Open Journal of Inorganic Chemistry
Vol.06 No.03(2016), Article ID:67767,8 pages
10.4236/ojic.2016.63011
Synthesis of NbFAPO-5 and NbFAPSO-5 Molecular Sieve by Hydrothermal Method and Comparison of Their XRD Patterns and Their Acidic Properties Evaluation by Infrared Spectroscopy
Mominou Nchare1*, Lei Wang2, Salomon Anagho3
1Department of Mining & Extractive Metallurgy, School of Geology & Mining Engineering, University of Ngaoundéré, Ngaoundéré, Cameroon
2Shanghai Institute of Technology, Shanghai, China
3Department of Chemistry, University of Dschang, Dschang, Cameroon

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 21 December 2015; accepted 25 June 2016; published 28 June 2016
ABSTRACT
Mesoporous molecular sieves, NbFAPO-5 and NbFAPSO-5 were hydrothermally synthesized with AlPO-5 type structure. Characterization of these molecular sieves was performed by X-ray diffraction to determine their structure, ICP-EAS for their elemental composition and infrared spectrometry to access their acidic properties. X-ray diffraction patterns confirmed well AlPO-5 type structure. ICP-EAS analysis confirmed the incorporation of silicon (12.9%), aluminium (15.4%), phosphorous (21.9%), iron (5.62%) and niobium (0.39%) into AlPO-5 framework. Infrared spectrometry analysis showed that both Bronsted and Lewis sites were found in the synthesized samples. The presence of both Bronsted and Lewis acid site led to bifunctional function of NbFAPO-5 and NbFAPSO-5 molecular sieve in promoting both oxidation and esterification reactions. NbFAPSO-5 Bronsted acidity was higher than that of NbFAPO-5 and for Lewis acidity, NbFAPO-5 was higher than that of NbFAPO-5.
Keywords:
Niobium, AlPO-5 Molecular Sieve, Hydrothermal Synthesis, Acid Properties

1. Introduction
Catalyst technology has increasingly played a key role in the economic development of countries around the word. Today, most of the chemicals, polymers, drugs, dyes and fabrics are prepared by using catalysts [1] . The discovery of new catalysts and their applications has historically led to major innovations in chemical processing. In recent years the isomorphous substitution in zeolite and aluminophosphate materials by other elements has been extensively studied and reviewed [2] . Isomorphous substitution of elements in these materials is carried out in order to modify their catalytic and shape selective properties. Different ways to perform such substitutions are well established either during hydrothermal synthesis or by post-synthesis methods in liquid and vapor phase [3] - [5] . Generally hydrothermal synthesis is preferred over post synthesis because it leads to better incorporation of a wide variety of metal ion into aluminophosphate materials which are of particular interest for the design of novel catalysts. The past decade has brought an increasing interest in niobium-containing materials, which can be applied within many fields [6] . Niobium compounds exhibit special properties not shown by the compounds of neighboring elements in the periodic table. Some of them like stability, or strong metal support interaction are very important for good quality catalyst. The unfavorable feature of niobium oxides, the biggest group of niobium compounds applied in heterogeneous catalysis is a low mobility and reducibility of niobium species.
In this work, Nb was successfully introduced into FAPO-5 and FAPSO-5 type structure leading to NbFAPO-5 and NbFAPSO-5 and their acidic properties studied.
2. Experimental Procedures
2.1. Synthesis of AlPO-5 Molecular Sieve [7] [8]
In 50 ml Erlern Mayer, a fixed amount of deionized water was added with 85% phosphoric acid and just after aluminum source was slowly added under stirring which took 20 minutes.
After adding the template (Triethylamine), the temperature was rised to 40˚C and the whole mixture was then stirred for another 30 minutes. The resulting mixture was then placed into a Teflon-line stainless steel autoclave and heated at 200˚C for 24 hours. After quenching to the room temperature, the product was washed repeatedly with deionized water, and then dried at 100˚C for 4 hours. The resulting product was calcined at 600˚C for 4 hours.
The synthesis of FeAIPO-5 follows the same procedure as described for AIPO4-5. With any priority, the iron source was added before aluminum source. The synthesis of FeAPSO-5 also follows the procedure described for FeAIPO4-5. Silicon source was added just after aluminum had form a homogenized mixture.
For the niobium solution preparation, a fixe quantity of niobium hydroxide was put in 80 g oxalic acid and 500 ml deionized water under stirring, and the temperature was rised to 70˚C - 80˚C. After a period of time, a clear solution of niobium complexed in oxacilic acid was obtained.
