Optics and Photonics Journal
Vol.07 No.12(2017), Article ID:81136,8 pages
10.4236/opj.2017.712022
Optical Gain Coefficient Measurements for 1-(4’-(Diphenylamino)- [1,1’-biphenyl]-4-yl)ethanone
F. Z. Henari, I. M. El-Deeb
Department of Medical Sciences, Royal College of Surgeons in Ireland, Medical University of Bahrain, Busaiteen, Kingdom of Bahrain

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: November 7, 2017; Accepted: December 15, 2017; Published: December 18, 2017
ABSTRACT
In this study, the optical gain coefficient due to spontaneous emission for the conjugated compound 1-(4’-(diphenylamino)-[1,1’-biphenyl]-4-yl)ethanone (DBE) in two solvents, Tetrahydrofuran (THF) and Dichloromethane (DCM) was investigated using the variable stripe length method. The solutions were placed in 10 mm cuvettes and pumped optically with N2 laser (337 nm) with pulse duration of 1.2 ns and repetition rate of 10 Hz. A maximum net gain of 12 cm−1 for the compound in THF, and 7 cm−1 for the compound in DCM were recorded at the input energy of 162 μJ. The fluorescence quantum yields (ff) of the compound were determined at the excitation wavelength of 337 nm using coumarin as a standard. The values of (ff) for the samples in DCM and THF solvents were found to be 0.68 and 0.61, respectively. The high values of quantum yields suggest the possibility of using this material as an active media for lasing and for LED.
Keywords:
Optical Gain, Variable Stripe Length Method, Conjugated Compound

1. Introduction
Conjugated polymers become an important class of materials and have attracted huge research interested worldwide for many years. These materials possess a notable photophysical and electrochemical properties, which make them useful in a wide range of optoelectronic devices, in particular, the use of these materials in light-emitting diodes, field-effect transistors, and photovoltaic cells [1] - [6] . The migration of the delocalized π-electrons along the conjugated backbones results in a gain of fluorescence signals and subsequent excellent performance in organic photoluminescence [4] , and electroluminescence devices [5] . Conjugated polymers proved to be good candidates for lasing medium due to their high photoluminescence quantum yields and their wide range tunability. There are several techniques available for measuring gain in such materials. A quite straightforward technique to determine the steady-state gain spectrum for an optically active medium is the variable stripe length method, originally presented by Shaklee and Leheny [7] . This technique involves the measurement of the amplified spontaneous (fluorescent) emission (ASE) from an illuminated stripe of varying length. The ASE measurements have been investigated in organic conjugated polymers [8] [9] , inorganic polymers [10] , and rare earth doped polymers [11] .
In this paper, we present the measurements of the optical gain due to spontaneous emission using the variable stripe length method of the conjugated compound 1-(4’-(diphenylamino)-[1,1’-biphenyl]-4-yl) ethanone (DBE, Figure 1) in two solvents: Tetrahydrofuran (THF) and Dichloromethane (DCM). The fluorescent intensity variation as a function focused on stripe length of the sample was measured. The fluorescent intensity of samples as a function of the input intensities for a fixed stripe length was also investigated. To the best of our knowledge, the experimental studies of the fluorescence properties and optical gain coefficient of this compound have not been reported previously.
2. Experiments and Results
The conjugated ethanone derivative (DBE) was synthesised according to the procedure described in [12] , applying Suzuki coupling of 4-bromo-N,N-diphenylaniline with 4-(acetylphenyl)boronic acid under palladium catalysis. The structure of the compound is shown in Figure 1.
Solutions of 1 g/l concentration were prepared in THF and DCM. The solutions were placed in 10 mm cuvettes. Emission and excitation spectra were obtained using 600 fluorometer. The fluorescence quantum yields (ff) of the compound were determined at excitation wavelength 337 nm using coumarin as
Figure 1. Structure of the fluorescent compound 1-(4’-(diphenylamino)-[1,1’-biphenyl]-4-yl)ethanone (DBE).
a standard. The values of (ff) for the samples in DCM and THF solvents were found to be 0.68 and 0.61, respectively. The high values of quantum yields suggest the possibility of using this material as an active media for lasing and for LED.
