Advances in Biological Chemistry
Vol.05 No.05(2015), Article ID:57966,11 pages
10.4236/abc.2015.55017
Glycation Induced Physicochemical Changes in Low-Density Lipoprotein and Its Role in Promoting Cholesterol Accumulation in Macrophages along with Antiglycation Effect of Aminoguanidine
D. S. Jairajpuri1*, S. Fatima2, Z. S. Jairajpuri3
1Department of Medical Biochemistry, College of Medicine and Medical Sciences, Arabian Gulf University, Manama, Kingdom of Bahrain
2Department of Biochemistry, Faculty of Life Science, Aligarh Muslim University, Aligarh, India
3Department of Pathology, Hamdard Institute of Medical Sciences and Research, Hamdard University, New Delhi, India
Email: *jairajpurids@gmail.com, shamila_3@rediffmail.com, zeebasj@rediffmail.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 14 June 2015; accepted 11 July 2015; published 15 July 2015
ABSTRACT
The present study aimed at investigating physicochemical changes in modified LDL by sugars specifically fructose due to recent reports on its involvement in cardiovascular diseases and also glucose and their role in subsequent in vitro accumulation of cholesterol in macrophages. Antiglycation action of aminoguanidine was also investigated. LDL isolated from human blood was incubated with fructose or glucose and aminoguanidine where indicated. The physicochemical changes in modified LDL were detected by electrophoretic, spectroscopic and chemical analysis. Accumulation of cholesterol and its inhibiton in human monocyte-derived macrophages incubated with modified LDL was determined by HPLC. Results showed increased relative electrophoretic mobility, hyperchromicity at 280 nm, development of AGE fluorescence, decrease in free amino groups and increased carbonyl content in glycated LDL as compared to native LDL. Also total cholesterol accumulated in macrophages was more for glycated LDL as compared to native LDL. The magnitude of changes was more prominent in case of fructose as compared to glucose. Aminoguanidine showed remarkable restriction of glycation-induced alterations in LDL and also in accumulation of cholesterol in macrophages. The study thus proclaims that LDL-AGEs formed by fructose may contribute to accelerated initiation of diabetes induced atherosclerosis via foam cells generation and aminoguanidine may have therapeutic potential against it.
Keywords:
Glycation, LDL-AGEs, D-Fructose, Aminoguanidine, Diabetes-Induced Atherosclerosis

1. Introduction
Non-enzymatic glycation involves a complex series of sequential reactions between reducing sugars and nucleophilic groups of various biomolecules like proteins, lipids and nucleic acids, giving rise to fluorescent and/or colored adducts known as AGEs [1] . AGEs, which are also termed as glycotoxins, are well-known triggers of excess reactive oxygen species and abnormally high oxidative stress. They have been shown to accumulate in the circulation and in various tissues with normal process of ageing [2] and also under number of pathological conditions like atherosclerosis [3] , osteoarthritis, retinopathy [4] and cancer.
Majorly glycation reactions focus on those mediated by glucose (glucation), since glucose is the most abundant monosaccharide in blood and tissues and its levels are elevated remarkably in diabetes [5] . However, recent years researchers have witnessed a remarkable interest in the glycation reactions mediated by fructose (fructation) as it is far more reactive than glucose [6] . Local increases in fructose concentrations have been demonstrated in diabetic-like conditions in peripheral nerves, blood vessels and erythrocytes [7] .
Lipoproteins like other proteins are susceptible to glycation-induced modifications resulting in formation of highly heterogeneous and complex lipid-AGEs [8] which affect the structural and functional attributes of lipoproteins [9] . Glycation reactions have been linked with the development of diabetes-associated cardiovascular diseases [10] . LDL is referred to as a prime target for oxidative modifications contributing to different processes that can be considered proatherogenic. This has led to the hypothesis of possible role of LDL-AGEs in increasing artherosclerotic risk of patients with diabetes and hypercholesterolemia [11] . Metabolic abnormalities associated with glycation of LDL include diminished recognition of LDL by the classic LDL receptor; increased covalent binding of LDL in vessel walls; enhanced uptake of LDL by macrophages, thus stimulating foam cell formation; increased platelet aggregation; formation of LDL-immune complexes; and generation of oxygen free radicals, resulting in oxidative damage to both the lipid and protein components of LDL [12] .
