Open Journal of Physical Chemistry
Vol. 2 No. 3 (2012) , Article ID: 22095 , 6 pages DOI:10.4236/ojpc.2012.23018
Evaluation of TiO2, ZnO, CuO and Ga2O3 on the Photocatalytic Degradation of Phenol Using an Annular-Flow Photocatalytic Reactor
1Department of Chemical Systems Engineering, Laboratory of Catalytic Processes Development (LDPC), Faculty of Chemical Engeneering, UNICAMP-University of Campinas, Campinas, Brazil
2Department of Analytical Chemistry Institute, UNICAMP-University of Campinas, Campinas, Brazil
Email: flaviasertori@feq.unicamp.br
Received May 12, 2012; revised June 15, 2012; accepted July 16, 2012
Keywords: Phenol; TiO2; ZnO; CuO; Ga2O3; heterogeneous photocatalysis
ABSTRACT
Even with rigorous environmental regulations, phenol still is a major contaminant. One possible solution is the use of heterogeneous photocatalysis due to low chemical addition, feasibility and reliability to be implanted on cost-effective industrial process. TiO2 is the most employed photocatalyst because of its favorable (photo) chemical properties and ZnO is considered one of the best alternative for that. Other oxides were tested in lesser proportions, like CuO and Ga2O3. When the photocatalyst is dispersed as slurry, higher degradation rates are achieved due to high solid to liquid contact area when compared with supported form. The aim of this work was to develop a batch recirculating photocatalytic reactor and evaluate its efficiency when assisted by the photocatalysts TiO2 P25, ZnO, CuO and β-Ga2O3. TiO2 achieved 95% mineralization after 200 min reaction in an average degradation rate of 0.68 mg∙L−1∙min−1 and ZnO was less efficient (0.41 mg∙L−1∙min−1). Ga2O3 and CuO presented poor performance, mainly due to low surface area for the CuO syntesized and the absorption of the UV radiation by the reactor walls, decreasing Ga2O3 activity. Degradation intermediates were detected in diverse concentrations and at different operational times for each oxide tested, which indicate different degradation mechanisms.
1. Introduction
Despite the increase of rigor about environmental regulations, phenols persist as one of the major contaminants to aquatic life [1,2]. These compounds are highly carcinogenic and toxic to all forms of life and can be detected in higher concentrations on the industrial wastewater from petrochemical, oil-refineries, paper-making, coking and iron-smelting processes [3]. Due to its importance and recalcitrance to traditional degradation processes, it is a common model compound adopted in advanced water studies, mainly those envolving Advanced Oxidation Processes (AOPs).
The AOPs are process that generate high reactive radicals, mainly hydroxyl (•OH), which could mineralize organic pollutants completely if all chemical and engineerng conditions are well stabilished. Between them, ozonization, Fenton process and heterogeneous photocatalysis (HP) are the most promising technologies for final wastewater treatment stage, but HP is becoming the future of water treatment due to low chemical addition, easibility to be implanted on cost-effective industrial process [3,4]. This technique combines the use of a radiation with adequate energy to activate a semiconductor resulting on the generation of oxidative and reuductive sites on the photocatalyst surface.
TiO2 P25 (70% anatase, 30% rutile) is the most employed photocatalytic semiconductor because of its chemical inertness, photostability, low cost, and atoxicity [5]. Anatase form has been successfully used for photocatalytic treatment of contaminants due to its faster electron transfer when exposed to UV radiation. As an alternative to TiO2, ZnO has also been reported as an effective photocatalyst, due to its wide band-gap energy (3.37 eV) and large exciton binding energy (60 meV) [6]. A few studies have supported the assertion that ZnO is a better photocatalyst than TiO2, especially for chlorinated compounds using hydroxyl radicals [7]. ZnO was also reported as being more efficient than TiO2 in visible light photocatalytic degradation of some organic compounds in aqueous solution [5,8,9], but certainly it is not stable as titanium dioxide [4].
Other oxides were tested in lesser proportions, like CuO and Ga2O3. Copper oxide (II) has low cost and toxicity, in addition to high availability. This oxide is among the few with the band gap energy near to visible light, on the degradation of methylene blue [10]. Gallium oxides exist in a lot of polymorphs, and the thermodynamic stable form is β-Ga2O3. This material has much wider bandgap (4.8 eV) than TiO2 doing that photogenerated electrons in the conductive band have much higher reductive capability. β-Ga2O3 is also an environmental friendly material according to Worksafe Australia criteria and some authors reported that it appears to be selective on the photocatalytic degradation of aromatics compounds, like benzene [11-13].