Synthesis of NbFeAPO-5 and NbFeAPSO-5 follows the same procedure as described for FeAPO-5 and Fe- APSO-5 except that niobium solution was added just after putting aluminum source for NbFeAPO-5 and silicon source for NbFeAPSO-5.
The raw materials for different synthesized catalysts are found in Table 1.
2.2. Product Analysis
X-ray powder diffraction data were obtained on a Rigaku D/max diffractometer using Cu ka radiation at 40 kv and 60 Ma. Elemental composition of different catalysts was accessed using ICP-AES performed on IRIS 1000 and the acidity by mean of infrared spectrometry performed on Nicolet Magna-IR 550.
3. Results and Discussion
3.1. Effect of Iron Content in NbFAPSO-5 Molecular Sieve
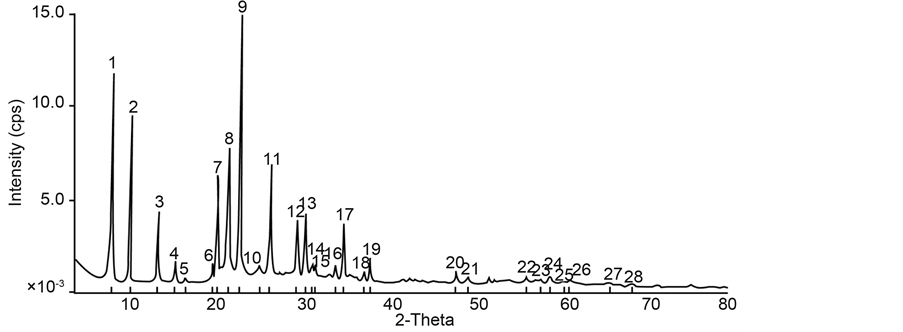
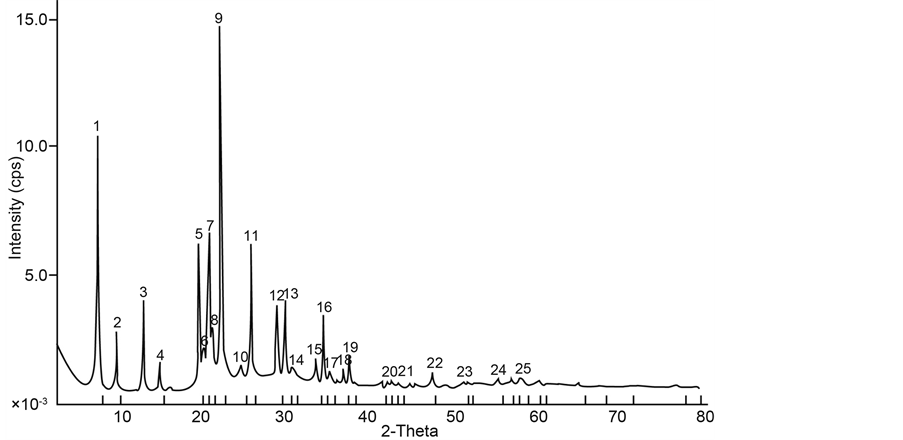
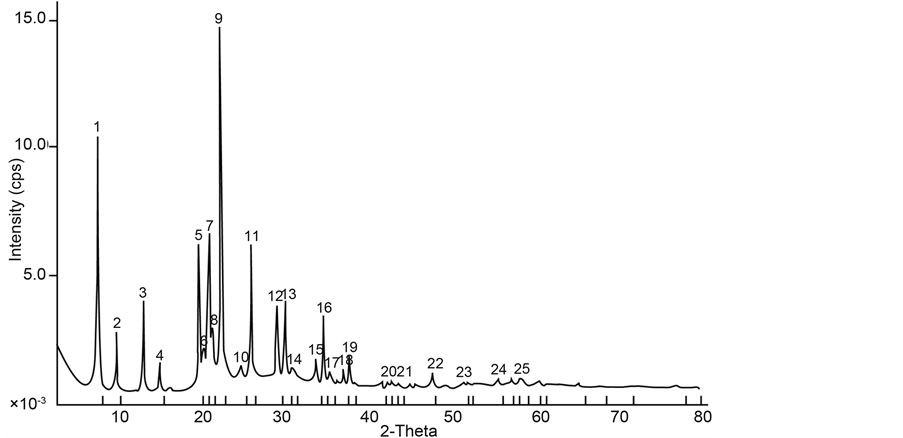
Samples with iron content of 2.02 g (Figure 1) and 4.04 g (Figure 2) were prepared and the resulting XRD patterns confirm well that of published data [9] .
