Open Journal of Inorganic Chemistry
Vol. 2 No. 4 (2012) , Article ID: 23411 , 6 pages DOI:10.4236/ojic.2012.24011
Preparation of N,N,N’,N’-tetrakis-(2-benzimidazolylmethyl)-1,2-ethanediamine and crystal assemblies of the relative complexes
![]()
1College of Light Industry & Chemical Engineering, Guangdong University of Technology, Guangzhou, China
2Logistics Group, Huainan Normal College, Huainan, China
Email: *yanyan600716@hotmail.com
Received 3 July 2012; revised 14 August 2012; accepted 3 September 2012
Keywords: Benzoimidazolmethyl Ethane Diamine; Solvothermal; Complex; Crystal Architecture
ABSTRACT
N,N,N’,N’-tetrakis-(2-benzimidazolylmethyl)-1,2-ethanediamine (TBIMEDA), was prepared by reaction of ethylenediamine tetra-acetic acid disodium salt (EDTA) with 1,2-diaminobenzene in a refluxed glycol solution, and furthermore, three allomeric complexes[(MII TBIMEDA) SO4·5H2O, M = Cd, Co, Ni] were selfassembled by solvothermal method based on reaction of this ligand with the relative sulfates respectively. These allomeric complexes were characterized by elemental analysis and IR spectroscopy and their crystal structures were determined by single crystal X-ray structural analysis. In the crystal architecture of these complexes, every metal(II) ion is chelated by one neutral TBIMEDA ligand to form an octahedral core with configuration of five heterocyclic rings (fivemember ring). These cores then were linked together by multi hydrogen bond interactions with sulfate ions and water molecules to construct their 3D crystal architectures.
1. INTRODUCTION
Azaheterocyclic compounds, with strong coordination ability during complex preparations and as better acceptors in hydrogen bonding formation, were more frequently selected in coordination research [1-4]. After selfassemblies, the conjugated p bonds in the heterocyclic ligands provided abounding information on electromagnetism and photoelectrochemistry, and these special complex’s aggregation was well known as functionalized material in future applications [5-9]. Meanwhile EDTA (ethylenediamine tetra-acetic acid) was regard as the most useful multidentate ligand using in analytic chemistry. In this work, based on reaction of EDTA with benzene-1,2-diamine, a multidentate ligand with four benzoimidazole groups, N,N,N’,N’-tetrakis-(2-benzim-idazolyl methyl)-1,2-ethanediamine(TBIMEDA), was prepared according to the reported method (Scheme 1) [10-13]. Furthermore, by self-assembly of TBIMEDA reacting with different sulfates, three single-nuclear complexes with same crystal configuration were constructed by sovothermal methods, and their crystal structures were well defined by X-ray analysis.
2. EXPERIMENTAL
2.1. Materials and Physical Measurements
All chemicals and solvents were of analytical reagent grade and used as received. Elemental analysis was performed in a Perkin-Elmer 240 elemental analyzer. IR spectrum was obtained using a Nicolet IR200 infrared spectrometer. The fluorescence spectra were taken on an Edinburgh Instruments FLS920 fluorescence spectrumeter respecttively.
2.2. Synthesis of Ligand
Preparation of the ligand, N,N,N’,N’-tetrakis-(2-benzimidazolylmethyl)-1,2-ethanediamine (TBIMEDA) was described as below [10-13]: A solution of EDTA (2.92 g, 0.01 mol) and 1, 2-diaminobenzene (4.32 g, 0.04 mol) in 100 mL of glycol was heated to boiling and kept in reflux for 16h. After cooling down to room temperature, the mixture was added to water (ac. 400 mL) for precipitation over night. The crude product was separated by filtration, purified by recrystallization with small volume of ethanol for three times and dried in air. A white powder product in yield of 70% was obtained with m. p. = 156˚C - 158˚C. IR data (KBr, cm−1): 3186 vs (nN-H), 2966 w, 2823 w, 1617 s, 1535 s, 1486 w(sN-H), 1454 s, 1433 vs, 1348 s, 1311 m, 1274 vs, 1245 m, 1217 w, 1094 s, 1049 m, 1021 m, 996 m, 963 w, 845 m, 747 vs, 616 w, 485 m. Calc. (found) for C34H32N10:C 72.88 (72.90), H 3.84 (3.82), N 7.08 (7.12). This IR data were similar to the reported values. [10] The preparation of TBIMEDA was described as Scheme 1.
