Optics and Photonics Journal
Vol.06 No.12(2016), Article ID:72895,10 pages
10.4236/opj.2016.612031
Change of Spectroscopic Properties of PU/PVC Doped by Single Walled Carbon Nanotubes
L. H. Gaabour
Physics Department, Faculty of Science, Alfaisaliah Campus, King Abdulaziz University, Jeddah, KSA

Copyright © 2016 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: November 23, 2016; Accepted: December 18, 2016; Published: December 21, 2016
ABSTRACT
Nanocomposite films based on polyurethane and polyvinyl chloride (PU/PVC) doped by low content (≤0.045 wt%) of single wall carbon nanotubes (SWCNTs) were prepared and characterized. The structural, optical, thermal and mechanical properties of the prepared nanocomposites were characterized by various tools. The IR band at 3437 cm−1 in SWCNTs vanishing for doped samples signified that SWCNTs were successfully covalently encapsulated with PU/PVC. The X-ray peaks broaden with reduced intensity which refers to a reduction of the crystallinity for the blend after increase of SWCNTs attributed to changing in the crosslink density. UV/Vis analysis reveals that the optical band gap values were decreased with increased SWCNT attributed to the formation of defects in polymeric matrix with increase in the degree of disorder in the films. These defects produce the localized states in the optical band gap. All results data showed well dispersion of SWCNTs in polymer matrix at all weight percentages and an interaction between SWCNTs and the blend occurred. The thermal analysis showed that the melting point (Tm) and degradation temperature (Td) of the obtained films were increased gradually with adding of SWCNTs. Furthermore, the thermal stability was improved after adding SWCNTs into the PU/PVC polymer blend.
Keywords:
Nanocomposites, SWCNTs, X-Ray, TGA, Mechanical Properties

1. Introduction
Polyurethane (PU) has attracted an exceptional measure of consideration because of a variety of applications such as thermoplastic, elastomers, scientific device, textiles and smart actuators adhesive [1] . The molecular structure of PU can be easily tailored to meet particular property requirements.
Poly vinyl chloride (PVC) is a polymer utilized as often as possible as a part of uses that require structural features (mechanical strength) abilities. Since PVC has attractive properties, it usually requires the incorporation of different additives [2] [3] .
Blending PU with other polymers has been of a considerable interest in recent years. There are many studies on the blends of PVC with PU [4] [5] . The presence of intermolecular bonding of hydrogen between carbonyl groups (C=O) of PU with active α-hydrogen in PVC chains makes PVC component and PU more compatible in the main blend chain [6] . Adding PVC to PU would be possible to expand the characteristics of two polymers to obtain compatibility of the blends and better mechanical property. Due to well dispersion, highly potential nanoparticles, or tubes in polymeric matrices, high-performance lightweight nanocomposites can be produced [7] .
Carbon nanotubes (CNTs) incorporating into the polymeric matrices is an attractive method to combine the optical, electrical and mechanical properties for CNTs with the advantages of the polymer [8] [9] . It has attracted numerous researchers because of their good properties. As a result of the properties with nanometer scale size and aspect ratio, they are seen perfect as a strong reinforcing specialist for high strength polymer nanocomposites.
Many researchers have focused on the preparation of polymer nanocomposites loaded with carbon nanotubes by casting technique, mixed by melt method and electro spinning with enhanced thermal and mechanical structure [10] [11] [12] . The present work seeks for synthesis and investigation of PU/PVC polymer blend doped with low content of single-walled carbon nanotubes (£0.045 wt%) and studies the changes of the structural and spectroscopic properties of the nanocomposites.
2. Experimental Work
Polyurethane (PU) have Mw » 330,600 from Cargill-Dow Company, South Korea and high molecular weight polyvinyl chloride (PVC) supplied by Fluka. Tetra hydro furan (THF) supplied from Duksan Company (Korea) and it is used as received. Purified single walled carbon nanotubes (SWCNTs) with COOH functional groups (NTX10) were obtained from Nanothinx, Greece. SWCNTs-COOH has a diameter of 8 - 14 nm, length ≥ 5 µm and a purity of 88%.
The PU/PVC (75:25) wt% blend composite was dissolved in Tetrahydrofuran (THF) as a solvent at 45˚C under stirring until 24hour to complete dissolution to form a homogeneous solution. The polymer blend solution was left without stirring at 30˚C @ 2 hour to remove the bubbles during stirring. Also, SWCNT functionalization was mixed with polymer solution under continuous stirring in an ultrasonic to disperse of these CNTs inside the solution. Different concentration of SWCNT (0.015, 0.030 and 0.045 wt%) were added. After that, the films of the (PU/PVC)-SWCNT were obtained. The nanocomposites solution was put in oven at room temperature to evaporate the solvent for about 36 h. The obtained films were kept in vacuum desiccators before their investigation and measurement.