With growing implications of glycation in number of pathological conditions, currently several strategies are being employed to control glycation. For the present study, AG has been chosen as the inhibitor, which is a highly reactive nucleophilic reagent that reacts with many biological molecules (pyridoxal phosphate, pyruvate, glucose etc.). AG is known to efficiently trap dicarbonyl intermediates to form substituted triazines preventing AGE formation and thus inhibiting many glycation induced chances in different biomolecules [13] . Also some studies on animals have shown positive effect of AG in retarding the process of glycation [14] . Panagiotopoulos et al. explored the inhibitory action of AG on accumulation of AGEs and on the development of artherosclerosis. It was observed in the study on male New Zealand white cross rabbits fed on a high cholesterol diet, that increased doses of AG (25, 50 and 100 mg/kg AG per body weight) prevented AGE accumulation and plaque formation in the aortic arch, thoracic and abdominal aorta in a concentration dependent manner [14] .
Recent evidences on LDL-AGEs as a prime link for diabetes-associated cardiovascular diseases have shifted the focus of glycobiology on studies involving glycation of LDL with different compounds like glucose [15] , ribose [16] , glycolaldehyde [17] , etc. Studies have also shown LDL-AGEs induced accelerated formation of cholesterol-laden foam cells which play a critical role in disease development [18] . Also the antagonistic role of various compounds like carnosine against LDL glycation and in turn preventing foam cell formation has been investigated [17] [19] . However, to best of our knowledge, none have been performed by using fructose and aminoguanidine.
Thus on the basis of above explanations, the present study focuses on examining the physicochemical changes due to in vitro treatment of LDL with glucose or fructose. The possible inhibitory effect of AG on physicochemical changes in glycated LDL and also on its in vitro accumulation of cholesterol in HMDM will also be investigated.
2. Materials and Methods
2.1. Materials
All the chemicals used during the study like D-glucose, D-fructose, AG, DETAPAC, TNBS, DNPH as well as salts for PBS were procured from Sigma Aldrich (St Louis MO, USA). Precast 1% agarose gels and PD10 columns were purchased from Helena Laboratories (Mt. Waverly, Vic). All chemicals were of analytical grade and all solvents of HPLC grade. Solutions were prepared using Nanopure water (Milli Q system, Millipore-Waters, Lane Cove, Australia).
2.2. In Vitro Glycation of LDL with Glucose and Fructose
LDL was isolated from healthy volunteers with normal basal metabolic rate and between age group of 25 - 32 years old working in HIMSR, Jamia Hamdard with normoglycaemic and normolipidaemic profiles [18] . Blood was collected after taking approval from the Jamia Hamdard Ethics and Research Committee as well as signed informed consent from the volunteers. Purified LDL (1 mg/ml) in 100 mM PBS, pH 7.2 was incubated with fructose or glucose at a final concentration of 25 mM. Where indicated 25 µM AG was included. The mixtures were sterilized by filtration through a 0.2 μm pore-size nitrocellulose filter and then incubated at 37˚C for 21 days under sterile aerobic conditions. LDL incubated with 25 µM DETAPAC under the same conditions served as control. The added reagents (DETAPAC, fructose, glucose, AG) were removed post incubation by PD10 chromatography.
2.3. Isolation and Culturing of HMDM
Monocytes were isolated from white cell concentrates by counter-current elutriation and then incubated (1 × 105/ml) in 12-well plates (Costar, Corning, USA) in serum-free RPMI-1640 for 2 hours. The plates were then washed and adhering cells were incubated (5% CO2 and 37˚C) in RPMI for 10 days to give HMDM [20] .
2.4. Electrophoretic Analysis
Changes in overall LDL particle charge in control as well as treated samples were quantified by electrophoretic mobility on agarose gels [18] .
2.5. Absorbance Measurements
The UV absorption measurements of control LDL and treated samples were obtained by measuring the ultraviolet absorption profile between the wavelength ranges of 200 - 400 nm on a Shimadzu spectrophotometer using a cuvette of 1.0 cm pathlength. Three hundred micrograms of samples in a total volume of 1.0 ml was taken for the analysis.
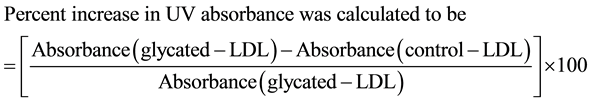 (1)
(1)
2.6. Fluorescence Measurements
Similarly control sample, sugar incubated samples with or without AG were studied by measuring fluorescence at 25˚C ± 0.2˚C on a Hitachi F2000 spectrofluorometer (Tokyo, Japan). The samples were excited at 370 nm excitation and an emission was recorded in the range of 350 - 500 nm. The development of AGE fluorescence of treated samples was recorded with emission at 450 nm.