2.3. Synthesis of Complexes
2.3.1. Co(TBIMEDA)·SO4·5H2O(1)
Keeping under stirring, TBIMEDA (0.0706 g, 0.1 mmol), CoSO4·7H2O (0.0422 g, 0.15 mmol) are mixed with water (8 mL). The mixture was sealed in an autoclave and the autoclave was placed in an oven at 120˚C for 60 h. After cooling down to room temperature at rate of 5˚C/h, filtrating and washing, several large and transparent orange crystals were collected (in yield of 34%). Found (Calc.) for CoC34H42N10O9S: C, 49.44(49.45); H, 5.11(5.13); N, 16.98(16.96)%. IR data(KBr, cm−1): 3415 s, 3101 w, 3056 w, 2917 w, 2774 w, 2643 w, 1621 m, 1540 m, 1470 m, 1450 vs,1392 m, 1274 vs, 1119 vs, 1029 m, 939 m, 910 w, 910 w, 857 w, 743 vs, 620 vs, 555 w, 514 w.
2.3.2. Ni(TBIMEDA)·SO4·5H2O(2)
Keeping under stirring, TBIMEDA (0.0706 g, 0.1 mmol), NiSO4·6H2O (0.0262 g, 0.1 mmol) were mixed with water (8 mL). The mixture was sealed in an autoclave and the autoclave was placed in an oven at 140˚C for 60 h. After cooling down to room temperature at rate of 5˚C/h, filtrating and washing, several large and transparent blue crystals were collected (in yield of 45%). Found (Calc.) for NiC34H42N10O9S:C, 46.48(46.47); H, 5.13(5.11); N, 16.97(16.99) %. IR data (KBr, cm−1): 3415 s, 3105 m, 3064 w, 2913 w, 2765 w, 2639 w, 1544 m, 1405 vs, 1392 s, 1331 vs, 1278 vs, 1221 w, 1115 vs, 1029 s, 988 w, 939 s, 906 m, 849 m, 743 vs, 616 vs, 545 w, 518 w.
2.3.3. Cd(TBIMEDA)·SO4·5H2O(3)
Similar to the assembly of 1, when NiSO4·6H2O was replaced by CdSO4·8H2O (0.0385 g,0.05 mmol), large and transparent blue crystals were obtained in yield of 45% by the same hydrothermal method. Found(Calc.) for CdC34H42N10O9S:C, 49.44(49.45); H, 5.07(5.09); N, 15.95(15.93)%. IR data (KBr, cm−1): 3395 s, 3096 m, 2761 w, 1621 m, 1540 m, 1446 vs, 1384 s, 1335 m, 1278 s, 1094 vs, 1025 s, 755 s, 620 m.
2.4. Single Crystal X-Ray Diffraction Analysis
The X-ray data collections and structure determinations were performed on a Bruker SMART CCD. The data were collected using graphite-monochromatic Mo-Ka radiation (l = 0.71073 Å). The crystal structure was solved by direct methods and refined by full-matrix least-square calculation on F2 with SHELX-97 program package. [14]. All non-hydrogen atoms were treated anisotropically. Hydrogen atoms were placed in calculated positions. The crystallographic data for 1 - 3 are summarized in Tables 1 and 2.
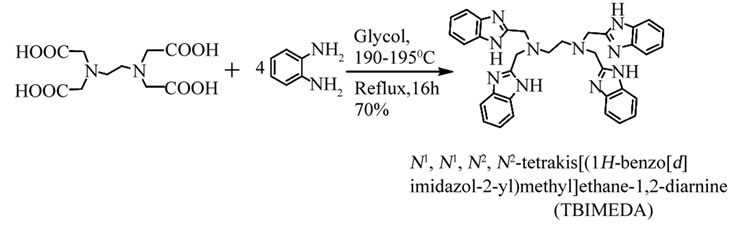
Scheme 1. Preparation of N,N,N,N-tetrakis-(2-benzimidazolylmethyl)-1,2-ethanediamine( TBIMEDA).
