Open Journal of Immunology
Vol.04 No.03(2014), Article ID:49406,6 pages
10.4236/oji.2014.43009
Impact of Smoking on Serum Immunoglobulin G Levels in Patients with Periodontitis
Charu Shrestha, Ashita Uppoor, Dilip Nayak
Kathmandu University, Kathmandu, Nepal
Email: charustha@gmail.com, uash55@hotmail.com, drdilipnayak@yahoo.com,
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 7 June 2014; revised 7 July 2014; accepted 7 August 2014
ABSTRACT
Tobacco smoking has been found to be a major environmental factor associated with generalized forms of severe periodontitis. Altered serum and gingival crevicular fluid inflammatory cytokine profiles, immune cell function, and altered proteolytic regulations are noticed in smokers. These observations are not consistent, and to date, there has been no clear mechanism to explain how smoking may affect periodontal disease. Hence, the present study was undertaken to assess the impact of smoking on serum immunoglobulin G (IgG) levels in smokers with periodontitis and its potential role as a risk indicator of the disease process. 40 subjects (15 smokers and 15 non- smokers with chronic periodontitis, 10 healthy controls) were included in the study. Smoking history was assessed according to a standardized interview and a questionnaire, Fagerstom Test for Nicotine Dependence. Serum immunoglobulin IgG was estimated with immunoturbidimetric assay. IgG levels were significantly decreased with longer duration of smoking. In addition levels of serum IgG were significantly lower in smokers compared to non-smokers with chronic periodontitis and healthy controls (P < 0.001). Current observations indicate that cigarette smoking may be associated with the suppression of B-cell function and immunoglobulin production. The alteration of antibody levels further explains the potential mechanism by which smoking exacerbates peri- odontal disease. Further studies at the molecular level may highlight the specific mechanism by which tobacco can interact with cells of the immune system and its impact on periodontal disease process. Additional controlled, longitudinal studies may expound the significance of serum anti- bodies as potential markers for periodontal disease.
Keywords:
Smoking, Chronic Periodontitis, Immunoglobulin G

1. Introduction
Chronic periodontitis is the result of a response of the host to bacterial aggregations on the tooth surfaces. Inflammation and destruction of periodontal tissues are largely considered to result from the response of a susceptible host to a microbial biofilm containing Gram-negative bacteria [1] . Among the risk factors identified for periodontitis are age, gender, socioeconomic status, and genetic predisposition, bacterial colonization, certain systemic conditions and smoking [2] - [4] .
Tobacco smoking has been found to be a major environmental factor associated with periodontal disease [5] - [7] . Epidemiological studies on the relationship between tobacco use and periodontal diseases consistently reported that cigarette smokers are five times more likely to develop severe periodontitis than non-smokers [8] - [10] . These investigators also reported a relationship between the duration of smoking and increased clinical attachment loss. The current understanding of the pathogenesis of periodontal disease suggests that periodontal tissue is destroyed by the modulation of host defences by bacterial products [4] . This stimulates the host inflammatory process and releases cytokines and enzymes capable of destroying the host tissues. This does not preclude a role for bacteria and virulence factors, but suggests that in most forms of periodontal disease, destruction is a consequence of the host response to these factors [11] . Further, this suggests that the most relevant virulence factors are those that strongly stimulate the destruction of host tissues by host-derived molecules [12] .
Systemic alterations of the cellular and humoral immune responses to periodontal pathogens in presence of environmental risk factors such as smoking, have been evaluated including immunosuppression, exaggerated inflammatory cell responses, impaired neutrophils, and reduced antibody production [13] . The exact mechanism by which tobacco smoking influences the periodontal tissues is still unclear. Biological plausible mechanisms for the effects of smoking on periodontium can be described with supportive evidence. The effects of smoking were remarkably selective with respect to both IgG and race. The present study is different from earlier studies because of variation in race. However, research is still required to more thoroughly clarify the pathways by which tobacco smoking and its constituents exert effects on the pathogenesis and treatment outcomes of periodontal disease. Hence in the present study the levels of serum IgG in response to chronic periodontitis was evaluated in smokers and non-smokers.