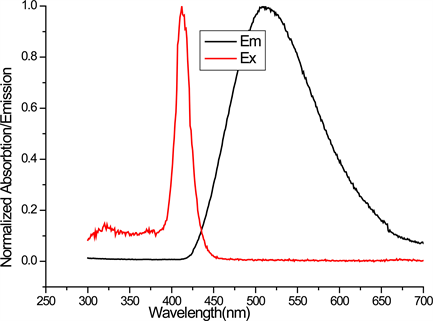
Figure 2 shows the absorption and emission spectra of DBE in THF. The emission spectra are a mirror image of the absorption spectra indicating that there are no other excited state involvements. Absorption maxima λmax occurs at 412 nm while the emission λmax occurs at 510 nm for excitation wavelength λ = 370 nm. The fluorescence spectra exhibit stokes shift of Δλmax = 98 nm (4664 cm−1) which is larger than that of organic fluorophores such as Rhodamine 6 G [13] Δλmax = 24 nm (Δυ = 823 cm−1). It is well known that the large Stokes shift eliminates spectral overlap between absorption and emission and allows detection of fluorescence while reducing interference [14] , therefore the studied compound may be considered for biological imaging and in vivo labelling.
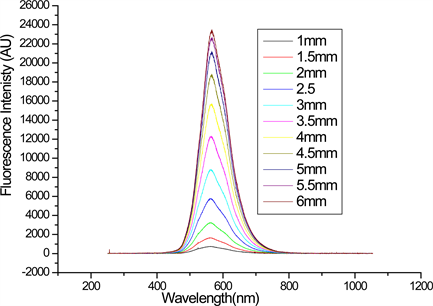
The variable stripe method was used to measure the optical gain from DBE. The laser beam from N2 laser (λ = 337 nm, pulse duration 1.2 ns and repetition rate 10 Hz) was used for photoexcitation. The beam was focused by a cylindrical lens with 10 cm focal length to form a rectangular stripe with a length of 5mm and width of 1 mm, in order to obtain the uniform intensity along the stripe length the two ends of the stripe were blocked and the experiment was performed with the central part of the stripe. The knife edge was placed on the translation stage and used to vary the excitation region. The emitted fluorescent was collected for different lengths of the stripe at a right angle to the direction of laser beam. The fluorescent signal was detected by CCD spectrometer. Figure 3 shows the evolution of the fluorescent intensity from the end of excitation stripe as a function of different stripe length at a fixed input energy (78 μJ) for the
Figure 2. Normalized absorption and emission of DBE in THF.
Figure 3. The emission spectra for different excitation stripe length at fixed input energy for DBE in DCM.
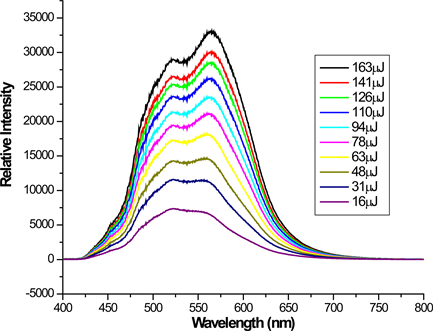
Figure 4. The emission spectra for different input energy at a fixed excitation stripe length for DBE in THF.
compound in DCM. As can be seen from the figure the emission intensities saturates for stripe length larger than 5 mm. This is probably due to the gain saturation, caused by depletion of excited state population by stimulated emission. This phenomenon was observed also when the intensity of the emission was studied as a function of the excitation length. Figure 4 shows the evolution of the emission spectra collected at different input energy and a fixed stripe length of 4 mm, for DBE in THF. The evolution of the emission may arise from the increase of the excitation population as input energy varied from 16 μJ to 163 μJ. It is known that the presence of the stimulated emission can be established from spectrum narrowing and from the super increase of the fluorescence output as the length of the stripe is varied [8] . In this experiment, the linear increase of the fluorescent output as a function of stripe length was observed, which is considered
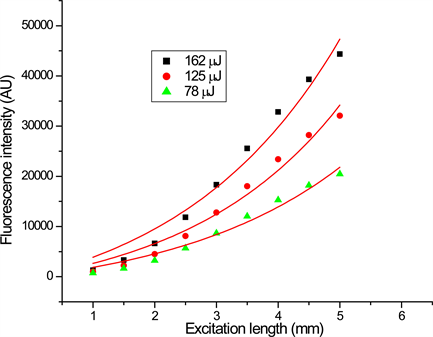
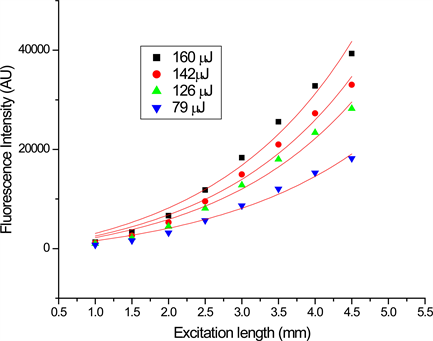
Figure 5. (a) Emission peak intensity of DBE in THF as a function of stripe length for three different input energies; (b) Emission peak intensity of DBE in DCM as a function of stripe length for three different input energies.