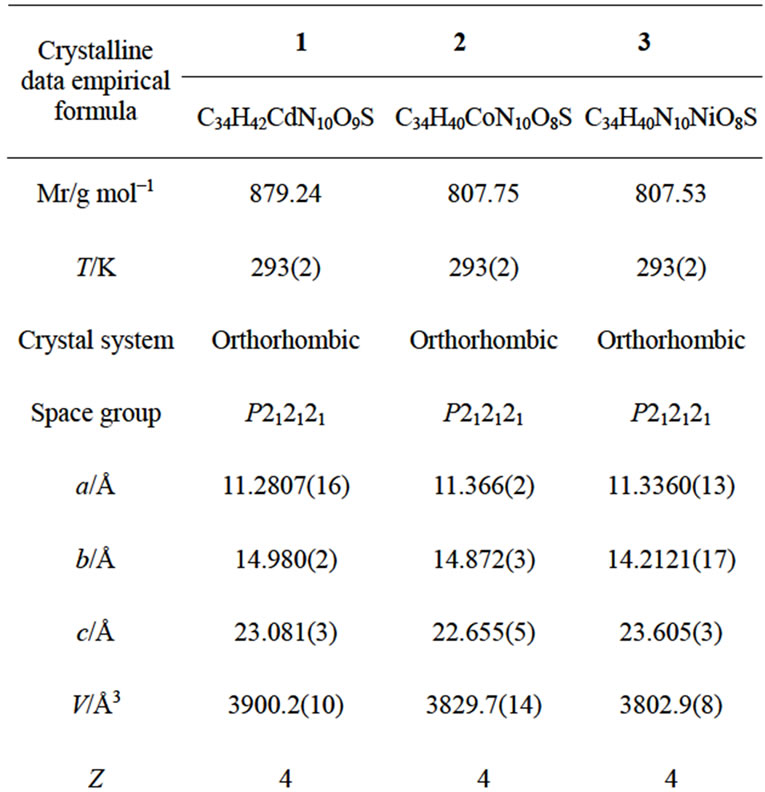
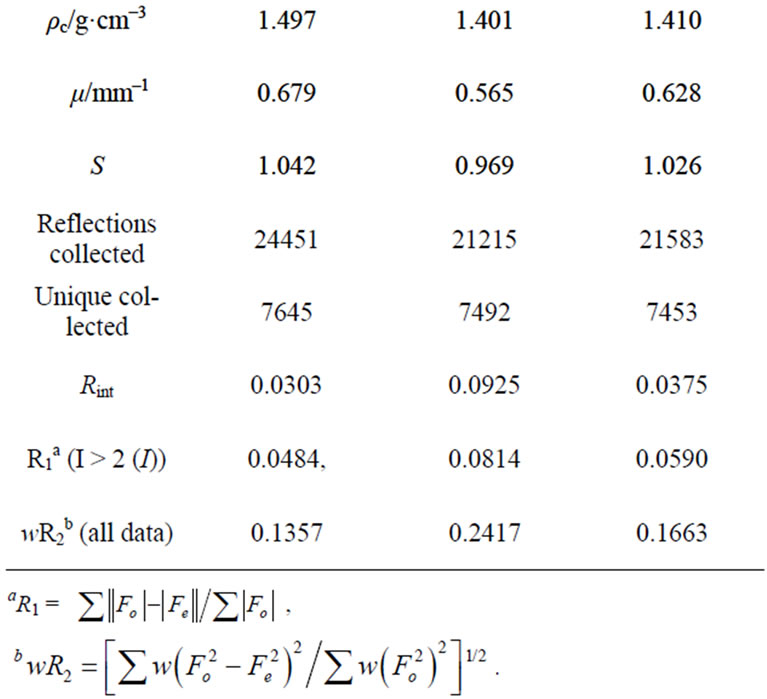
Table 1. Crystallographic data for 1 - 3.
.
3. RESULTS AND DISCUSSION
3.1. Crystal Analyses for 1 - 3
Complexes 1 - 3 are alloisomers with each other, 1 is therefore selected as a typical example for discussing their crystal configurations. The view of the single core of 1 is depicted in Figure 1. Some selected bond distances and angles are listed in Table 1, and the hydrogen bonding data are listed in Table 2. 1 adopted orthorhombic system with P212121 space group (unit cell parameters: a = 11.2807(16) Å, b = 14.980(2) Å, c = 23.081(3) Å, β = 90.00˚, V = 3900.2(10) Å3, Z = 4, Dc = 1.497 mg/cm3). Cadmium(II) ion in the core of complex 1 is coordinated by one neutral TBIMEDA ligand, forming five of fivemember rings (Figure 1). The octahedral coordination core around cadmium(II) ion includes six N atoms, two of them originate from the chain of ethanediamine and the others originate from the unsaturated N atoms in imidazole rings. All distances of Cd-N described above are near to or shorter than the sum of Van der Waals radii for Cd and N, and the effective chelations result in distorted octahedron around Cd(II) ions. However, the saturated N atoms in imidazoles, include N3, N5, N11 and N12, are protonated and without any coordination contribution.