Because the amount of iron incorporated is rather low, therefore changes in XRD patterns are not observed. Access inside the behavior of the iron incorporating AFI framework, using energy minimization technique [6] , shows that the substitution of Fe (III) for Al (III) in the AIPO-5 framework is energetically unfavorable, producing
Table 1. Catalysts raw materials.
Figure 1. XRD patterns of synthesized samples with iron content of 2.02 g.
Figure 2. XRD patterns of synthesized sample with iron content of 4.04 g.
strong distortion around the Fe atom. According to this study, Fe (III) in tetrahedral sites destabilizes, but does not disrupt the structure. Fe incorporation increases the T-O distance, resulting in strong distortion of the frame- work. This is due to the fact that Fe-O distance is approximately larger than the Al-O one, and also owing to differences in tetrahedral atoms (Al, Fe)-Oxygen distances, the cell parameters will vary.
3.2. Effect of Silicon Content in NbFAPSO-5 Molecular Sieve
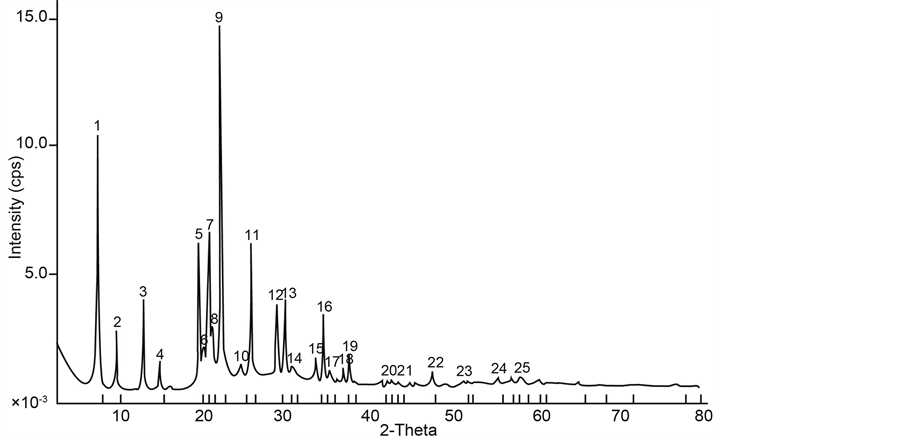
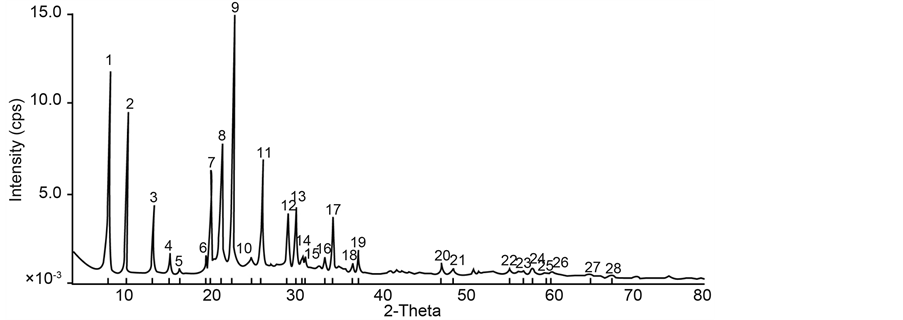
High silicon content in NbFAPSO-5 molecular sieve result in the disturbance of XRD patterns. In this study, 1 ml to 3 ml was successfully introduced into FAPO-5 molecular sieve without resulting in XRD patterns disturbance as shown in Figure 3 and Figure 4.
Adding silicon into FAPO-5 resulted in the increase of acidic properties of these materials, by the formation of SiO2-Al2O3 oxides, which also increase the stability of this molecular sieve. Elemental analysis using ICP- AES technique shows that silicon content up to 12.9% was successfully incorporated into our prepared samples, maintaining AFI type structure. A fundamental question that arises regarding the substitution of silicon into the AIPO-5 structural framework is the location of this substituted ion. Silicon theoretically can substitute for aluminum or phosphorous, or both. If the silicon substitutes for aluminum, the charged on the framework will be positive, giving rise to anion exchange properties; substitution for phosphorous will result in anionic framework
Figure 3. XRD patterns of silicon containing NbFAPSO-5 molecular sieve with silicon content of 1 ml.
