported that injection of PRP on injured Achilles tendon, there was significant increase in number of fibroblast and cellular density and collagen deposition, whereas Venter et al. reported that use of PRP after deep burns leads to faster wound closure and thicker epidermis than control group [11] . From these reports, it is suggested that PRP leads to fast and better healing of soft tissue, which is similar to the results of our present study.
As we ranged over former studies, the research paper which clearly expressed the effect of PRP to oral mucosa, in vitro and/or in vivo studies in the maxillofacial fields was rarely seen. PRP is often used in clinical studies, such as application to periodontal wound healing [6] , alveolar bone healing after extraction [4] [12] [13] , etc. Dutta, et al. reported that they used PRP in empty extraction socket of impacted mandibular third molars, and has significant improvement in soft tissue healing and bone density in extraction sockets [12] . Gawai KT, et al. concluded that application PRP to extraction socket leads to significant improvement of the soft tissue healing, but there was no added benefit in late bone healing [13] .
In our study, the results demonstrated the presence of early inflammatory reactions and a rich fibrin network in the experimental group, which led to the formation of thick, vessel-rich granulation tissue. The findings of day-5 specimens showed remaining of granulation tissue on wider area were seen in control group. Meanwhile, the basement membrane regenerated in wider area in control groups, than in the experimental groups in day-7 specimens. Re-epithelializa- tions in the same period both in the control and experimental group lead


Figure 5. Findings of H-E stain (a), (c) and Elastica van Gieson stain (b), (d) of day 5. The granulation tissue (shown in double-head arrows) over the bone defects in the experimental group (b), was thicker than that observed in the control group (d). Granulation tissue was not detected in the middle part of the bone defects in the control group (c), (d). Original magnification ×20. Scale bar; 500 μm.


Figure 6. Findings of H-E stain (a), (c) and Type 4 collagen (b), (d) of day 7. Granulation tissue was seen in the bone defects in both the control group (a), (b) and experimental group (c), (d). Granulation tissue is shown in double head arrows (a), (c). The defects were covered with epithelium, and thick granulation layer was observed in the experimental group, although concavity was noted in the control group. The basement membrane (red arrows) regenerated in wider area in control groups (d), than in the experimental groups (b). Original magnification ×20 (a), (c) and ×40 (b), (d). Scale bar; 500 μm.
to excavation in bone defect area. From these results, formation of thick, vessel-rich granulation tissue in the early stage of healing may lead to better wound healing in experimental group than in control group, without excavation in bone defect area. Seppa et al. showed PDGF could lead fibroblasts to migrate into the clot, and induce their proliferation [14] . Our results may also support the rich PDGF of PRP stimulates early inflammatory reactions and formation of fibrin, which leads to wound healing stimulation.
One of the reasons of variety of results in PRP studies is high heterogeneity, and this leads to the different conclusions in many studies [15] [16] . In this study, we showed that the application of PRP stimulates wound healing in tooth extraction bone defects; however, the mode of action of the growth factors remains unclear. Therefore, PRP may confer some beneficial effects on the outcomes. This issue must be analyzed in further detail, for example, using standardized models to test its effectiveness in vivo as well as the regenerative effect with different biomaterials.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding
JSPS KAKENHI Grant Number 20791498, 23592983.
Cite this paper
Niimi, K., Yoshizawa, M., Koyama, T., Funayama, A., Mikami, T. and Kobayashi, T. (2017) An Experimental Study on the Effects of Platelet Rich Plasma on the Wound Healing of Tooth Extraction-Related Bone Defects. Open Journal of Stomatology, 7, 327-335. https://doi.org/10.4236/ojst.2017.78027
References
- 1. Marx, R.E., Carson, E.R., Eichstaedt, R.M., Schimmele, S.R., Strauss, J.E. and Geogeff, K.R. (1998) Platelet-Rich Plasma: Growth Factor Enhancement for Bone Grafts. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 85, 638-646.
https://doi.org/10.1016/S1079-2104(98)90029-4 - 2. Marx, R.E. (2001) Platelet-Rich Plasma (PRP): What Is PRP and What Is Not PRP? Implant Dentistry, 10, 225-228.
https://doi.org/10.1097/00008505-200110000-00002 - 3. Lynch, M.D. and Bashir, S. (2015) Applications of Platelet-Rich Plasma in Dermatology: A Critical Appraisal of the Literature. Journal of Dermatological Treatment, 14, 1-5.