3.2. Hydrogen Bond Interactions in 1
In the crystal structure of complex 1, the Cd(II) coordination cores are linked through intermolecular N-H···O and O-H···O hydrogen bonds with sulfate ions and water molecules (Figure 2), forming a complicate 3D architecture. If all water molecules are omitted, the Cd(II)
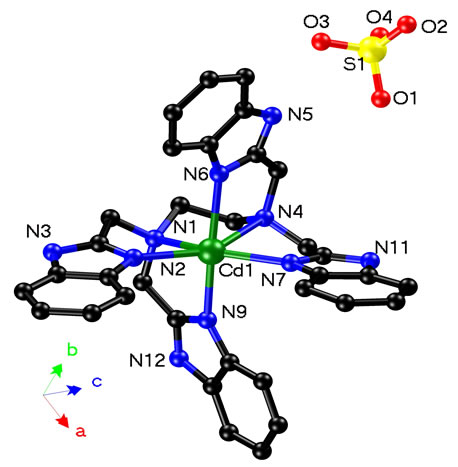
Figure 1. Diagram of single core in crystal architecture of 1. All H atoms and 5 water molecules (O5-O9) are omitted for clarity. Just like the chelation of EDTA with metal ion, CdII in 1 adopting an octahedral configuration is chelated by five of 5-membered rings, where the two positive charges are counteracted by the sulfate anion.
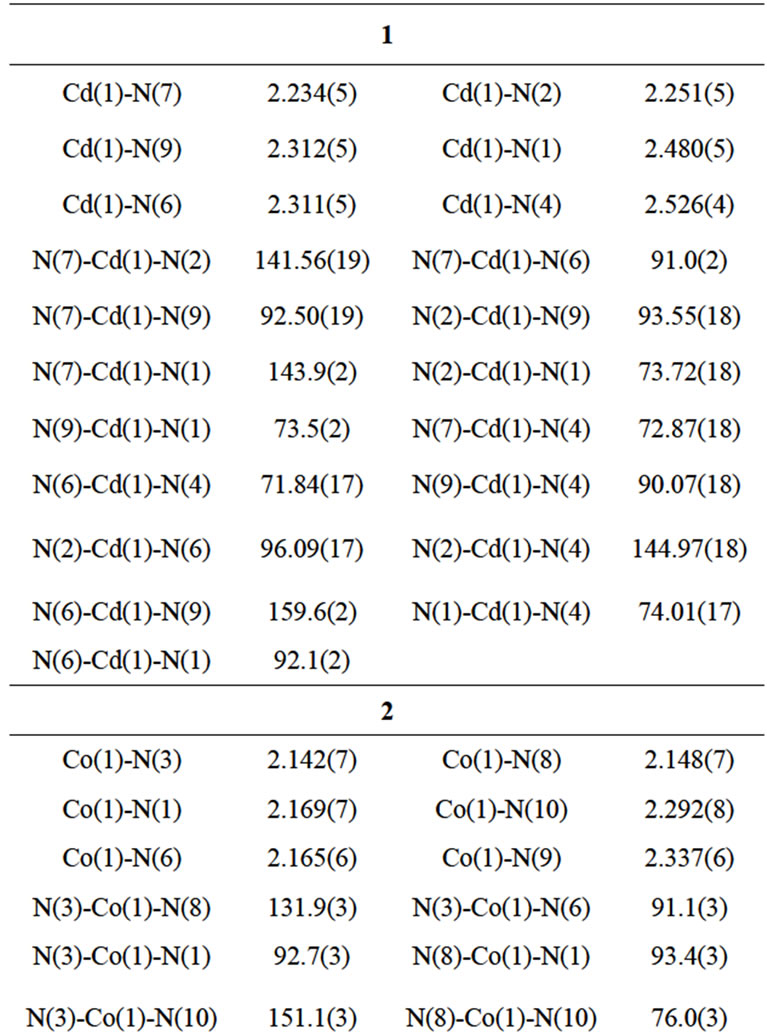
Table 2. Select bond lengths (Å) and angles (˚) for 1 - 3.
coordination water molecules are omitted, the Cd(II) coordination cores and sulfate ions will be linked by hydrogen bonding interactions to form a metal organic framework (MOF, as describing in Figure 3), and this MOF can be simplified as a topologic diagram with characters of 3 × 72 around Cd cores and 32 × 75 × 83 around sulfate cores (Figure 4). Meanwhile, if all Cd cores were ignored, the sulfate ions and water molecules will be linked together to construct a 2D chain running along the a axis (Figure 5).