as an indication of the occurrence of amplified spontaneous emission. The spectra narrowing was not observed due the followings: the low input energy used in the experiment which is limited by output energy of the laser and the ns pulse duration used in the experiment which, may have taken longer time to resolve the emission processes in this compound [15] (too long and not quite clear sentence). However, the spectral narrowing was observed for polymers using fs pulses [16] [17] .
In the limit of the experimental conditions, the fluorescence intensity from stripe length is related to optical gain by equation [6]
(1)
where is the amplified emission, is spontaneous emission, g is the net gain coefficient and is the stripe length. By fitting the experimental data to Equation (1), and taking as a fitting parameter, the gain coefficient for different input energy can be measured.
Figure 5(a), Figure 5(b) shows the emission intensity as a function of excited strip length for DBE in DCM and THF respectively. The solid lines in the figures are the best fit to the experimental data to the Equation (1), which yield the maximum gain of 12 cm−1 for the compound in THF and 7 cm−1 for the compound in DCM at the input energy of 162 μJ. Saturation was observed for longer excitation lengths and the fitting graph departs from the experiment data (not shown). The reason for this behaviour is explained above.
Figure 6 shows the gain measured as a function of the input energy at the emission maximum (λmax = 561 nm) for 5 mm stripe length. As can be seen from
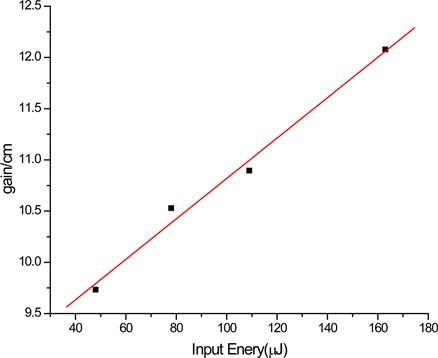
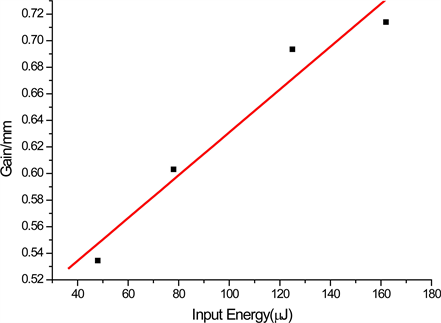
Figure 6. Gain as a function of input energy at emission maxima; (a) for DBE in THF, (b) for DBE in DCM.
the figure, the gain increases as the input energy increase. Maximum output energy recorded was 11.2 μJ, for input energy of 162 μJ (efficiency of 6.8%). This is an indication of possible use of this compound as active media for laser action.
3. Conclusion
In conclusion, we studied spontaneous emission and gain measurement for 1-(4’-(diphenylamino)-[1,1’-biphenyl]-4-yl)ethanone (DBE) in two solvents, Tetrahydrofuran (THF) and Dichloromethane (DCM) using the variable stripe length method. The net gain of 12 cm−1 for the compound in THF and 7 cm−1 for the compound in DCM respectively at the input energy of 162 μJ have been recorded. The differences in gains may be related to different absorptions of the pumping wavelength. This compound may be considered as a potential candidate as active media for a laser action and for LED devices.
Cite this paper
Henari, F.Z. and El-Deeb, I.M. (2017) Optical Gain Coefficient Measurements for 1-(4’-(Diphenylami- no)-[1,1’-biphenyl]-4-yl)ethanone. Optics and Photonics Journal, 7, 245-252. https://doi.org/10.4236/opj.2017.712022
References
- 1. Braun, D. and Heeger, A.J. (1991) Visible Light Emission from Semiconducting Polymer Diodes. Applied Physics Letters, 58, 19882. https://doi.org/10.1063/1.105039
- 2. Moses, D. (1992) High Quantum Efficiency Luminescence from a Conducting Polymer in Solution: A Novel Polymer Laser Dye. Applied Physics Letters, 60, 3215. https://doi.org/10.1063/1.106743
- 3. Manna, H., Henari, F.Z., Al-Saie, A., Drury, A., Kobayashi, T. and Blau, W.J. (2003) Light Amplification and Lasing in a (Meta-Phenylene Vinylene) Copolymer. Journal of Applied Physics, 93, 1871. https://doi.org/10.1063/1.1537463
- 4. Sheridan, A.K., Buckley, A.R., Fox, A.M., Bacher, A., Bradley, D.D.C. and Samuel, I.D. (2002) Efficient Energy Transfer in Organic Thin Films—Implications for Organic Lasers. Journal of Applied Physics, 92, 6367. https://doi.org/10.1063/1.1516270
- 5. Wang, L., Fang, G. and Cao, D. (2014) Amplified Spontaneous Emission and Gain from Optically Pumped Films of Dye-Doped Polymers. Applied Optics, 43, 5074.