The photocatalyst could be used in slurry or supported forms. When the photocatalyst is dispersed as slurry inside the reactor, higher degradation rates are achieved due to high solid to liquid contact area and high axial flow rates are necessary to prevent the catalyst from settling. If the conversion per pass is low, recycling of the process fluid becomes necessary [14]. Some inconvenients of slurries are that the powders are not easy to precipitate and recover from water, preventing their regeneration and reuse, but several engineering/chemical solutions are being investigated, from incorporating titania on the reactor walls and the use of slurry reactors, to immobilization techniques on different supports [15-18].
In this context, the aim of this work was to develop a batch recirculating photocatalytic reactor and evaluate its efficiency when assisted by the photocatalysts TiO2 P25, ZnO, CuO and β-Ga2O3.
2. Experimental
2.1. Photocatalytic Reactor Assembly
All the experiment were carried out in a batch recirculation mode. It was built a reactor with recirculation fluid composed by three main elements: 1) Photoreactor: consisting of PyrexTM cylindrical glass with hollow center formed by three concentric 3.5 mm layers isolating two chambers, the first with a 290 mL volume for the test solution flow and the second for a cold water stream to exchange heat between fluids and maintaining the system temperature at 30˚C. In the reactor central annulus it was coupled a mercury vapor lamp (250 W, Osram) without bulbe involucres. The reactor was covered with aluminum to prevent radiation loss, and thus allowing a greater use of the emitted photons. The diagram of photoreactor with some measures is showed on Figure 1; 2) Recirculation pump (60 Hz) to promote the test solution flow
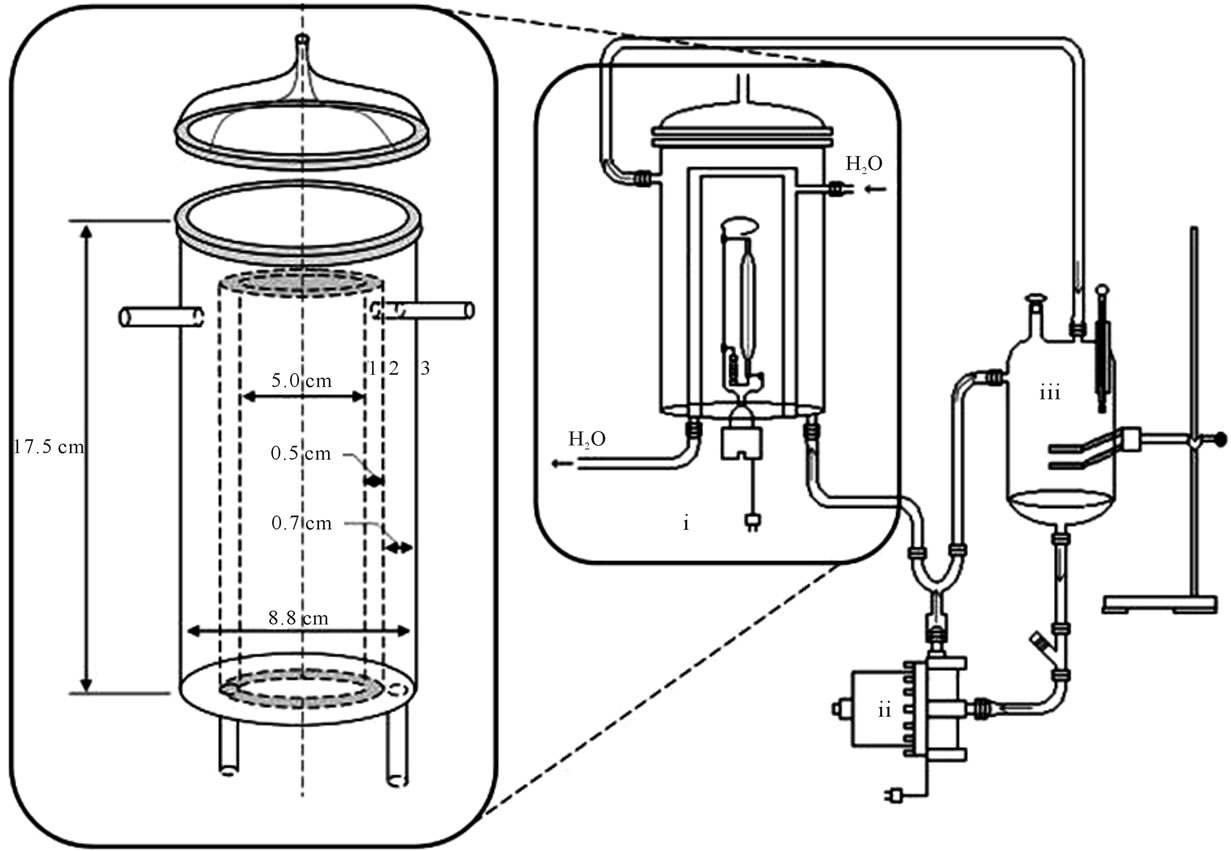
Figure 1. Scheme of the photocatalytic reactor assembled for the trials: (i) photocatalytic reactor and its detailed chambers; (ii) recirculation pump; (iii) recycling tank.