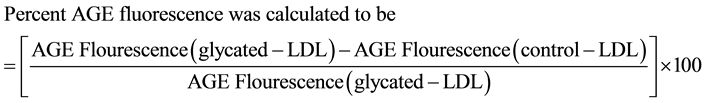 (2)
(2)
2.7. Amino Group Estimation
The amino groups of control and treated samples were determined by the method of Habeeb and Hiramoto [21] with slight modification. Suitable aliquots of sample were dissolved in 1.0 ml of 0.1 M sodium tetraborate buffer, pH 9.3, 25 µl of 0.3 M TNBS added and the tubes agitated instantly to ensure complete mixing and allowed to stand for 30 minutes at room temperature. Absorbance of the yellow colour developed was recorded at 420 nm against a reagent blank. Glycine was used as a standard amino acid. The results were expressed as total number of amino groups per molecule of LDL.
2.8. Determination of Protein Bound Carbonyl Groups
Protein-bound carbonyl groups were estimated according to the protocol of Levine et al. by using DNPH. The samples were read at 379 nm and the results were expressed as the number of nanomoles of carbonyl per mg of sample LDL using a e379 nm = 22,000 M−1∙cm−1 [22] .
2.9. Cellular Cholesterol-Loading Studies
HMDM were exposed to control as well as treated LDL samples (150 µg/ml) in media containing 10% lipoprotein-deficient serum. After 72 hours, cells were washed with 20 mM PBS and lysed in water. Total cholesterol concentration was quantified by HPLC [23] .
2.10. Data Analysis
Data analysis was performed using one-way analysis of variance using the Newman-Keuls multiple comparison test. P ≤ 0.05 was taken as significant.
3. Results and Discussion
In order to compare the physicochemical changes between native LDL and modified LDL (incubated in the absence as well as presence of AG) electrophoretic, spectroscopic and chemical analysis was carried out.
3.1. Electrophoretic Analysis
Incubation of LDL with 25 mM glucose or fructose resulted in a significant increase in REM and thus indicative of an overall decrease in positive charge (Table 1). This decrease in charge is more prominent in case of fructose treated sample as compared to one treated with glucose. On the other hand, presence of 25 µM AG decreased particle modification as evident by low REM for samples with sugar + AG in comparison to those with sugar only (Table 1).
The greater shift in REM observed with sugar treated LDL compared to samples with AG represents the formation of an adduct that contributes to charge modification which is more in case of fructose than in glucose. This is expected due to high reactivity of fructose [24] . On the other hand, charge modification for samples with AG was fairly less than samples with only sugars, thus highlighting the ability of AG to prevent formation of
Table 1. Change in LDL particle charge. Overall LDL particle charge was determined by REM for sugar- modified LDL both in the absence or presence of AG and that incubated with DETAPAC (control).
Results are means + SEM from 3 - 4 separate experiments each with 3 samples per treatment. #P < 0.001 compared with control.
AGEs significantly [25] . AG has been reported to react with sugars and aldehydes and detoxify aldehyde-/sugar- modified proteins [26] .
3.2. Absorbance and Fluorescence Studies
The possible sugar-induced structural changes in the LDL were examined with respect to changes in the chromophoric and fluorophoric properties. In both the parameters, i.e. UV absorbance and fluorescence, an increase was observed in the sugar-treated samples as compared to the control sample. However, the changes in the spectroscopic properties of the samples incubated with sugars along with AG were significantly less as compared to the sample treated with sugars alone.
3.2.1. UV Absorbance Measurements
The magnitude of hyperchromicity was more in case of fructose (75% + 3) as compared to glucose (65% + 3). The presence of AG in LDL samples showed small increase in the absorbance, as a mere 15% + 2 hyperchromicity in case glucose + AG and 22% + 3 hyperchromicity in case of fructose + AG samples (Figure 1). Percent hyperchromicity seen in the UV region can be due to sugar induced unfolding of the LDL molecules resulting in exposure of the chromophoric aromatic residues [27] . The large variations observed in the hyperchromicity among fructose treated and glucose treated LDL are suggestive of major differences in conformational alterations resulting from sugar-mediated reactions. AG was highly effective in restricting sugar-induced alterations as evident by marked decrease in the sugar-mediated hyperchromicity in samples were it was included. AG is
Figure 1. Percent Hyperchromicity. Absorbance in UV region was measured for sugar-modified LDL both in absence or presence of AG and that incubated with DETAPAC (control). LDL (1 mg/ml) was incubated with glucose/fructose (25 mM) either in the absence or in the presence of aminoguanidine (25 µM) and that incubated DETAPAC was taken as control. Incubation was for 21 days at 37˚C. Percent hyperchromicity was calculated according to the formula stated in the materials and methods section (Equation (1)). Results are expressed as the means + SEM from 3 - 4 separate experiments each with 3 samples per treatment. P < 0.05 for glucose + AG and fructose + AG are compared with glucose treated and fructose treated samples respectively.
considered as a putative antiglycation agent resulting in prevention of formation of AGEs by reacting rapidly with α, β-dicarbonyl compounds [28] [29] .