2. Materials and Methods
2.1. Study Population
The present study was conducted in the Department of Periodontics, Manipal College of Dental Sciences, Mangalore, India. It was approved by Institutional Ethics Committee, Kasturba Medical College, Mangalore, India. A total of 40 systemically healthy males, smokers and non-smokers, aged between 25 to 55 years old, were included in the study. The healthy subjects were included in the control category and cases were subjects with chronic periodontitis (smokers and non-smokers). Subjects who were former smokers, those taking smokeless tobacco, and those who have undergone periodontal treatment or were on antibiotic therapy within the preceding 3 months were excluded from the study.
Patients were informed about the purpose and design of the study prior to initiation of the study and were required to sign an informed consent. The study protocol and consent form were approved by the Institutional Ethics Committee. 15 smoker patients with clinical attachment loss of >3 mm and probing pocket depth of ≥4 mm in at least 30% of the sites were included in Group 1 [14] . Subjects who smoked minimum of 10 cigarettes per day for not less than two years were included in Group 2 [15] . 15 patients with clinical attachment loss >3 mm and probing pocket depth of ≥4 mm in at least 30% of the sites were included in this group and remainder 10 non smoker subjects who have clinically healthy gingiva and no clinical attachment loss were included in control group.
Smoking status was determined according to daily consumption and period of cigarettes smoking [15] . The smoking history was assessed according to a standardized interview and a questionnaire, Fagerstom Test for Nicotine Dependence [16] . The smoking exposure was expressed in terms of consumption (number of cigarette per day) and duration (in years). Following this patients were classified as either smokers or non-smokers. Those who were included smoked a minimum of 10 cigarettes per day for a minimum period of two years.
For each patient, a set of complete examination of extraoral and intraoral full-mouth clinical assessment and the individual number of teeth present; excluding the third molars, were documented. The examination sets and clinical measurements were taken by the same examiner for all the patients. The following clinical indices Plaque index (PI), Gingival index (GI), Modified sulcular bleeding index (mSBI), Probing pocket depth (PPD) and Clinical attachment level (CAL) were measured.
Ten millilitres of venous blood sample were collected from each patient by venipuncture in the antecubital fossa without excessive venous stasis. The blood samples were drawn by well-trained medical assistant using Vacutainer containing no anticoagulant and allowed to clot. The serum samples were collected and then centrifuged at 3000 rpm for 10 min. The samples were stored in plastic vials at −20˚C [17] .
2.2. Immunoturbidimetric Assay
Estimation of total serum IgG levels were performed by immunoturbidimetry. The method is based on assessing the immunoprecipitation reaction by measuring the intensity of transmitted light measured as absorbance with the help of immunoturbidimetric method using Automated Analyser (Cobas Integra® 800) [18] .
Sample containing human IgG is suitably diluted and then reacted with specific antiserum to form a precipitate which is measured turbidimetrically at 340 nm. By constructing a standard curve from the absorbances of standards, the IgG concentration of sample can be determined. Normal range: 800 - 1800 mg/dl.
3. Statistical Analysis
Means and standard deviations for age, number of teeth, Plaque Index, Gingival Index, modified Sulcular Bleeding Index, Periodontal Probing Depths, Clinical Attachment Levels, and serum IgG of the subjects (Group 1, 2 and 3) were analyzed. Differences between the three study groups for all variables were determined with Kruskal Wallis test (H) (to explore the differences between any two groups) Mann Whitney U test (z) was used to analyze the mean differences of PPD and CAL between Group 1 and 2. Correlation between IgG level and pack years was determined using Spearman’s correlation coefficient. P values <0.05 were considered significant. For all statistical calculations, the statistical Program for Social Sciences (version 11.5, SPSS Inc., India) was used.
4. Results
A total of 40 systemically healthy male patients were included in this study of which 15 patients in Group 1 (smoker with periodontitis), 15 in Group 2 (non-smoker with periodontitis) and 10 patients were in Group 3 (healthy controls). The mean age of Group 1, Group 2 and Group 3 were 42.93 ± 8.09, 45.66 ± 8.63 and 39.30 ± 10.45 years, respectively. The mean Plaque Index (PI), gingival index (GI) and modified sulcular bleeding index (mSBI) in non-smoker with periodontitis was higher as compared to smoker with periodontitis and healthy individuals.