throughout the system, and to provide an adequate turbulence to keep catalyst in suspension; 3) Recycling tank: a 550 mL cylindrical glass container where the test solution passed through during the recirculation process in order to create enlightened and non-illuminated regimes during the process.
2.2. Photocatalytic Trials
Before each experiment, a catalyst (TiO2 P25, ZnO, CuO or β-Ga2O3) amount of 0.3 g was added to distilled water and the resulting slurry was sonicated for 10 min to ensure an uniform catalyst dispersion and the recirculation flow was setted to 80 L∙h−1, verified (checked) with a online rotameter. 1.2 mL of a phenol stok solution (50 g∙L−1) was added to this slurry and mixed well to get the initial phenol concentration (100 mg∙L−1). P25 and ZnO were purchased from Degussa and Sigma-Aldrich, respectively. CuO and Ga2O3 samples were synthesized in accord [17,18], respectively. The photocatalytic reaction was initiated once the Hg-lamp was turned on. Under these conditions, illuminated working volume was 290 mL, and trials time was standardized at 135 min after phenol addition. Preliminary tests were performed in order to recycle, where the solution was continuously through the reactor, and samples of 3 mL were withdrawn periodically and immediately filtered through 0.45 µm syringe filter (PTFE) for further analysis.
During the experiments, three different processes were evaluated and two of them used as controls the photocatalytic experiment: 1) photolysis: test solution treatment without using catalyst, but with the light on; 2) photocatalysis: It involved the treatment of test solution with the use of a catalyst, and the light on; 3) adsorption: consisted of test solution with the same amount of catalyst of photocatalysis but with the lamp off.
2.3. Analytical Procedures
The concentration of phenol and the intermediates (hydroquinone, benzoquinone and catechol) formed during the processes were evaluated by high performance liquid chromatography (HPLC) on a Shimadzu (SCL 10AVP) with C18 column and photodiode array detector (DAD, SPD-M10VP). Methanol-water at gradient elution of 40% - 100% in 10 min was used as the solvent. The injection volume was 20 µL. Figure 2 shows a typical chromatogram for those analytes separation. Total organic carbon (TOC) also was measured before and after the trials using a Shimadzu TOC-V CPN Total Organic Carbon Analyser.
2.4. Radiation Source Characterization
UV intensity was measured using a radiometer Cole-
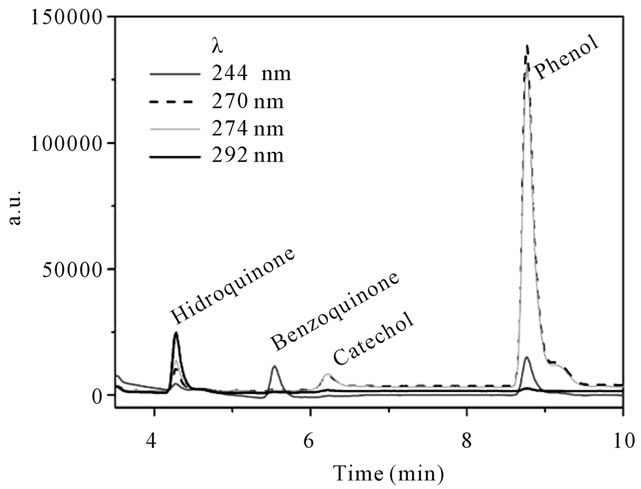
Figure 2. Typical HPLC chromatogram for separation of phenol and the degradation intermediates (hydroquinone, benzoquinone and catechol). HPLC configuration: Shimadzu (SCL 10AVP), C18 column; photodiode array detector (DAD, SPD-M10VP). Methanol-water at gradient ellution of 40% - 100% in 10 min; injection volume was 20 µL.