Figure 4. XRD patterns of silicon containing NbFAPSO-5 molecular sieve with silicon content of 3 ml.
similar to the zeolite molecular sieve; no net change in the framework will be observed if both aluminum and phosphorous are simultaneously substituted with two silicon atoms. The ability to exchange cations as well as observed acid activity in SAPO-5 materials indicates that the silicon does, indeed, substitute for phosphorous. SAPO-5 (AFI) has mole fraction for SixAIyPz with x + y greater than z, which is evidence for substitution of two silicon atoms for (AI + P) in addition to substitution of silicon for phosphorous.
3.3. Effect of Nb Content in NbFAPSO-5 Molecular Sieve
Two sources of niobium were tested, niobium pentaoxide and niobium hydroxide. The first resulted in low solubility; niobium hydroxide complexed easily in oxalic acid as described in experimental section. The XRD patterns of NbFAPO-5 are shown in Figure 5 and that of NbFAPSO-5 in Figure 6. Compositional analysis of the synthesized samples NbFAPO-5 and NbFAPSO-5 showed that 39% of niobium was confirmed to be incorporated while maintaining AIPO-5 type structure.
3.4. Effects of Crystallization Temperature, Time and Template in NbFAPO-5 and NbFAPSO-5
The synthesis of NbFAPO-5 and NbFAPSO-5 exhibits many similarities to that of aluminosilicate molecular sieves. All are synthesized from reactive gels under the pH of 4 to 7, with the presence of organic additives helping
Figure 5. XRD patterns of niobium containing NbFAPO-5.
Figure 6. XRD patterns of niobium containing NbFAPSO-5.
to promote crystallization of a specific phase.
Temperature plays an important role in the crystallization of these synthesized samples. At lower temperature (100˚C - 125˚C) AFI type structure was not obtained; this structure occurred only for temperature ranging from 150˚C to 200˚C. At higher crystallization temperature (200˚C), other non-zeolitic phases are observed to crystalllize with increased crystallization time. The optimum time needed to promote crystallization of these phases appears to be dependent on both the temperature and the nature of the organic species used in the reaction mixture.
The organic additives appear to promote crystallization of a specific aluminophosphates structure, although it is unclear if it is in the same way that the organic additive promotes crystallization of a specific structure in the synthesis of the aluminosilicates. Many organic amines have been claimed to promote the crystallization of the AIPO4-5 structure; but there appears to be little correlation among them. A wider range of neutral organic amines have been found to aid in crystallization of aluminophosphates structures, compared to the aluminosilicate system; however, this may be a result of the initially acidic environment of the aluminophosphates gel, which would encourage the formation of a protonated amine, thus generating the cationic form in situ. The preparation of several structures from a given organic amine is a result of changing other synthesis conditions.
3.5. Acidic Properties
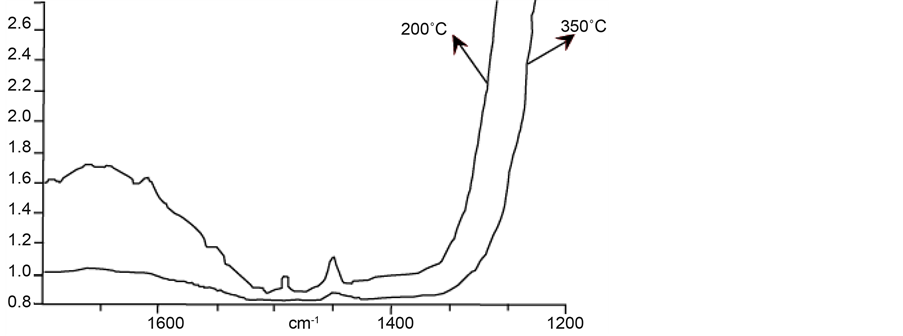
Infrared spectra of different synthesized samples show three distinct regions. Peaks at 1450 and 1540 cm−1 are assigned to Lewis and Brönsted acid sites respectively. According to Fateley [10] , peak at 1500 cm−1 is assigned to the C-H bending vibrations of triethylamine template incorporated in AFI channels.
The infrared spectrum of FAPO-5 molecular sieve is shown in Figure 7.
From Figure 7, we can see that there is an expressed peak at ca. 1450 cm−1 after evacuation at 200˚C and no peak at ca. 1450 cm−1. Evacuation at 350˚C shows a light peak at ca. 1450 cm−1 which mean that in this molecular sieve, exist only strong and weak Lewis acidity. FAPO-5 molecular sieve has small number of the aluminum atoms substituted with iron while retaining the charge neutrality of the framework; therefore, no Brönsted sites are present.