The periodontal pocket depth was measured at six sites and the mean values in millimetres were calculated for each subject in Group 1 and 2. The mean probing pocket depth and clinical attachment level of Group 1 were 5.78 ± 0.71 and 5.99 ± 0.79 respectively and that of Group 2 were 5.29 ± 0.40 and 5.84 ± 0.48 respectively. Group 1 had a significantly higher probing pocket depth compared to Group 2 (p > 0.05) and the clinical attachment loss was also higher than that of Group 2 but not statistically significant (p = 0.52).
The correlation of PPD with PI, GI and mSBI in Group 1 and 2 was also analysed using Spearman’s correlation coefficient (Table 1). PPD was negatively correlated with PI, GI and mSBI in Group 1 and positively correlated with PI, GI and mSBI in group 2.
The mean duration of smoking was 15 years and IgG levels was significantly decreased with longer duration of smoking (p = 0.041) (Table 2).
The mean IgG levels were 1176.46 ± 242.49 mg/dl in Group 1, 1500.66 ± 178.22 mg/dl in Group 2 and 1340.00 ± 200.66 mg/dl in Group 3 (Graph 1). The serum IgG antibody showed significantly lower levels in Group 1 compared to Group 2 (p < 0.001). Group 3 showed lower IgG levels when compared with the values of Group 1 and 2 but was statistically not significant.
5. Discussion
Evidence obtained from cross-sectional risk assessment studies and several longitudinal studies have suggested the causal role of association between tobacco smoking and the progression of periodontitis in humans [19] . The
Table 1. Spearman’s correlation of PPD in Group 1 and 2 with PI, GI and mSBI.
Table 2. Spearman’s correlation of IgG levels in Group 1 with duration of smoking.
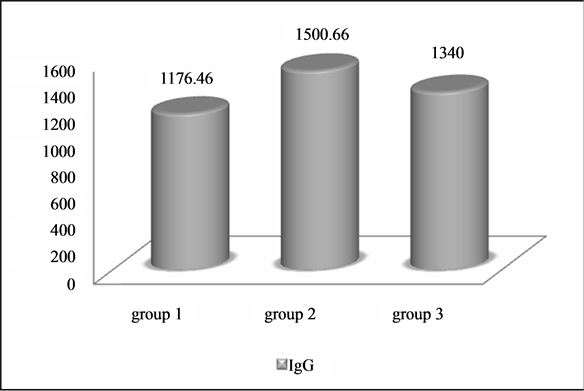
Graph 1. Comparison of IgG level between Group 1, 2 and 3.
exact mechanism by which tobacco exerts its influence on oral health has not been fully understood or explained by experiments. Smoking has been shown to have an adverse effect on fibroblast function, chemotaxis and phagocytosis by neutrophils [20] and immunoglobulin production [21] .
The purpose of the present study was to evaluate impact of smoking on serum IgG levels in smokers with periodontitis and its potential role as a risk indicator of the disease process. This cross sectional study included 40 systemically healthy male patients who were subdivided into 3 Groups, Group 1 (smoker with periodontitis), Group 2 (non smoker with chronic periodontitis) and Group 3 (controls). Because of the low prevalence of female smokers in India [22] , this study examined only male patients. Similarly, Yanagisawa T et al. (2010) [23] conducted a study to determine the relationships between the number of teeth present and periodontal diseases with smoking habits in a cohort of Japanese men and they have mentioned the same reasons for examining only male patients in their study.
In the present study, correlation between IgG level and duration of smoking was analysed and we found that the mean duration of smoking was 15 years and IgG levels was significantly decreased with longer duration of smoking and this result is in agreement with the results of the previous study by P. Moszczynski et al. (2001) [24] .
Group 1 showed lower GI and mSBI compared with Group 2. Smoking suppresses the inflammatory response to plaque challenge, and therefore masks clinical signs of gingival inflammation [13] . The correlation of PPD with PI, GI and mSBI in Group 1 and 2 was also analysed. PPD was negatively correlated with PI, GI and mSBI in group which is consistent with other reports [13] , implying that the harmful effects of smoking on periodontal health may not be associated with plaque accumulation and poor oral hygiene.