Parmer Instruments Company, at a wavelength of 254 nm (model 9811-56), 312 nm (model 9811-54) and 365 nm (model 9811-50). Measurements were performed 15 minutes after the lamp accionament and also for the radiation transmitted through the glassy walls (three 3.5 mm layers and 0.5 cm of flowing water). The integral radiant flux of the incident light was measured with a power-meter (Newport 1830-C USA).
3. Results and Discussion
The reactor was operated in batch mode to prevent catalyst settling and simultaneously provide atmospheric oxygen absorption to guarantee the minimal leves necessary to generate hydroxyl radicals [14]. Despite the pH influence, no pH alterations were made to simulate realistic interactions between the produced species and the photocatalysts [1].
Control experiments using the photocatalysts without radiation exposition (dark) showed adsorption values varying from 0.8% to 3.2% (not showed here).
Despite the use of lamp with an irradiation spectrum varying from UV to visible regions, it is observed on Figure 3 that only the photocatalysts which are activated mainly by UV-A radiations (TiO2 and ZnO) were capable to produce significant results on the mineralization of phenol.
Analyzing Figures 3 and 4 simultaneously it is possible to relationate mineralization with phenol degradation, and also adquire information about mechanisms differences. Photolysis graphs indicate 15% TOC remotion and 20% degradation, respectively. Considering high volatility of phenol and the fact that reactor used operated
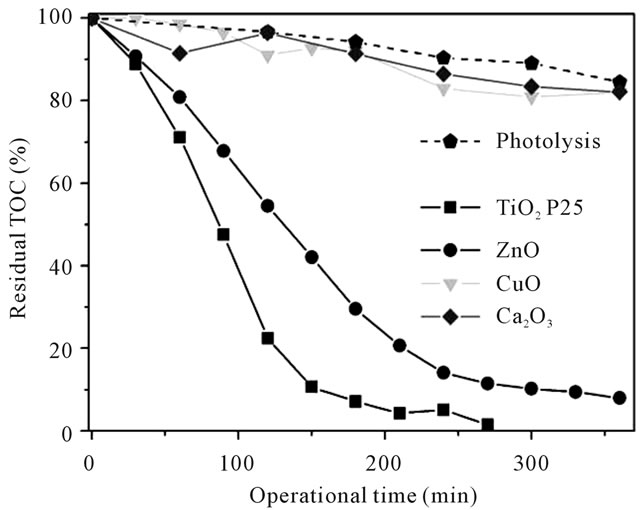
Figure 3. Mineralization results for the photocatalytic and photolysis processes, Residual TOC in accord with operational time.
not sealed, it is adequate consider this value as a combination of photolysis plus volatilization process. The indicative of phenol partial photolysis is the higher degradation than mineralization and the detection of hidroquinone intermediate in trace levels (1 to 2.5 mg∙L−1).
TiO2 achieved more than 95% mineralization after 200 min reaction in an average degradation rate of 0.68 mg∙L−1∙min−1. During the first hour, after achieved 30% mineralization and 60% degradation it is observed the intermediates generation peak (catechol 2 mg∙L−1; benzoquinone 4 mg∙L−1; hifroquinone 14 mg∙L−1). The intermediates profile for TiO2 is unique because all intermediates are formed at the same time, suggesting that the generation of radicals is different from the other catalysts and the intermediates considered here are the main formed compounds after phenol degradation, since mass balance between mineralization, degradation and intermediates generated are perfectly accurate. These results are similar to other studies using different reactor, as [16] that identified benzoquinone and hidroquinone as the predominant intermediates for P25, mainly during the first hour of irradiation [14].