The FT-IR absorption spectra were carried out using the single beam Fourier transform-infrared spectrometer (FT-IR-430, JASCO, Japan). The FT-IR spectra of the samples were obtained in the spectral range of 2000 - 400 cm−1. The X-Ray diffraction scans were obtained using DIANO corporation-USA equipped using Cu-Ka radiation (l = 1.540 Å, at 30 kV, the Bragg angle 2q = 5˚ - 60˚. The UV-vis. spectra were measured in wavelength region of 190 - 900 nm by spectrophotometer (V-570 UV-VIS-NIR, JASCO, Japan). Thermogravimetric analysis (TGA) used to characterize the decomposition and thermal stability of prepared samples was carried out using A Perkin-Elmer TGA-7 from 30˚C to 550˚C with a heating rate of 5˚C/min.
3. Results and Discussion
3.1. Fourier Transform Infrared (FT-IR)
Figure 1 displays FT-IR spectrum of both polyurethane (PU) and poly vinyl chloride (PVC). The spectrum of PU shows IR absorption bands at 3340 cm−1 due to NH stretching, at 2950 cm−1 corresponding to CH asymmetric stretching, the band at 2860 cm−1 due to CH symmetric stretching, 1730 cm−1 for C=O stretching of urethane groups, 1602 cm−1 due to NH bonding), 1530 cm−1 attributed to C-C stretching (aromatic)) and at 1065 cm−1 C-O assigned to stretching of urethane groups [13] [14] . The spectrum of pure PVC shows the main characteristic bands at 2960 cm−1 due to CH stretching, 2910 cm−1 assigned to CH2 asymmetric stretching, 2861 cm−1, 1434 cm−1 due to CH bending and at 837 cm−1 attributing to C-Cl stretching [15] [16] .
The spectrum for PU/PVC blend shows characteristic absorption bands of both PU and. However, the band corresponding to C-N-H bonding vibrations modes at 1602 cm−1 and 837 cm−1 were slightly shift to 1594 cm−1 and 817 cm−1 assigned to hydrogen bonded interactions between C-N-H in PU and C-Cl in PVC groups. The shift of IR absorption band can be used as measure intermolecular interactions between two polymers.

Figure 1.FT-IR spectra of pure PU, pure PVC and pure PU/PVC.
It know that, pure single walled carbon nanotubes have function group at 3437 cm−1 refers to OH stretching of the hydroxyl group which can be attributed to the oscillation of carboxyl groups (O=C-OH and C-OH). The main band which observed at 2922 cm−1 is attributed to asymmetric stretching of CH2 and at 2861 cm−1 is attributed to symmetric stretching of CH2. The absorption band at 1735 cm−1 is corresponding to C=O stretching of COOH. The band at 1630 cm−1 can be associated with the stretching of the CNTs backbone and band at 1579 cm−1 is C=C vibration.
Figure 2 depicts the FT-IR spectra of PU/PVC doped with low contents of SWCNT. It can be seen that, the intensity of the band at 3437 cm−1 which shows strongest band in SWCNTs was vanishes after addition of SWCNTs signified that SWCNTs were successfully covalently encapsulated by two layers of polymeric matrices. The carboxylic acid group may have reacted with OH group in the blend to form ester groups and consist broad band at 3320 - 3400 cm−1 was overlapping of free amine group (NH) in urethane linkage and hydroxyl group (OH) in SWCNT-COOH.
3.2. X-Ray Diffraction (XRD)
Figure 3 display the X-ray diffraction of PU/PVC doped SWNTs in the range of 2θ = 5˚ to 70˚. The spectra show the semicrystalline behavior and characterized by two peaks at 2θ = 21.6˚ and 22.3˚. The spectra which contain SWCNTs showed much lower diffraction intensity compared to the blend and these peaks become broadened with reduced intensity refers to a reduction of the crystallinity of the blend with increase of SWCNTs contents. This change can be attributed to changing in the crosslink density of the polymer blend with increase in SWCNTs. The SWCNTs considerably affect the well short-range microstructural phases of both soft and hard segments of polymeric matrices attributed to the presence of strong interfacial interactions between components. No peaks pertaining to SWCNT appeared in the spectra indicating the complete/partial dissolution of the SWCNT in the polymer matrix.

Figure 2.FT-IR spectra of PU/PVC doped SWCNT.
Figure 3. X-ray spectra of PU/PVC doped SWCNT.