The relationship between plaque accumulation and development of inflammation in smokers has been studied in classical experimental gingivitis studies [25] . They demonstrated that there is no difference in plaque accumulation when comparing smokers and non-smokers. However, the development of inflammation was very much retarded in the smoking group with less sites exhibiting bleeding on probing. The reduced bleeding has previously been proposed to be caused by nicotine-induced vasoconstriction, but more recent evidence has failed to show a reduction in blood flow to the gingiva following smoking a cigarette in regular smokers [13] .
Group 1 had a significantly higher PPD compared to Group 2 and CAL was also higher as compared to Group 2. Previous study by Haber J and Kent RL (1992) [26] showed that the proportion of PPDs ≥ 4 mm is higher in current smokers than never smokers in all regions and on all tooth surfaces suggests that smoking is associated with increased disease severity.
IgG antibodies have been considered important in preventing periodontal destruction in patients with aggressive and chronic periodontitis [27] . It was also observed that the serum IgG levels were elevated in individuals suffering from chronic periodontitis. The high serum level of IgG antibodies in chronic periodontitis may simply represent an inflammatory reaction to the external microbial challenge. In the present study, serum IgG level was found to be higher in non smokers as compared with periodontally intact control subjects and this result is in agreement with the previous studies [17] [28] .
Plasma levels of antibodies are regarded as indices of immunity and smoking is now an acceptable risk factor for reduced level of IgG [24] . The serum IgG was found to be significantly lower among Group 1 compared to Group 2 and 3. One of the mechanisms to explain this finding is that smoking decreases the proliferative capacity of T-cells and T-cell-dependent antibody responses which affects B-cell function and antibody generation. It is possible that B-cells are functionally compromised by the reduced proliferative responses to oral pathogens, resulting in decreased production of serum antibodies. In addition, it has been shown that alveolar macrophages from smokers exhibit reduced expression as antigen-presenting cells. This may eventually lead to a reduction in the humoral immune response to invading organisms in periodontitis patients.
The results of the present study are in agreement with the observations of the previous researchers [5] [17] [27] . More specifically, smokers have been shown to have reduced titers of serum IgG to P. intermedia and F. Nucleatum [26] . The major antibody response in periodontitis is IgG, presumably in response to bacterial polysaccharide and outer membrane protein of gram negative periodontal pathogen. There are conflicting data regarding the influence of cigarette smoking on the levels of serum IgA and IgM. While some reports suggest that smoking suppresses serum levels of IgM and IgA [29] , others indicate that one or another of these isotypes is not affected by smoking.
There were certain limitations of this study. The sample size of the study was less. Larger sample sizes could have permitted greater confidence in the results. Because of the low prevalence of female smokers, this study examined only men. However, the prevalence of smoking among women in their 20 s and 30 s has recently increased [30] . In this study we have used a questionnaire to assess the smoking status of the patients which is a subjective measure.
Due to the relationship between antibodies and periodontal infection, the potential application of antibody titers for periodontal diagnostic purposes has received considerable research attention. Individuals with periodontitis often have elevated antibody titers to periodontal pathogens, as compared to periodontally healthy controls. Precisely the serum IgG may be reflective of the destructive periodontal disease and its levels can be considered a risk indicator for the disease progression. The possible influence of smoking on serum IgG and IgG2 antibody levels noted in the present and previous studies stress the significance of smoking as a potential risk factor for the development and progression of periodontitis among smokers [5] [17] [27] .
Furthermore, Smoking cessation significantly improves the outcome of non-surgical periodontal therapy [31] . Further studies can be done for the effect of periodontal therapy and smoking cessation.
6. Conclusion
Current observations indicate that cigarette smoking may be associated with the suppression of B-cell function and immunoglobulin production. The alteration of antibody levels further explains the potential mechanism by which smoking exacerbates periodontal disease. Further research will elucidate the molecular mechanism by which smoking affects the immune system. Additional controlled, longitudinal studies may expound the significance of serum antibodies as potential markers for periodontal disease.