Degradation results with ZnO were similar to TiO2, but mineralization was less efficient (0.41 mg∙L−1∙min−1). This fact reflected on intermediates profile, starting with catechol (1.5 mg∙L−1) after 30 min, followed by hidroquinone (2 mg∙L−1) at 120 min and benzoquinone (0.9 mg∙L−1). Considering mass balance it is possible to infer that other intermediates might be present than the substances considered here. Organic acids such as the formic acid, acetic acid, oxalic acid and succinic acid are substances already reported in other works using different process as final intermediates before CO2 formation [3]. ZnO is considered a promising photocatalyst as an alternative to TiO2, showing effective oxidation of phenol even under low-powered UV radiation [7].
Gallium oxide is expected to be stimulated by UV-C and copper oxide is activated by visible radiation, but despite the radiation source be adequate to activate these photocatalysts, its performances were similar to mineralization behaviors for photolysis process. The poor performance of Ga2O3 could be due to glass absorption of the UV-C radiation from 30 to 10 mW∙cm−2, even with
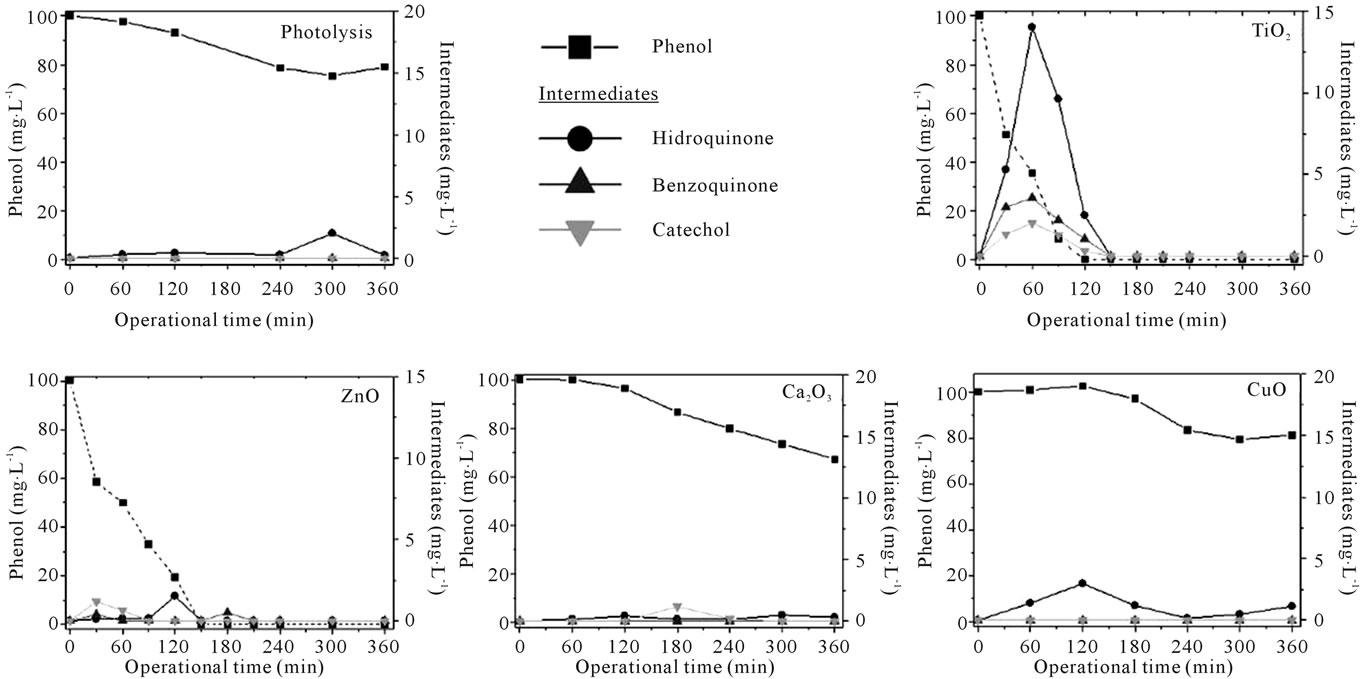
Figure 4. Degradation results for the photocatalytic and photolysis processes, Phenol and degradation intermediates (benzoquinone, hidroquinone, catechol) concentration in accord with operation time.
the use of PYREX glass. Zhang et al. [12] also reported a low catalytic activity for gallium oxide, being necessary 8 h irradiation using a 300 W lamp to obtain degradation of similar compounds. Hou et al. [19] revealed that β-Ga2O3 sample prepared with ethylene glycol showed the highest photocatalytic activity and this could be due to high surface area, abundant hydroxyl groups, and wide band gap in this case.