3.3. Photoluminescence
Excitation under lEx = 341 nm, ligand TBIMEDA gave a emission at lEm = 375 nm, which was contributed from its conjugated configuration with π-π* electron transition (Figure 6). After crystal assembly using the ligand with the metal ions with d10 configuration, such as Cd(II) in 1, its crystal state also presented a strong emission peaks at lEm =417 nm (lEx = 339 nm). Obviously, compared with
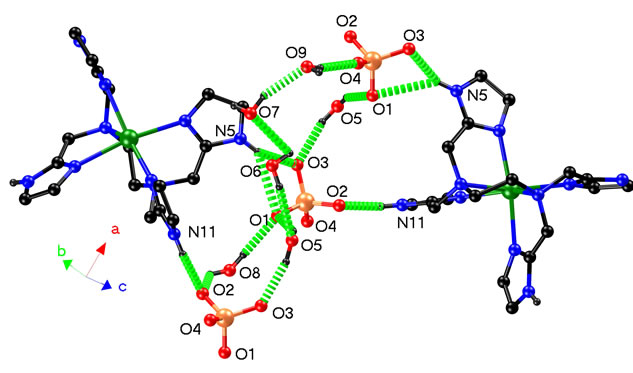
Figure 2. Hydrogen bonding interactions in 1’s crystal architecture.
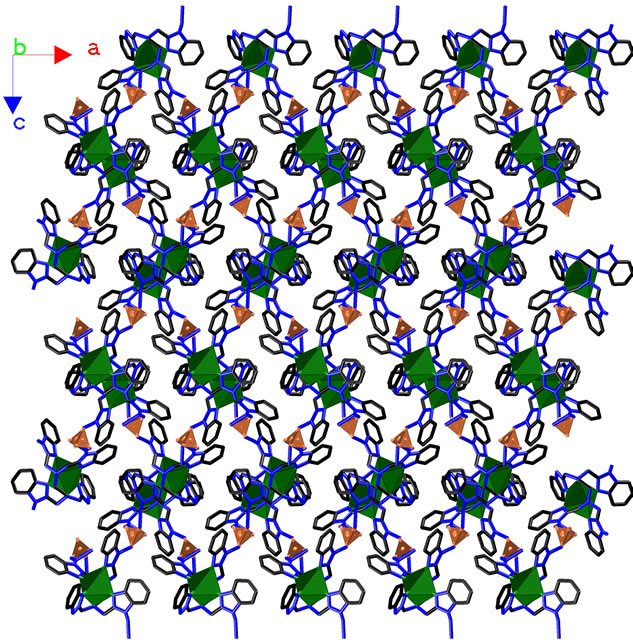
Figure 3. Crystal architecture of 1 constructed by hydrogen bonding interactions. CdII cores and 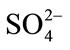 ions are linked together to form a network stretched in the ac plane. All H2O molecules and H atoms without hydrogen bonding contributions are omitted for clarity.
ions are linked together to form a network stretched in the ac plane. All H2O molecules and H atoms without hydrogen bonding contributions are omitted for clarity.
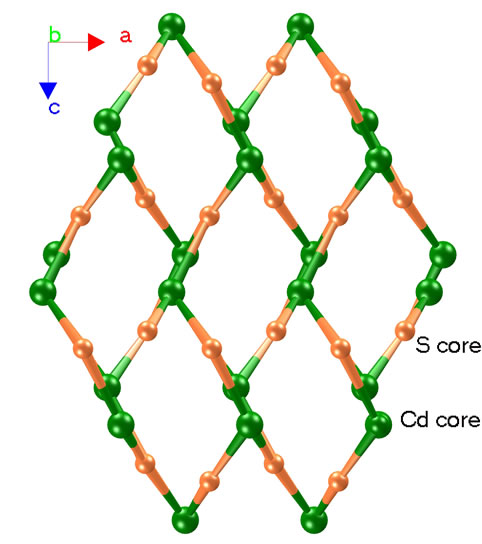
Figure 4. Simplified graph of for complex 1 with topologic characters of 3 × 7 × 8 around Cd(II) cores and 32 × 74 × 84 around sulfate anions.