3.2.2. Fluorescence Measurements
Development of AGE fluorescence (370 nm excitation/450 nm emission) is a well established measure of protein glycation/AGE formation [16] . Similar to UV absorbance, the magnitude of new fluorescence developed was more in case of fructose (83% + 3) as compared to glucose (70% + 2). Also as expected, AG was highly effective in restricting sugar-induced alterations as samples showed negligible development of new fluorescence. Percent new fluorescence was 5% + 1 only for both glucose and fructose treated LDL samples in the presence of AG (Figure 2). Development of AGE fluorescence is attributed to formation of heterogeneous cyclic structures during the process of glycation which make adducts fluoresce [30] . The fluorophores relative contribution to AGE fluorescence can vary widely depending on the site and extent of glycation [27] . This is why the percent development of AGE fluorescence is seen exponentially high in case of fructose as compared to glucose [31] . Modified LDL with AG, however, showed only negligible development of AGE fluorescence thus confirming antiglycation effect of AG [25] .
3.3. Chemical Modifications
The extent of glycation in the treated samples was also determined chemically by estimation of free amino groups and carbonyl group content. In general the results obtained were complementary to the observations in
Figure 2. Percent AGE Fluorescence. Development of fluorescence was measured for sugar-modified LDL both in absence or presence of AG and that incubated with DETAPAC (control). LDL (1 mg/ml) was incubated with glucose/fructose (25 mM) either in the absence or in the presence of aminoguanidine (25 µM) and that incubated DETAPAC was taken as control. Incubation was for 21 days at 37˚C. Percent AGE fluorescence was calculated according to the formula stated in the materials and methods section (Equation (2)). Results are expressed as the means + SEM from 3 - 4 separate experiments each with 3 samples per treatment. P < 0.05 for glucose + AG and fructose + AG are compared with glucose treated and fructose treated samples respectively.
spectroscopic studies and electrophoretic analysis confirming sugar induced LDL glycation in the treated samples. As compared to the control sample of LDL, a decrease in the free amino groups and an increase in the carbonyl content were seen in sugar treated samples. However, marked restriction of chemical modifications of LDL was seen in samples which had AG along with sugars in them.
3.3.1. Determination of Free Amino Groups
Figure 3 shows the amino groups of native LDL and those in treated samples accessible to reaction with TNBS under the conditions used. Control LDL revealed about 40 + 2 free amino groups per molecule of LDL whereas a steep decrease in the free amino groups was seen in LDL treated with glucose (26 + 1 per molecule of LDL) and fructose (18 + 2 per molecule of LDL). Samples with AG showed less reduction in free amino groups as 35 + 2 and 32 + 2 amino groups per molecule of LDL in glucose + AG and fructose + AG samples respectively. Decrease in free amino groups in modified LDL molecules observed is not unexpected since glycation begins with the reaction of sugars mainly with the free amino groups present of various biomolecules and supports the earlier observation of Lapolla et al. [32] , that exposed amino groups may play an important role in determining the susceptibility to glycation by the sugars.
3.3.2. Determination of Carbonyl Content
Sugar-treated LDL samples were further analyzed for their carbonyl content by reaction with DNPH. Results are exhibited in Figure 4. As evident, fructose caused exponential increase in the formation of carbonyl groups in the LDL molecule as compared to glucose. The carbonyl content was 30.33 + 1.01 nmol∙mg−1 LDL and 22.14 + 1.22 nmol∙mg−1 LDL for fructose and glucose respectively. Carbonyl group content is a consequence of glycation,
Figure 3. Free Amino Group Content. Free amino groups were measured for sugar-modified LDL both in absence or presence of AG and that incubated with DETAPAC (control). LDL (1 mg/ml) was incubated with glucose/fructose (25 mM) either in the absence or in the presence of aminoguanidine (25 µM) and that incubated DETAPAC was taken as control. Incubation was for 21 days at 37˚C. Results are expressed as the means + SEM from 3 - 4 separate experiments each with 3 samples per treatment. P < 0.05 for glucose + AG and fructose + AG are compared with glucose treated and fructose treated samples respectively.