References
- Socransky, S.S. and Haffajee, A.D. (2005) Periodontal Microbial Ecology. Periodontology 2000, 38, 135-187. http://dx.doi.org/10.1111/j.1600-0757.2005.00107.x
- Page, R.C. and Kornman, K.S. (1997) The Pathogenesis of Human Periodontitis: An Introduction. Periodontology 2000, 14, 9-11. http://dx.doi.org/10.1111/j.1600-0757.1997.tb00189.x
- Page, R.C., Offenbacher, S., Schroeder, H.E., Seymour, G.J. and Kornman, K.S. (1997) Advances in the Pathogenesis of Periodontitis: Summary of Developments, Clinical Implications and Future Directions. Periodontology 2000, 14, 216-248. http://dx.doi.org/10.1111/j.1600-0757.1997.tb00199.x
- Schenkein, H.A. (2006) Host Responses in Maintaining Periodontal Health and Determining Periodontal Disease. Periodontology 2000, 40, 77-93. http://dx.doi.org/10.1111/j.1600-0757.2005.00144.x
- Apatzidou, D.A., Riggio, M.P. and Kinane, D.F. (2005) Impact of Smoking on the Clinical, Microbiological and Immunological Parameters of Adult Patients with Periodontitis. Journal of Clinical Periodontology, 32, 973-983. http://dx.doi.org/10.1111/j.1600-051X.2005.00788.x
- Haber, J., Wattles, J., Crowley, M., Mandell, R., Joshipura, K. and Kent, R.L. (1993) Evidence for Cigarette Smoking as a Major Risk Factor for Periodontitis. Journal of Periodontology, 64, 16-23. http://dx.doi.org/10.1902/jop.1993.64.1.16
- Martinez-Canut, P., Lorca, A. and Magan, R. (1995) Smoking and Periodontal Disease Severity. Journal of Clinical Periodontology, 22, 743-749. http://dx.doi.org/10.1111/j.1600-051X.1995.tb00256.x
- Bergstrom, J. and Preber, H. (1994) Tobacco Use as a Risk Factor. Journal of Periodontology, 65, 545-550. http://dx.doi.org/10.1902/jop.1994.65.5.545
- Gonzalez, Y.M., De Nardin, A., Grossi, S.G., Machtei, E.E., Genco, R.J. and De Nardin, E. (1996) Serum Cotinine Levels, Smoking and Periodontal Attachment Loss. Journal of Dental Research, 75, 796-802. http://dx.doi.org/10.1177/00220345960750021001
- Salvi, G.E., Lawrence, H.P., Offenbacher, S. and Beck, J.D. (1997) Influence of Risk Factors on the Pathogenesis of Periodontitis. Periodontology 2000, 14, 173-201. http://dx.doi.org/10.1111/j.1600-0757.1997.tb00197.x
- Van Dyke, T.E. and Sheilesh, D. (2005) Risk Factors for Periodontitis. Journal of the International Academy of Periodontology, 7, 3-7.
- Bascones, A., Noronha, S., Gomez, M., Mota, P., Gónzalez Moles, M.A. and Dorrego, M.V. (2005) Tissue Destruction in Periodontitis: Bacteria or Cytokines Fault? Quintessence International, 36, 299-306.
- Palmer, R.M., Wilson, R.F., Hasan, A.S. and Scott, D.A. (2005) Mechanisms of Action of Environmental Factors― Tobacco Smoking. Journal of Clinical Periodontology, 32, 180-195. http://dx.doi.org/10.1111/j.1600-051X.2005.00786.x
- Armitage, G.C. (1999) Development of a Classification System for Periodontal Diseases and Conditions. Annals of Periodontology, 4, 1-6. http://dx.doi.org/10.1902/annals.1999.4.1.1
- Xu, L., Loos, B.G., Craandijk, J., Ritsema, E., Huffels, R.A. and van der Velden, U. (2002) Teeth with Periodontal Bone Loss, Cigarette Smoking and Plasma Cotinine Levels. Journal of the International Academy of Periodontology, 4, 39-43.