Figure 5. Simplified graph of for 1 only with water molecules and sulfate anions.
the ligand’s emission, the fluorescent peaks of 1 emerged with red shift. This photoluminescence mechanism originated from ligand-metal charge transition (LMCT) [15], and its emission peak position was decided by the coordination situation of ligand with metal ion [16].
4. CONCLUSION
Ligands of diamine with imidazole group exhibited potentially application in biodegradation. In this work, after preparation of N,N,N’,N’-tetrakis-(2-benzimidazolylmethyl)-1,2-ethanediamine(TBIMEDA), and using it as ligand reacting with different salts, three crystal architectures were self-assembled under solvothermal conditions. In crystal assembly, beside coordination between metal and ligands, the crystal architectures were also sustained by multi hydrogen bonding interactions from the complex cores with sulfate anions and water molecules. Three crystal architectures of M(TBIMEDA) SO4·5H2O(M = CdII, CoII, NiII) all adopted orthorhombic crystalline with P212121 space group. The metal center was chelated by three TBIMEDA to construct an octahedral configuration with five 5-numbered chelating rings, where the coordinating atoms were unsaturated N in imidazole ring and saturated N from ethylenediamine chain. These complexes can be used as a model to study
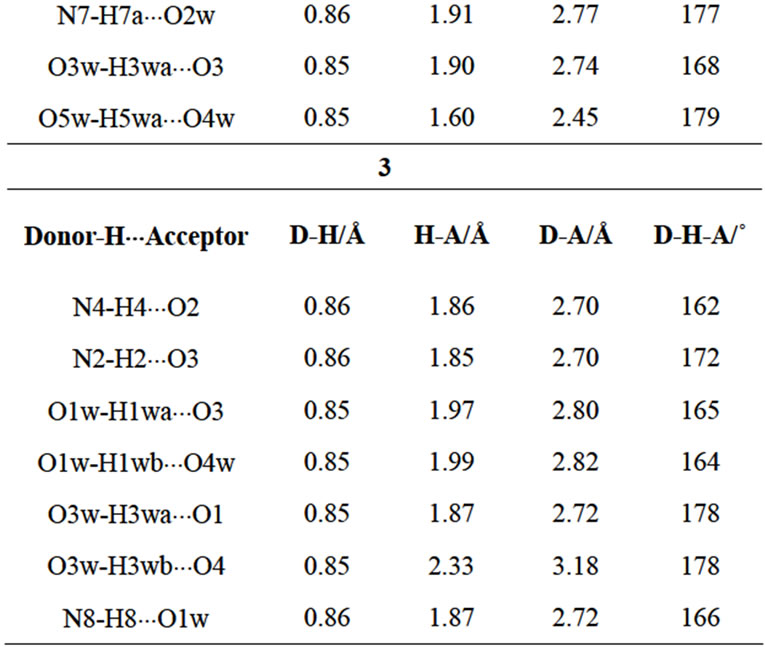
Table 3. Typical hydrogen bonding interactions in 1 - 3.

Figure 6. Emission spectra of ligand TBIMEDA (lexa = 341 nm) and 1 (lexa = 339 nm).
the effect of stereochemistry on the coordination polyhedron of M(II) ions. Although these M(II) ions were coordinated by six atoms, their coordination bonds were different in length, and therefore they may use different hybrid obits to form inner orbital or outer orbital coordination compounds. Obviously, these conclusions should be supported by magnetic determination and thermal gravimetric analysis. Further study should be intensively investigated.
5. ACKNOWLEDGEMENTS
This work was financially supported by the National Natural Science Foundation of China (No. 20771073); by the “211” Project of Guangdong Province (the third time): The Key and Common Technologies in Fine Chemical Engineering; and by The Key Project of Education Office from Guangdong Province, China.