Figure 4. Total Carbonyl Content. Total carbonyl content was measured for sugar-modified LDL both in absence or presence of AG and that incubated with DETAPAC (control). LDL (1 mg/ml) was incubated with glucose/fructose (25 mM) either in the absence or in the presence of aminoguanidine (25 µM) and that incubated DETAPAC was taken as control. Incubation was for 21 days at 37˚C. Results are expressed as the means + SEM from 3 - 4 separate experiments each with 3 samples per treatment. P < 0.05 for glucose + AG and fructose + AG are compared with glucose treated and fructose treated samples respectively.
thus LDL incubated with DETAPAC alone show negligible carbonyl group content of 5.24 + 0.84 nmol∙mg−1 LDL. LDL incubated with respective sugars in the presence of AG showed generation of very small amount of carbonyl content as 7.12 + 0.77 nmol∙mg−1 LDL in case of glucose and 8.01 + 0.54 nmol∙mg−1 LDL in case of fructose. DNPH is a standard method for detecting carbonyl groups and can be used to follow changes on glycation of lipoproteins [16] . The carbonyls detected under the conditions are clearly the result of sugar-induced glycation [2] , since there is little DNPH-reactivity in the LDL incubated with DETAPAC (control). It is well recognized that carbonyl groups are incorporated in proteins as a consequence of glycation and protein carbonyl content is considered as a reliable measure of glycation [33] . Also low levels of carbonyl group content in AG treated samples are again in agreement with previous studies which have identified it as forming discrete adducts with carbonyl compounds during glycation, confirming its role as true scavenger of carbonyl compounds [13] [26] .
3.4. Total Cholesterol Content in HMDM
Incubation of HMDM with sugar treated LDL (150 µg) for 48 hours resulted in a significant accumulation of cellular cholesterol as evident in Figure 5. In accordance to earlier observations fructose treated LDL resulted in far more cholesterol accumulation (220 + 4 nmoles/mg cell protein) as compared to glucose treated LDL (170 + 3 nmoles/mg cell protein). However, the samples with AG during the LDL modification phase showed significantly low levels of cholesterol accumulation by HMDM as the amount of total cholesterol obtained in case of glucose + AG (110 + 3 nmoles/mg cell protein) and fructose + AG (116 + 4 nmoles/mg cell protein) was not significantly different from that of control (95 + 3 nmoles/mg cell protein). The total cholesterol content obtained post incubation of HMDM with sugar-modified LDL strongly embarked the implications of LDL glycation
Figure 5. Total Cholesterol Concentration Present in HMDM. Total cholesterol content in HMDM was measured after incubation with sugar-modified LDL both in absence or presence of AG and that incubated with DETAPAC (control). LDL (1 mg/ml) was incubated with glucose/fructose (25 mM) either in the absence or in the presence of aminoguanidine (25 µM) and that incubated DETAPAC was taken as control. Incubation was for 21 days at 37˚C. HMDM were exposed to control as well as treated LDL samples (150 µg/ml) for 72 hours and then total cholesterol content was quantified by HPLC. Results are expressed as the means + SEM from 3 - 4 separate experiments each with 3 samples per treatment. P < 0.05 for glucose + AG and fructose + AG are compared with glucose treated and fructose treated samples respectively.
on macrophagial accumulation of cholesterol, in turn resulting in the formation of foam cells; a prominent hallmark of atherosclerosis [34] .
The accumulation observed more in case of fructose is due to modification of LDL to an exaggerated extent owing to high reactivity of fructose [35] . However, LDL-modified by both the sugars in the presence of AG were able to achieve almost complete inhibition of lipid loading even when complete prevention of LDL modification was not achieved, as evident in physicochemical analysis. This indicated that all that was required to prevent lipid loading was reduction of damage below a key threshold related to modification of key residues on apolipoprotein B-100 [17] . This has potential therapeutic significance.
It is now recognized, that in vitro glycation of LDL can induce intracellular cholesterol accumulation in macrophages resulting in foam cell formation which is a characteristic of atherosclerosis [23] . Fructose has been taken as the prime sugar for the study due to numerous reports on its increased consumption in the form of high fructose corn syrup in processed foods [36] and also it has been recently implicated as a risk factor for cardiovascular diseases [37] . Moreover during diabetic conditions excessive blood glucose levels automatically result in high levels of fructose in tissues/organs were polyol pathway is active [38] . Fructose is known to be almost 300 times more reactive as compared to glucose due to presence of greater number of reactive open chain structures [39] , thus causing far more AGE formation and oxidative stress than glucose [6] .
AG is a well established therapeutic agent for the prevention of glycation process which is a characteristic of normal aging as well as different pathological conditions like diabetes, atherosclerosis [28] . It reacts rapidly with different sugars and dicarbonyl compounds to prevent the formation of AGEs. Adducts formed are substituted 3-amino-1, 2, 4-triazine derivatives [13] . Inhibition of disease mechanisms, especiallly vascular complications in experimental diabetes, by AG has provided evidence that accumulation of AGEs is a risk factor for disease progression and AG is effective in preventing it [28] [29] and is currently undergoing clinical trials to be used as a therapeutic drug for AGE associated pathologies.