3.3. Ultraviolet and Visible Study
Figure 4(a), Figure 4(b) shows UV-Vis spectra of PU/PVC doped with low content of SWCNTs (≤0.045 wt%) in the range of 190 - 600 nm. All spectra exhibit very small absorbance in the ultra-violet range from 200 to 380 nm. The absorption band at 200 nm for the blend was assigned to n → π* transitions and the band at 290 nm was attributed to π → π* transition [17] . The main absorption edge for all curves was observed at about 208 nm which shifted toward longer wavelengths by about 20 nm with increasing SWCNT content. This shift in the bands indicates interactions between the polymers chains with the nanotube surface.
The optical energy band gap (Eg) for transitions can be determined by using the equations [18] :
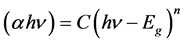 (1)
(1)
where C is a constant and n is the order which characterizes the optical absorption processes. It's that can take values as: n = 1/2 for indirect allowed transition, n = 1 for nonmetallic materials, n = 3/2 for direct forbidden transition, n = 2 for direct allowed transition and n = 3 for indirect forbidden transition [19] .
The plot of (ahn)1/2 from Equation (1) depends on of photon energy (hn) as shown in Figure 5 is used to calculate the indirect optical energy gap. The estimated values of indirect energy gap are recorded in Table 1. The indirect band gap values was decreased with increase SWCNT content attributed to the formation of defects in the polymeric matrix with increase in the degree of disorder in the films. These defects pro- duce the localized states in the optical band gap.
3.4. Thermogravimetric Analysis (TGA)
Figure 6 shows the TGA thermograms of PU/PVC doped with single walled carbon nanotubes from 30˚C to 650˚C. Two major weight losses were observed in all curves. The first weight loss of pure blend was observed from 220˚C to 350˚C attributed to losses of vapors and gasses from the blend. The initial weight loss were observed for all


Figure 4. (a) UV-Vis spectra of pure PU, pure PVC and pure PU/PVC; (b) UV-Vis spectra of PU/PVC doped SWCNT.
Figure 5. The relation between (ahn)1/2 versus (hn) of PU/PVC doped SWCNT.
Table 1. The calculated values of direct band energy and the activation energy of PU/PVC doped SWCNTs.

Figure 6. TGA thermograms of PU/PVC doped SWCNT.
prepared nanocomposites at lower temperatures may be attributed to the residual solvent evolution. The second weight loss from 360˚C to 420˚C corresponding to burning of organic phase and the thermal dehydration of inorganic particles. The weight loss at t > 420˚C (»3%) due to degradation of large polymer chains into small fragments which subsequently undergo further decomposition forming carbonaceous matter at around 510˚C and remains constant there after showing the plateau behavior. For doped samples, there is higher stability attributed to the combined effect of the excellent thermal stability of CNTs and their strong interfacial interactions with the polymeric matrix. It is possible that the formation of compact char of carbon nanotubes and polymer matrix during the thermal degradation is beneficial to the improvement of thermal stability of the composites. It is important to point out that the extent of interaction between SWNTs and polymer matrix with good dispersion could be responsible for a higher thermal stability of the composite system.
3.5. Calculation of the Activation Energy
The thermodynamics parameters of main decomposition process in the second degradation region of the present samples can be calculated using two different methods:
3.5.1. Coats-Redfern Method
Coats-Redfern method is a typical integral method and can be represented in the following form [20] :
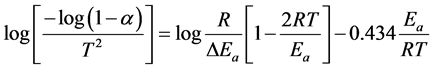 (2)
(2)
where, T is the absolute temperature in Kelvin, Ea is the activation energy in J/mol, R is the universal gas constant (8.3136 J/mol K), and the fraction of conversion (a) for a weight loss.
The plot between 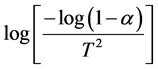 vs
vs  of the samples, the relation give a
of the samples, the relation give a
parallel straight lines. So, the activation energies are determined from slopes of these lines as:
Ea = 2.303R × slope (3)
3.5.2. Broido Method
Broido introduce a model to evaluate the activation energy associated with second stage of decomposition using equation [21] :
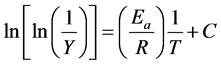 (4)
(4)
Y is given by :
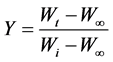 (5)
(5)
where, Y is the fraction of the number of initial molecules not yet decomposed; Wt is the weight at any time t; W∞ is the weight at infinite time (=zero) and Wi is the initial weight. A plot between 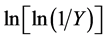 vs. 1/T gives an excellent approximation to a straight line. The slope is related to the activation energy. The values of the activation energy (Ea) for both methods are recorded in Table 1. From the Table 1, the calculated values of the activation energy were decreased with increasing of SWCNTs addition indicates that SWCNTs intensively affect the polymer.
vs. 1/T gives an excellent approximation to a straight line. The slope is related to the activation energy. The values of the activation energy (Ea) for both methods are recorded in Table 1. From the Table 1, the calculated values of the activation energy were decreased with increasing of SWCNTs addition indicates that SWCNTs intensively affect the polymer.