REFERENCES
- Huang, X.-Ch., Zhang, J.-P. and Chen, X.-M. (2004) A new route to supramolecular isomers via molecular templating: Nanosized molecular polygons of copper(I) 2- methylimidazolates. Journal of the American Chemical Society, 126, 13218-13219. doi:10.1021/ja045249l
- Moon, D., Kang, S., Park, J., Lee, K., John, R. P., Won, H., Seong, G.H., Kim, Y.S., Kim, G.H., Rhee, H. and Lah, M.S. (2006) Face-driven corner-linked octahedral nanocages: M6L8 cages formed by C3-symmetric triangular facial ligands linked via C4-symmetric square tetratopic PdII ions at truncated octahedron corners. Journal of the Ame rican Chemical Society, 128, 3530-3531. doi:10.1021/ja060051h
- Luo, F., Zheng, J.-M. and Batten, S.R. (2007) Unprecedented (3, 4)-connected metal-organic frameworks(MOFs) with 3-fold interpenetration and considerable solvent-accessible void space. Chemical Communications, 36, 3744-3746. doi:10.1039/B706177C
- Britt, D., Tranchemontagne, D. and Yaghi, O.M. (2008) Metal-organic frameworks with high capacity and selectivity for harmful gases. PNAS, 105, 11623-11627. doi: 10.1073/pnas.0804900105
- Wu, A.J., Hahn, J.E.P. and Pecoraro, V.L. (2005) Structural, spectroscopic, and reactivity models for the manganese catalase. Chemical Reviews, 104, 903-938. doi: 10.1021/cr020627v
- Maji, T.K., Ohba, M. and Kitagawa, S. (2005) Transformation from a 2D stacked layer to 3D interpenetrated framework by changing the spacer functionality: Synthesis, structure, adsorption, and magnetic properties. Inorganic Chemistry, 44, 9225-9231. doi: 10.1021/ic050835g
- Lopez, X., Benard, M. and Rohmer, M.M. (2007) Influence of electron-attractor substituents on the magnetic properties of Ni(II) string complexes. Inorganic Chemistry, 46, 5-7. doi: 10.1021/ic061705q
- Belloni, M., Kariuki, B.M., Manickam, M., Wilkie, J. and Preece, J. A. (2005) Design of potentially photorefractive liquid crystalline materials: Derivatives of 3,6-disubstituted carbazole. Crystal Growth & Design, 5, 1443-1450. doi: 10.1021/cg049580s
- Kjlo, J. and Okamoto, Y. (2002) Organo lanthanide complexes for electroluminescent materials. Chemical Reviews, 102, 2357-2368. doi: 10.1021/cr010448y
- Hendriks, H.M.J., Bokkel, H.W.O. and Reedijk, J. (1979) Synthesis, characterisation and complex formation of N,N,N’,N’-tetrakis-(2-benzimidazolylmethyl)-1,2-ethanediamine, a new hexadentate ligand. Recueil des Travaux Chimiques des Pays-Bas, 98, 499-500.
- Vishweshwar, P., Nangia, A. and Lynch, V.M. (2002) Recurrence of carboxylic acid-pyridine supramolecular synthon in the crystal structures of some pyrazinecarboxylic acids. The Journal of Organic Chemistry, 67, 556-565. doi: 10.1021/jo0162484
- Addison, A.W., Hendriks, H.M.J., Reedijk, J. and Thompson, L.K. (1981) Copper complexes of the “tripod” ligand tris(2-benzimidazolylmethyl)amine: fiveand sixcoordinate copper(II) derivatives and some copper(I) derivatives. Inorganic Chemistry, 20, 103-110. doi: 10.1021/ic50215a024
- Wahlgren, C.G. and Addison, A.W. (1989) Synthesis of some benzimidazole-, pyridineand imidazole-derived chelating agents. Journal of Heterocyclic Chemistry, 26, 541-543. doi:10.1002/jhet.5570260303
- Sheldrick G.M. (1997) SHELXL-97, program for the refinement of the crystal structures. University of Göttingen, Göttingen.
- Guo, C.Y., Wang, Y.Y., Xu, K.Z., Zhu, H.L., Liu, P., Shi, Q.Z. and Peng, S.M (2007) Crystal structures, bioactivities and fluorescent properties of four diverse complexes with a new symmetric benzimidazolic ligand. Polyhedron, 27, 3529-3536. doi:10.1016/j.poly.2008.08.018
- Wang, X.L., Bi, Y.F., Liu, G.C., Lin, H.Y., Hu, T.L. and Bu, X.H. (2008). Zn(II) coordination architectures with mixed ligands of dipyrido [3,2-d:2′,3′-f] quinoxaline/2,3-di-2-pyridylquinoxaline and benzenedicarboxylate: Syntheses, crystal structures, and photoluminescence properties. CrystEngCommunity, 10, 349-356. doi:10.1039/B706071H
NOTES
*Corresponding author.