4. Conclusion
The study gives strong evidence that fructose-induced alterations in LDL are far more prominent than that by glucose. This is of high significance owing to numerous reports about the role of fructose in lipid metabolism and also in cardiovascular disorders [37] [39] . However, the key finding from the data obtained is that aminoguanidine is effective at very low concentrations to combat glycation even with a sugar as potentially reactive as fructose. Thus, aminoguanidine may serve as effective scavengers of glycation agents hence of protein glycation and as potential therapeutic agents to inhibit diabetes-induced atherosclerosis.
Conflict of Interest
There is no conflict of interest that can be perceived as prejudicing the impartiality of the present research study.
Acknowledgements
The authors are grateful to the Aligarh Muslim University (Aligarh, India) and Hamdard Institute of Medical Sciences and Research, Hamdard University (New Delhi, India) for the research facilities. Acknowledgment is also extended to College of Medicine and Medical Sciences, Arabian Gulf University (Manama, Bahrain) for other facilities.
Cite this paper
D. S.Jairajpuri,S.Fatima,Z. S.Jairajpuri, (2015) Glycation Induced Physicochemical Changes in Low-Density Lipoprotein and Its Role in Promoting Cholesterol Accumulation in Macrophages along with Antiglycation Effect of Aminoguanidine. Advances in Biological Chemistry,05,203-214. doi: 10.4236/abc.2015.55017
References
- 1. Cerami, A. and Ulrich, P. (2001) Pharmaceutical Intervention of Advanced Glycation Endproducts. Novartis Foundation Symposia, 235, 202-212.
- 2. Anguizola, J., Matsuda, R., Barnaby, O.S., Hoy, K.S., Wa, C.L., DeBolt, E., Koke, M. and Hage, D.S. (2013) Review: Glycation of Human Serum Albumin. Clinica Chimica Acta, 425, 64-76.
http://dx.doi.org/10.1016/j.cca.2013.07.013 - 3. Yokoyama, H., Araki, S., Haneda, M., Matsushima, M., Kawai, K., Hirao, K., Oishi, M., Sugimoto, K., Sone, H., Maegawa, H. and Kashiwagi, A. (2012) Chronic Kidney Disease Categories and Renal-Cardiovascular Outcomes in Type 2 Diabetes without Prevalent Cardiovascular Disease: A Prospective Cohort Study (JDDM25). Diabetologia, 55, 1911-1918.
http://dx.doi.org/10.1007/s00125-012-2536-y - 4. Yamagishi, S., Nakamura, K. and Matsui, T. (2006) Advanced Glycation End Products (AGEs) and Their Receptor (RAGE) System in Diabetic Retinopathy. Current Drug Discovery Technologies, 3, 83-88.
http://dx.doi.org/10.2174/157016306776637555 - 5. Ghosh-Moulick, R., Bhattacharya, J., Roy, S., Basak, S. and Dasgupta, A.K. (2007) Compensatory Secondary Structure Alterations in Protein Glycation. Biochimica et Biophysica Acta (BBA)—Proteins and Proteomics, 1774, 233-242.
http://dx.doi.org/10.1016/j.bbapap.2006.11.018 - 6. Sakai, M., Oumomi, M. and Kasuga, M. (2002) Experimental Studies on the Role of Fructose in the Development of Diabetic Complications. Kobe Journal of Medical Sciences, 48, 125-136.
- 7. Poulsom, R., Boot-Handford, R.P. and Heath, H. (1983) The Effects of Long-Term Treatment of Streptozotocin-Diabetic Rats with an Aldose Reductase Inhibitor. Experimental Eye Research, 37, 507-515.
http://dx.doi.org/10.1016/0014-4835(83)90027-1 - 8. Gasser, A. and Forbes, J.M. (2008) Advanced Glycation: Implications in Tissue Damage and Disease. Protein and Peptide Letters, 15, 385-391.
http://dx.doi.org/10.2174/092986608784246515 - 9. Stitt, A.W. (2003) The Role of Advanced Glycation in the Path-ogenesis of Diabetic Retinopathy. Experimental and Molecular Pathology, 75, 95-108.
http://dx.doi.org/10.1016/S0014-4800(03)00035-2 - 10. Rashid, I., Brown, B.E., van Reyk, D.M. and Davies, M.J. (2006) The Roles of Glycation, Glycoxidation and Advanced Glycation End-Product Formation in Diabetes-Induced Ather-osclerosis. In: Cheema, S.K., Ed., Biochemistry of Artherosclerosis, Springer Verlag, New York, 247-283.
http://dx.doi.org/10.1007/0-387-36279-3_12 - 11. Zimmermann, R., Panzenbock, U., Wintersperger, A., Levak-Frank, S., Graier, W., Glatter, O., Fritz, G., Kostner, G.M. and Zechner, R. (2001) Lipoprotein Lipase Mediates the Uptake of Glycated LDL in Fibroblasts, Endothelial Cells, and Macrophages. Diabetes, 50, 1643-1653.