- 4. Mariano, R.C., Melo, W.M. and Avelino, C.C. (2012) Comparative Radiographic Evaluation of Alveolar Bone Healing Associated with Autologous Platelet-Rich Plasma after Impacted Mandibular Third Molar Surgery. Journal of Oral and Maxillofacial Surgery, 70, 19-24.
https://doi.org/10.1016/j.joms.2011.03.028 - 5. Mooren, R.E.C.M., Merkx, M.A.W., Bronkhorst, E.M., Jansen, J.A. and Stoelinga, P.J.W. (2007) The Effect of Platelet-Rich Plasma on Early and Late Bone Healing: An Experimental Study in Goats. Journal of Oral and Maxillofacial Surgery, 36, 626-631.
https://doi.org/10.1016/j.ijom.2007.03.013 - 6. Powell, C.A., Bannister, S.R., Mackey, S.A., Maller, S.C., McDonnell, H.T. and Deas, D.E. (2009) Periodontal Wound Healing with and without Platelet-Rich Plasma: Histologic Observations and Assessment of Flap Tensile Strength. Journal of Periodontology, 80, 986-982.
https://doi.org/10.1902/jop.2009.080626 - 7. Xie, Z., Paras, C.B., Weng, H., Punnakitikashem, P., Su, L.C., Vu, K., Tang, L., Yang, J. and Nguyen, K.T. (2013) Dual Growth Factor Releasing Multi-Functional Nanofibers for Wound Healing. Acta Biomaterialia, 12, 9351-9359.
https://doi.org/10.1016/j.actbio.2013.07.030 - 8. Kim, T.H., Kim, S.H., Sandor, G.K. and Kim, Y.D. (2014) Comparison of Platelet-Rich Plasma (PRP), Platelet-Rich Fibrin (PRF), and Concentrated Growth Factor (CGF) in Rabbit-Skull Defect Healing. Archives of Oral Biology, 59, 550-558.
https://doi.org/10.1016/j.archoralbio.2014.02.004 - 9. Hatakeyama, I., Marukawa, E., Takahashi, Y. and Omura, K. (2014) Effects of Platelet-Poor Plasma, Platelet-Rich Plasma, and Platelet-Rich Fibrin on Healing of Extraction Socket with Buccal Dehiscence in Dogs. Tissue Engineering: Part A, 20, 874-882.
- 10. Rajabi, H., Shahin, H.S., Norouzian, M., Mehrabani, D. and Nazhvani, S.D. (2015) The Healing Effects of Aquatic Activities and Allogenic Injection of Platelet Rich Plasma (PRP) on Injuries of Achilles Tendon in Experimental Rat. World Journal of Plastic Surgery, 4, 66-73.
- 11. Venter, N.G., Marques, R.G., dos Santos, J.S. and Monte-Alto-Costa, A. (2016) Use of Platelet-Rich Plasma in Deep Second- and Third-Degree Burns. Burns, 42, 807-814.
https://doi.org/10.1016/j.burns.2016.01.002 - 12. Dutta, S.R., Singh, P., Passi, D. and Pattter, P. (2015) Mandiblar Third Molar Extraction Wound Healing with and without Platelet Rich Plasma: A Comparative Prospective Study. Journal of Oral and Maxillofacial Surgery, 14, 808-815.
https://doi.org/10.1007/s12663-014-0738-1 - 13. Gawai, K.T. and Sobhana, C.R. (2015) Clinical Evaluation of Use of Platelet Rich Plasma in Bone Healing. Journal of Oral and Maxillofacial Surgery, 14, 67-80.
https://doi.org/10.1007/s12663-013-0605-5 - 14. Seppa, H., Grotendorst, G., Seppa, S., Schiffmann, E. and Martin, G.R. (1982) Platelet-Derived Growth Factor is Chemotactic for Fibroblasts. Journal of Cell Biology, 92, 584-588.
https://doi.org/10.1083/jcb.92.2.584 - 15. Jovani-Sancho, M.M., Sheth, C.C., Marques-Mateo, M. and Puche-Torres, M. (2016) Platelet-Rich Plasma: A Study of the Variables that May Influence Its Effect on Bone Regeneration. Clinical Implant Dentistry and Related Research, 18, 1051-1064. https://doi.org/10.1111/cid.12361
- 16. Roselló-Camps, à., Monje, A., Lin, G.H., VahidKhoshkam, V., Chávez-Gatty, M., Wang, H.L., Gargallo-Albiol, J. and Hernandez-Alfaro, F. (2015) Platelet-Rich Plasma for Periodontal Regeneration in the Treatment of Intrabony Defects: A Meta-Analysis on Prospective Clinical Trials. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology, 120, 562-574.
https://doi.org/10.1016/j.oooo.2015.06.035