The difference between CuO and Ga2O3 degradation mechanisms is evident on Figure 4. Surface area and microstructure could be also influenced the reaction performance. CuO is recognized as active when surface área is higher than 40 m2∙g−1, a big difference when compared with the CuO tested (0.5 m2∙g−1). Karunakaran et al. [20] tested TiO2, Fe2O3, CuO, ZnO, ZnS, CdO and Nb2O5 on the phenol degradation under UV-A and observed that the use of two semiconductors together in suspension could enhance photocatalytic activity due to interparticle electron-transfer.
4. Conclusion
The process described here is ecofriendly, cost-effective, and reusable for the treatment of phenol-contaminated water. Results showed that when feeding the reactor with TiO2 the efficiency is higher than the other oxides but very similar to ZnO. Other interesting information about degradation mechanisms is that the degradation intermediates concentration varies differently for each photocatalyst tested, inferring different mechanism even when reaction rates were similar.
5. Acknowledgements
This project was financially supported by National Council for Scientific and Technological Development (CNPq) and National Council for the Improvement of Higher Education (CAPES).
REFERENCES
- K. M. Parida and A. C. Pradhan, “Fe/meso-Al2O3: An Efficient Photo-Fenton Catalyst for the Adsorptive-Degradation of Phenol,” Industrial & Engineering Chemistry Research, Vol. 49, No. 18, 2010, pp. 8310-8318. HUdoi:10.1021/ie902049sU
- H. G. Oliveira, D. C. Nery and C. Longo, “Effect of Applied Potential on Photocatalytic Phenol Degradation Using Nanocrystalline TiO2 Electrodes,” Applied Catalysis B: Environmental, Vol. 93, No. 3-4, 2010, pp. 205-211. HUdoi:10.1016/j.apcatb.2009.09.030U
- Y. H. Huang, Y. J. Huang, H. C. Tsai and H. T. Chen, “Degradation of Phenol Using Low Concentration of Ferric Ions by the Photo-Fenton Process,” Journal of the Taiwan Institute of Chemical Engineers, Vol. 41, No. 6, 2010, pp. 699-704. HUdoi:10.1016/j.jtice.2010.01.012U
- U. I. Gaya, A. H. Abdullah, M. Z. Hussein and Z. Zainal, “Photocatalytic Removal of 2,4,6-Trichlorophenol from Water Exploiting Commercial ZnO Powder,” Desalization, Vol. 263, No. 1-3, 2010, pp. 176-182. HUdoi:10.1016/j.desal.2010.06.055U
- L. C. Chen, C. M. HuhghhhjjijjiHuang, M. C. Hsiao and F. R. Tsai, “Mixture Design Optimization of the Composition of S, C, SnO2-Codoped TiO2 for Degradation of Phenol under Visible Light,” Chemical Engineering Journal, Vol. 165, No. 2, 2010, pp. 482-489. HUdoi:10.1016/j.cej.2010.09.044U
- J. Rodríguez, F. Paraguay-Delgado, A. López, J. Alarcón and W. Estrada, “Syntesis and Characterization of ZnO Nanorod Films for Photocatalytic Disinfection of Contaminated Water,” Thin Solid Films, Vol. 519, No. 2, 2010, pp. 729-735. HUdoi:10.1016/j.tsf.2010.08.139U
- P. Shukla, I. Fatimah, S. Wang, H. M. Ang and M. O. Tadé, “Photocatalytic Generation of Sulphate and Hydroxyl Radicals Using Zinc Oxide under Low-Power UV to Oxidise Phenolic Contaminants in Wastewater,” Catalysis Today, Vol. 157, No. 1-4, 2010, pp. 410-414. HUdoi:10.1016/j.cattod.2010.04.015U
- B. Krishnakumar and M. Swaminathan, “Solar Photocatalytic Degradation of Acid Black 1 with ZnO,” Indian Journal of Chemistry, Vol. 49a, 2010, pp. 1035-1040.