http://dx.doi.org/10.2337/diabetes.50.7.1643 - 12. Lyons, T.J. (1993) Glycation and Oxidation: A Role in the Patho-genesis of Atherosclerosis. American Journal of Cardiology, 71, 26-31.
http://dx.doi.org/10.1016/0002-9149(93)90142-Y - 13. Majd, A.A., Goodarzi, M.T., Hassanzadeh, T., Tavilani, H. and Karimi, J. (2014) Aminoguanidine Partially Prevents the Reduction in Liver Pyruvate Kinase Activity in Diabetic Rats. Advances in Biomedical Research, 3, 260-265.
http://dx.doi.org/10.4103/2277-9175.148233 - 14. Panagiotopoulos, S., Brien, R.C.O., Bucala, R., Cooper, M.E. and Jerums, G. (1998) Aminoguanidine Has an Anti-Atherogenic Effect in the Cholesterol-Fed Rabbit. Atherosclerosis, 136, 125-131.
http://dx.doi.org/10.1016/S0021-9150(97)00192-5 - 15. Rabbani, N., Chittari, M.V., Bodmer, C.W., Zehnder, D., Ceriello, A. and Thornalley, P.J. (2010) Increased Glycation and Oxidative Damage to Apolipoprotein B100 of LDL Cholesterol in Patients with Type 2 Diabetes and Effect of Metformin. Diabetes, 59, 1038-1045.
http://dx.doi.org/10.2337/db09-1455 - 16. Ahmad, S., Akhter, F., Moinuddin, Shahab, U. and Khan, M.S. (2013) Studies on Glycation of Human Low Density Lipoprotein: A Functional Insight into Physic-Chemical Analysis. International Journal of Biological Biomolecules, 62, 167-171.
- 17. Rashid, I., van Reyk, D.M. and Davies, M.J. (2007) Carnosine and Its Constituents Inhibit Glycation of Low-Density Lipoproteins That Promotes Foam Cell Formation in Vitro. FEBS Letters, 581, 1067-1070.
http://dx.doi.org/10.1016/j.febslet.2007.01.082 - 18. Brown, B.E., Dean, R.T. and Davies, M.J. (2005) Glycation of Low-Density Lipoproteins by Methylglyoxal and Glycoaldehyde Gives Rise to the in Vitro Formation of Lipid-Laden Cells. Diabetologia, 48, 361-369.
http://dx.doi.org/10.1007/s00125-004-1648-4 - 19. Younis, N.N., Soran, H., Pemberton, P., Charlton-Menys, V., Elseweidy, M.M. and Durrington, P.N. (2013) Small Dense LDL Is More Susceptible to Glycation than More Buoyant LDL in Type 2 Diabetes. Clinical Science (London), 124, 343-349.
http://dx.doi.org/10.1042/CS20120304 - 20. Garner, B., Dean, R.T. and Jessup, W. (1994) Human Macro-phage-Mediated Oxidation of Low-Density Lipoprotein Is Delayed and Independent of Superoxide Production. Journal of Biochemistry, 301, 421-428.
- 21. Habeeb, A.F.S.A. and Hiramoto, R. (1968) Reaction of Proteins with Glutaraldehyde. Archives of Biochemistry and Biophysics, 126, 16-26.
http://dx.doi.org/10.1016/0003-9861(68)90554-7 - 22. Levine, R.L., Williams, J., Stadtman, E.R. and Shacter, E. (1994) Carbonyl Assays for Determination of Oxidatively Modified Proteins. Methods in Enzymology, 233, 346-357.
http://dx.doi.org/10.1016/S0076-6879(94)33040-9 - 23. Brown, B.E., Mahroof, F.M., Cook, N.L., van Reyk, D.M. and Davies, M.J. (2006) Hydrazine Compounds Inhibit Glycation of Low-Density Lipo-proteins and Prevent the in Vitro Formation of Model Foam Cells from Glycolaldehyde-Modified Low-Density Lipoproteins. Diabetologia, 49, 775-783.
http://dx.doi.org/10.1007/s00125-006-0137-3 - 24. Jairajpuri, D.S., Fatima, S. and Saleemuddin, M. (2008) Complexing of Glucose Oxidase with Anti-Glucose Oxidase Antibodies or the F(ab)’2/F(ab)’ Fragments Derived There from Protects Both the Enzyme and Antibody/Antibody Fragments. Biochemistry, 73, 1235-1241.
http://dx.doi.org/10.1134/S0006297908110102 - 25. Labieniec-Watala, M., Siewiera, K. and Jozwiak, Z. (2011) Resorcylidene Aminoguanidine (RAG) Improves Cardiac Mitochondrial Bioenergetics Impaired by Hyperglycaemia in a Model of Experimental Diabetes. International Journal of Molecular Sciences, 12, 8013-8026.