- Heatherton, T.F., Kozlowski, L.T., Frecker, R.C. and Fagerstrom, K.O. (1991) The Fagerstrom Test for Nicotine Dependence: A Revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86, 1119-1127. http://dx.doi.org/10.1111/j.1360-0443.1991.tb01879.x
- Graswinckel, J.E., van der Velden, U., van Winkelhoff, A.J., Hoek, F.J. and Loos, B.G. (2004) Plasma Antibody Levels in Periodontitis Patients and Controls. Journal of Clinical Periodontology, 31, 562-578. http://dx.doi.org/10.1111/j.1600-051X.2004.00522.x
- Redondo, F.L., Bermudez, P., Cocco, C., Colella, F., Graziani, M.S., Fiehn, W., Hierla, T., Lemoel, G., Belliard, A., Manene, D., Meziani, M., Liebel, M., McQueen, M.J. and Stockmann, W. (2003) Evaluation of Cobas Integra 800 Under Simulated Routine Conditions in Six Laboratories. Clinical Chemistry and Laboratory Medicine, 41, 365-381. http://dx.doi.org/10.1515/CCLM.2003.058
- Bergstrom, J., Eliasson, S. and Dock, J. (2000) A 10-Year Prospective Study of Tobacco Smoking and Periodontal Health. Journal of Periodontology, 71, 1338-1347. http://dx.doi.org/10.1902/jop.2000.71.8.1338
- Kenney, E.B., Kraal, I.H., Saxe, S.R. and Jones, L. (1977) The Effect of Cigarette Smoke on Human Oral Polymorphonuclear Leukocytes. Journal of Periodontal Research, 12, 227-234. http://dx.doi.org/10.1111/j.1600-0765.1977.tb00126.x
- Johnson, J.D., Houchens, D.P., Kluwe, W.M., Craig, D.K. and Fisher, G.L. (1990) Effects of Mainstream and Environmental Tobacco Smoke on the Immune System in Animals and Humans: A Review. Toxicology, 20, 369-395.
- Rani, M., Bonu, S., Jha, P., Nguyen, S. and Jamjoum, L. (2003) Tobacco Use in India: Prevalence and Predictors of Smoking and Chewing in a National Cross Sectional Household Survey. Tobacco Control, 12, e4. http://dx.doi.org/10.1136/tc.12.4.e4
- Yanagisawa, T., Ueno, M., Shinada, K., Ohara, S., Wright, F.A.C. and Kawaguchi, Y. (2010) Relationship of Smoking and Smoking Cessation with Oral Health Status in Japanese Men. Journal of Periodontal Research, 45, 277-283. http://dx.doi.org/10.1111/j.1600-0765.2009.01233.x
- Moszczynski, P., Zabinski, Z., Moszczynski Jr., P., Rutowski, J., Slowinski, S. and Tabarowski, Z. (2001) Immunological Findings in Cigarette Smokers. Toxicology Letters, 118, 121-127. http://dx.doi.org/10.1016/S0378-4274(00)00270-8
- Bergström, J. and Preber, H. (1986) The Influence of Cigarette Smoking on the Development of Experimental Gingivitis. Journal of Periodontal Research, 21, 668-676. http://dx.doi.org/10.1111/j.1600-0765.1986.tb01504.x
- Haber, J. and Kent, R.L. (1992) Cigarette Smoking in Periodontal Practice. Journal of Periodontology, 63, 100-106. http://dx.doi.org/10.1902/jop.1992.63.2.100
- Quinn, S.M., Zhang, J.B., Gunsolley, J.C., Schenkein, H.A. and Tew, J.G. (1998) The Influence of Smoking and Race on Adult Periodontitis and Serum IgG2 Levels. Journal of Periodontology, 69, 171-177. http://dx.doi.org/10.1902/jop.1998.69.2.171
- Chung, H.-Y., Lu, H.-C., Chen, W.-L., Lu, C.-T., Yang, Y.-H. and Tsai, C.-C. (2003) Immunoglobulin G Profiles in Different Forms of Periodontitis. Journal of Periodontal Research, 38, 471-476. http://dx.doi.org/10.1034/j.1600-0765.2003.00675.x
- Hersey, P., Prendergast, D. and Edwards, A. (1983) Effects of Cigarette Smoking on the Immune System. Follow-Up Studies in Normal Subjects after Cessation of Smoking. Medical Journal of Australia, 2, 425-429.
- The Information of Health and Nutrition Committee (2004) The National Health and Nutrition Survey in Japan. Daiichi Shuppan Co., Tokyo.
- Chambrone, L., Preshaw, P.M., Rosa, E.F., Heasman, P.A., Romito, G.A., Pannuti, C.M. and Tu, Y.K. (2013) Effects of Smoking Cessation on the Outcomes of Non-Surgical Periodontal Therapy: A Systematic Review and Individual Patient Data Meta-Analysis. Journal of Clinical Periodontology, 40, 607-615. http://dx.doi.org/10.1111/jcpe.12106