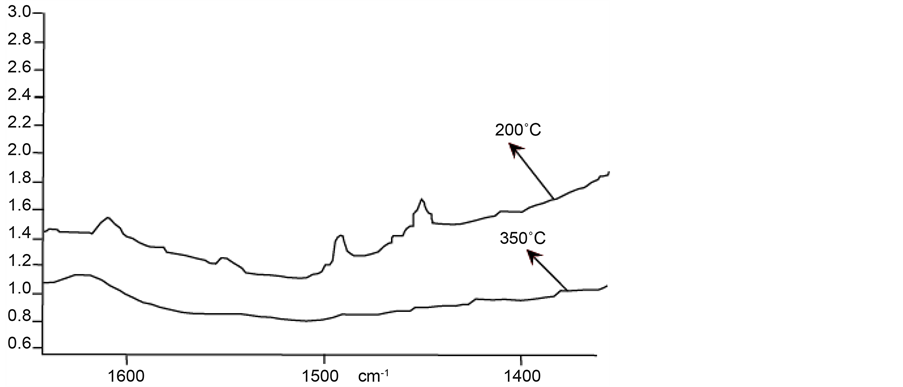
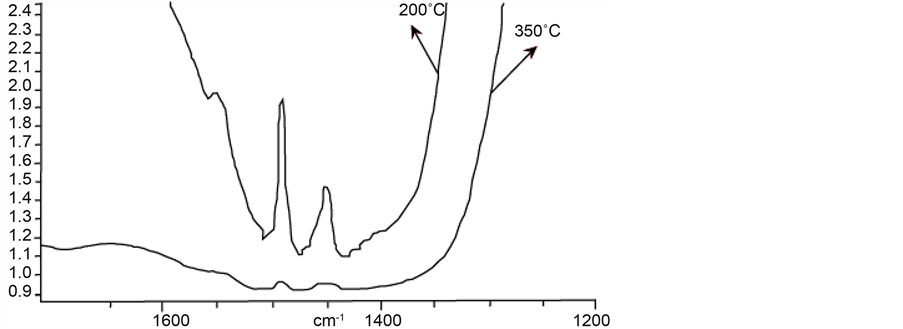
After incorporation of Nb into FAPO-5 molecular sieve, NbFAPO-5 sample is obtained. The resulting infra- red spectrum of pyridine chemisorption is shown in Figure 8.
From Figure 8, we can see that at 200˚C, two peaks are observed; one at 1450 cm−1 and another with low intensity at 1450 cm−1. Treatment at 350˚C does not show peak, both at 1450 and 1540 cm−1, meaning that there exist only weak Brönsted and Lewis acidity in this molecular sieve.
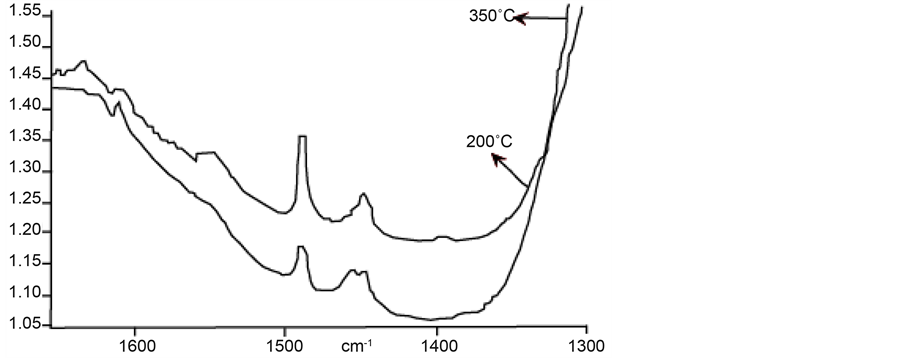
Infrared spectra of FAPSO-5 are shown in Figure 9. Treatment at 200˚C shows an expressed peak at 1450 and light intensity peak at 1450 cm−1. Treatment at 350˚C does not show peak at 1450 cm−1 meaning that in this molecular sieve both strong and weak Lewis acid sites exist with strong Brönsted acid sites.
Incorporation of Nb into FAPSO-5, leading to NbFAPSO-5 molecular sieve, infrared spectrum is shown in Figure 10.
Figure 7. Infrared spectrum of FAPO-5.
Figure 8. Infrared spectrum of NbFAPO-5.
Figure 9. Infrared spectrum of FAPSO-5.