http://dx.doi.org/10.3390/ijms12118013 - 26. Price, D.L., Rhett, P.M., Thorpe, S.R. and Baynes, J.W. (2001) Chelating Activity of Advanced Glycation End-Product Inhibitors. Journal of Biological Chemistry, 276, 48967-48972.
http://dx.doi.org/10.1074/jbc.M108196200 - 27. Bachetti, T., Masciangelo, S., Armeni, T., Bicchiega, V. and Ferretti, G. (2014) Glycation of High Density Lipoprotein by Methylglyoxal: Effect on HDL-Paraoxonase Activity. Metabolism, 63, 307-311.
http://dx.doi.org/10.1016/j.metabol.2013.10.013 - 28. Koh, G., Lee, D. and Woo, J. (2010) 2-Deoxy-D-Ribose Induces Cellular Damage by Increasing Oxidative Stress and Protein Glycation in a Pancreatic Beta-Cell Line. Metabolism—Clinical and Experimental, 59, 325-332.
http://dx.doi.org/10.1016/j.metabol.2009.07.028 - 29. Thornalley, P.J. (2003) Use of Aminoguanidine (Pimagedine) to Prevent the Formation of Advanced Glycation Endproducts. Archives of Biochemistry and Biophysics, 419, 31-40.
http://dx.doi.org/10.1016/j.abb.2003.08.013 - 30. Ansari, N.A., Moinuddin, A.K. and Ali, A. (2009) Preferential Recognition of Amadori-Rich Lysine Residues by Serum Antibodies in Diabetes Mellitus: Role of Protein Glycation in the Disease Process. Human Immunology, 70, 417-424.
http://dx.doi.org/10.1016/j.humimm.2009.03.015 - 31. Jakus, V. and Rietbrock, N. (2004) Advanced Glycation End-Products and the Progress of Diabetic Vascular Complications. Physiological Research, 53, 131-142.
- 32. Lapolla, A., Molin, L. and Traldi, P. (2013) Protein Glycation in Diabetes as Determined by Mass Spectrometry. International Journal of Endocrinology, 2013, 1-11.
http://dx.doi.org/10.1155/2013/412103 - 33. Beal, M.F. (2002) Oxidatively Modified Proteins in Aging and Disease. Free Radical Biology and Medicine, 32, 797- 803.
http://dx.doi.org/10.1016/S0891-5849(02)00780-3 - 34. Li, A.C. and Glass, C.K. (2002) The Macrophage Foam Cell as a Target for Therapeutic Intervention. Nature Medicine, 8, 1235-1242.
http://dx.doi.org/10.1038/nm1102-1235 - 35. Zhang, Y.H., An, T., Zhang, R.C., Zhou, Q., Huang, Y. and Zhang, J. (2013) Very High Fructose Intake Increases Serum LDL-Cholesterol and Total Cholesterol: A Meta-Analysis of Controlled Feeding Trials. Journal of Nutrition, 143, 1391-1398.
http://dx.doi.org/10.3945/jn.113.175323 - 36. Bray, G.A., Nielsen, S.J. and Popkin, B.M. (2004) Consumption of High-Fructose Corn Syrup in Beverages May Play a Role in the Epidemic of Obesity. American Journal of Clinical Nutrition, 79, 537-543.
- 37. Aeberli, I., Zimmermann, M.B., Molinari, L., Lehmann, R., L’Allemand, D., Spinas, G.A. and Berneis, K. (2007) Fructose Intake Is a Predictor of LDL Particle Size in Overweight Schoolchildren. American Journal of Clinical Nutrition, 86, 1174-1178.
- 38. Miyazawa, N., Kawasaki, Y. and Fuji, J. (1998) Immunological Detection of Fructated Proteins in Vivo and in Vitro. Biochemistry Journal, 336, 101-107.
- 39. Bantle, J.P., Raatz, S.K., Thomas, W. and Georgopoulos, A. (2000) Effects of Dietary Fructose on Plasma Lipids in Healthy Subjects. American Journal of Clinical Nutrition, 72, 1128-1134.
Abbreviations
AGEs: Advanced glycated end-products;
AG: Aminoguanidine;
DETAPAC: Diethyleneaminepenta acetic acid;
DNPH: 2, 4-dinitrophenylhydrazine;
HMDM: Human monocyte-derived macrophages;
LDL: Low density lipoprotein;
LDL-AGEs: low density lipoprotein advanced glycation end products;
PBS: Phosphate buffer saline;
REM: relative electrophoretic mobility;
RPMI: Roswell Park Memorial Institute;
TNBS: Trinitrobenzene sulphonic acid.
NOTES

*Corresponding author.