- E. S. Elmolla and M. Chaudhuri, “Effect of Fenton Operating Conditions on the Performance of the Combined Fenton-SBR Process for Antibiotic Wastewater Treatment,” Journal of Hazardous Materials, Vol. 173, No. 1-3, 2010, pp. 445-449. HUdoi:10.1016/j.jhazmat.2009.08.104U
- A. P. L. Batista, H. W. P. Carvalho, G. H. P. Luz, P. F. Q. Martins, M. Gonçalves and L. C. A. Oliveira, “Preparation of CuO/SiO2 and Photocatalytic Activity by Degradation of Methylene Blue,” Environmental Chemistry Letters, Vol. 8, No. 1, 2010, pp. 63-67. HUdoi:10.1007/s10311-008-0192-8U
- Y. Hou, X. Wang, L. Wu, Z. Ding and X. Fu, “Efficient Decomposition of Benzene over a β-Ga2O3 Photocatalyst under Ambient Conditions,” Environmental Science & Technology, Vol. 40, No. 18, 2006, pp. 5799-5803. HUdoi:10.1021/es061004sU
- H. Zhang, Y. Chen, L. Fu and J. Ma, “Syntesis, Thermal Stability, and Photocatalytic Activity of Nanocrystalline Gallium Nitrite via the Reaction of Ga2O3 and NH4Cl at Low Temperature,” Journal of Alloys and Compounds, Vol. 499, No. 2, 2010, pp. 269-272. HUdoi:10.1016/j.jallcom.2010.03.185U
- B. Zhao and P. Zhang, “Photocatalytic Decomposition of Perfluorooctanoic Acid with β-Ga2O3 Wide Bandgap Photocatalyst,” Catalysis Communications, Vol. 10, No. 8, 2009, pp. 1184-1187. HUdoi:10.1016/j.catcom.2009.01.017U
- M. Subramanian and A. Kannan, “Photocatalytic Degradation of Phenol in a Rotating Annular Reactor,” Chemical Engineering Science, Vol. 65, No. 9, 2010, pp. 2727- 2740. HUdoi:10.1016/j.ces.2010.01.004U
- A. Akyol and M. Bayramoglu, “Photocatalytic Performance of ZnO Coated Tubular Reactor,” Journal of Hazardous Materials, Vol. 180, No. 1-3, 2010, pp. 466-473. HUdoi:10.1016/j.jhazmat.2010.04.053U
- L. F. Velasco, B. Tsyntsarski, B. Petrova, T. Budinova and N. Petrov, “Carbon Foams as Catalyst Supports for Phenol Photodegradation,” Journal of Hazardous Materials, Vol. 184, No. 1-3, 2010, pp. 843-848. HUdoi:10.1016/j.jhazmat.2010.08.118U
- M. P. Paschoalino, J. Kiwi and W. F. Jardim, “Gas-Phase Photocatalytic Decontamination Using Polymer Supported TiO2,” Applied Catalysis B: Environmental, Vol. 68, No. 1-2, 2006, pp. 68-73. HUdoi:10.1016/j.apcatb.2006.08.001U
- V. P. Silva, M. P. Paschoalino, M. C. Gonçalves, M. I. Felisberti, W. F. Jardim and I. V. P. Yoshida, “Silicone Rubbers Filled with TiO2: Characterization and Photocatalytic Activity,” Materials Chemistry and Physics, Vol. 113, No. 1, 2009, pp. 395-400. HUdoi:10.1016/j.matchemphys.2008.07.104U
- Y. Hou, J. Zhang, Z. Ding and L. Wu, “Syntesis, Characterization and Photocatalytic Activity of β-Ga2O3 Nanostructures,” Powder Technology, Vol. 203, No. 3, 2010, pp. 440-446. HUdoi:10.1016/j.powtec.2010.06.004U
- C. Karunakaran, R. Dhanalakshmi, P. Gomathisankar and G. Manikandan, “Enhanced Phenol-Photodegradation by Particulate Semicondustor Mixtures: Interparticle Electron-Jump,” Journal of Hazardous Materials, Vol. 176, No. 1-3, 2010, pp. 799-806. HUdoi:10.1016/j.jhazmat.2009.11.105U