Figure 10. Infrared spectrum of NbFAPSO-5.
Adsorption of probe molecule behaves in the same manner as in FAPSO-5. Brönsted and Lewis acidity of different synthesized samples are shown in Table 2.
From Table 2, we can see that incorporating Nb in FAPO-5 catalysts increases both Lewis and Brönsted acidity,
Table 2. Lewis and Brönsted acid in FAPO-5, NbFAPO-5, FAPSO-5 and NbFAPSO-5.
TL = total Lewis acid, TB = total Brönsted acid, SL = strong Lewis acid, SB = strong Brönsted acid, WL = weak Lewis acid, WB = weak Brönsted acid.
meaning furthermore his presence in FAPO-5 framework. Incorporation in FAPSO-5 leads to a decrease in acidity, where Brönsted and Lewis acid are nearly at equilibrium. This behavior is of great importance for this molecular sieve which should promote both oxidation and esterification reactions. The presence of only weak Brönsted acidity can be attributed to structural defects leading to terminal P-OH and Al-OH. The occurrence of weak and strong Lewis sites on this solid supports the idea of structural breakage after calcinations to yield defective Al sites. The incorporation of silicon in FAPO-5 catalyst generates a relatively high proportion of Brönsted acid sites.
4. Conclusion
Using triethylamine as template, boemite as aluminium source, phosphoric acid as phosphorous source, ferricni- trate as iron source, orthosilicate as silicate source, niobium hydroxide as niobium source, it was possible to hydrothermally synthesize AlPO-5, FAPO-5, NbFAPO-5 and NbFAPSO-5 molecular sieve with AFI type structure. Both Lewis and Brönsted acidity were found in the synthesized samples. Incorporation of niobium into FAPSO-5 framework should be done carefully because high content will lower the acidity, and also diminish the molecular sieve crystallinity.
Acknowledgements
Special thanks are due to Mr. Toumbe Mama and Dr. Mbowou Isaack for their technical assistance to accomplish this work.
Cite this paper
Mominou Nchare,Lei Wang,Salomon Anagho, (2016) Synthesis of NbFAPO-5 and NbFAPSO-5 Molecular Sieve by Hydrothermal Method and Comparison of Their XRD Patterns and Their Acidic Properties Evaluation by Infrared Spectroscopy. Open Journal of Inorganic Chemistry,06,155-162. doi: 10.4236/ojic.2016.63011
References
- 1. Fleischmann, C., Lievenbrück, M. and Ritter, H. (2015) Polymers and Dyes: Developments and Applications. Polymers, 7, 717-746.
http://dx.doi.org/10.3390/polym7040717 - 2. Hartman, M. and Kevan, L. (2002) Substitution of Transition Metal Ions into Aluminophosphates and Silicoaluminophosphates: Characterization and Relation to Catalysis. Research on Chemical Intermediates, 28, 625-695.
http://dx.doi.org/10.1163/15685670260469357 - 3. Tuel, A. (1995) Fe3+ and Ti4+ Incorporating Zeolite Framework. Zeolites, 15, 228-235.
http://dx.doi.org/10.1163/15685670260469357 - 4. Flanigen. (1986) New Development in Zeolites. Studies in Surface Science and Catalysis, 28, 103.
- 5. Sheldon, R.A. (1997) Innovation in Zeolites Materials Sciences. Catalysis, 68, 381-388.
- 6. Ziolek, M. (2003) Niobium-Containg Catalysts—The State of the Art. Catalysis Today, 78, 47-64.
http://dx.doi.org/10.1016/S0920-5861(02)00340-1 - 7. Gonzales, J.G. and Delacruz, J. (1999) Computational Study of Substitution of Al by Fe3+ in the AlPO-5 Framework. Microporous and Mesoporous Materials, 29, 361-365.
http://dx.doi.org/10.1016/S1387-1811(99)00005-0 - 8. Wilson, S.T., Flanigen, E.M. and Pfaff, C. (1982) Crystalline Metallophosphate Composition. USP 4310440.
- 9. Qiu, S., Pang, W., Kessler, H. and Guth, J.L. (1989) Collection of Simulated XRD Powders Patterns for Zeolites. Zeolites, 9, 440-444.
- 10. Perez O., J.O., Borade, R.B. and Clearfield, A. (1998) Synthesis of a Mesoporous Aluminophosphate. Journal of Molecular Structure, 470, 221-228.
http://dx.doi.org/10.1016/S0022-2860(98)00484-0
NOTES
*Corresponding author.