- 6. Guo, X., Baumgarten, M. and Müllen, K. (2013) Designing π-Conjugated Polymers for Organic Electronics. Progress in Polymer Science, 38, 1832. https://doi.org/10.1016/j.progpolymsci.2013.09.005
- 7. Shaklee, K.L. and Leheny, L.F. (1971) Direct Determination of Optical Gain in Semiconductor Crystals. Applied Physics Letters, 18, 475. https://doi.org/10.1063/1.1653501
- 8. Costela, A., García, O., Cerdán, L., García-Moreno, I. and Sastre, R. (2008) Amplified Spontaneous Emission and Optical Gain Measurements from Pyrromethene 567-Doped Polymer Waveguides and Quasi-Waveguides. Optics Express, 16, 7023. https://doi.org/10.1364/OE.16.007023
- 9. Thompson, J., Anni, M., Lattante, S., Pisignano, D., Blyth, R.I.R., Gigli, G. and Cingolani, R. (2004) Amplified Spontaneous Emission in the Near Infrared from a Dye-Doped Polymer Thin Film. Synthetic Metals, 143, 305-307. https://doi.org/10.1016/j.synthmet.2003.12.012
- 10. Calzado, E.M., Boj, P.G. and Díaz-García, M.A. (2010) Amplified Spontaneous Emission Properties of Semiconducting Organic Materials. International Journal of Molecular Sciences, 11, 2546-2565. https://doi.org/10.3390/ijms11062546
- 11. Zhang, Q.J., Wang, P., Sun, X.F., Zhai, Y., Dai, P., Yang, B., Hai, M. and Xie, J.P. (1998) Amplified Spontaneous Emission of an Nd3+Nd3+-Doped Poly(Methyl Methacrylate) Optical Fiber at Ambient Temperature. Applied Physics Letters, 72, 407. https://doi.org/10.1063/1.120772
- 12. El-Deeb, I.M. and Lee, S.H. (2010) A New Efficient Convergent Synthesis of Conjugated Aryl-Containing Dendrimers. Bulletin of the Korean Chemical Society, 31, 1757-1760. https://doi.org/10.5012/bkcs.2010.31.6.1757
- 13. Gao, Z., Hao, Y.C., Zheng, M.L. and Chen, Y. (2017) A Fluorescent Dye with Large Stokes Shift and High Stability: Synthesis and Application to Live Cell Imaging. RSC Advances, 7, 7604. https://doi.org/10.1039/C6RA27547H
- 14. Sednev, M.V., Belov, V.N. and Hell, S.W. (2015) Fluorescent Dyes with Large Stokes Shifts for Super-Resolution Optical Microscopy of Biological Objects: A Review. Methods and Applications in Fluorescence, 3, Article ID: 42004. https://doi.org/10.1088/2050-6120/3/4/042004
- 15. de la Rosa-Fox, N. (1999) Optical Gain at Room Temperature in PPV-Related Materials. Optical Materials, 12, 267-271. https://doi.org/10.1016/S0925-3467(99)00053-1
- 16. Li, W., Zhang, C., Chen, Q., Wang, X. and Xiao, M. (2012) Two-Photon-Pumped Optical Gain in Dye-Polymer Composite Materials. Applied Physics Letters, 100, Article ID: 133305. https://doi.org/10.1063/1.3697706
- 17. Spiegelberg, Ch., Schulzgen, A., Allemand, P.M., Kippelen, B. and Peyghambarian, N. (1998) Gain Dynamics in Conjugated Polymers at Room Temperature. Physica Status Solidi B, 206, 131-138. https://doi.org/10.1002/(SICI)1521-3951(199803)206:1<131::AID-PSSB131>3.0.CO;2-K