4. Conclusion
Nanocomposites of PU/PVC-doped SWCNTs were prepared and characterized using different techniques. The FT-IR spectra show shift and disappearance of some bands after adding SWCNTs in PU/PVC blend. It indicates a good physical entanglement between the constituents and clearly the interaction between the SWCNT and the blend. The XRD results revealed broad peaks with lower intensity in relation with pure blend, which inferred that the SWCNTs considerably affect the well short range microstructural phases of both the soft and the hard segments of the polymer matrix, resulting in strong interfacial interactions. The UV-Vis shows shift of the bands towards longer wavelength. The TGA study shows that the incorporation of SWCNTs significantly improved the thermal stability caused by the high thermal conductivity. The calculated values of energies gap were decreased with increasing of SWCNTs.
Cite this paper
Gaabour, L.H. (2016) Change of Spectroscopic Properties of PU/PVC Doped by Single Walled Carbon Nanotubes. Optics and Photonics Journal, 6, 305-314. http://dx.doi.org/10.4236/opj.2016.612031
References
- 1. Wang, C., Chen, X., Xie, H. and Cheng, R. (2011) Effects of Carbon Nanotube Diameter and Functionality on the Properties of Soy Polyol-Based Polyurethane. Composites: Part A, 42, 1620.
- 2. Wu, X.L. (2010) Poly(vinyl Chloride)-Grafted Multi-Walled Carbon Nanotubes via Friedel-Crafts Alkylation. eXPRESS Polymer Letters, 4, 723-728.
https://doi.org/10.3144/expresspolymlett.2010.87 - 3. Soudais, Y., Moga, L., Blazek, J. and Lemort, F. (2007) Coupled DTA-TGA-FT-IR Investigation of Pyrolytic Decomposition of EVA, PVC and Cellulose. Journal of Analytical and Applied Pyrolysis, 78, 46.
- 4. Haponiuk, J.T. and Balas, A. (1995) Thermal Properties of Poly(vinyl Chloride)/Polyure-thane Blends. Journal of Thermal Analysis and Calorimetry, 43, 215-218.
https://doi.org/10.1007/BF02635987 - 5. Kolesov, S.V., Neboilova, I.V., Steklova, A.M., Vladychina, S.V. and Minsker, K.S. (1989) Kinetics of Thermal Degradation of Poly(vinyl Chloride)-Polyurethane Blends. Polymer Science U.S.S.R., 31, 476.
- 6. Malysheva, T.L., Golovan, S.V. and Starokadomsky, D.L. (2011) The Effect of Interfacial Interactions on a Structure and Properties of Polyurethane Elastomer/Poly(Vinyl Chloride) Blends. Open Journal of Organic Polymer Materials, 3, 1-7.
https://doi.org/10.4236/ojopm.2011.11001 - 7. Foy, J.V. and Lindt, J.T. (1987) Electrical Properties of Exfoliated-Graphite Filled Polyester Based Composites. Polymer Composites, 8, 419-426.
https://doi.org/10.1002/pc.750080608 - 8. Elashmawi, I.S. and Gaabour, L.H. (2015) Raman, Morphology and Electrical Behavior of Nanocomposites Based on PEO/PVDF with Multi-Walled Carbon Nanotubes. Results in Physics, 5, 105-110.
https://doi.org/10.1016/j.rinp.2015.04.005 - 9. Wang, Y.S.O., Gurkis, M.A. and Lindt, J.T. (1986) Electrical Properties of Exfoliated-Gra-phite Filled Polyethylene Composites. Polymer Composites, 7, 449-454.
- 10. Haggenmueller, R., Fischer, J.E. and Winey, K.I. (2006) Single Wall Carbon Nanotube/Po-lyethylene Nanocomposites: Nucleating and Templating Polyethylene Crystallites. Macromolecules, 39, 2964-2971.
https://doi.org/10.1021/ma0527698 - 11. Haggenmueller, R., Gommans, H.H., Rinzler, A.G., Fischer, J.E. and Winey, K.I. (2000) Aligned Single-Wall Carbon Nanotubes in Composites by Melt Processing Methods. Chemical Physics Letters, 330, 219-225.
https://doi.org/10.1016/S0009-2614(00)01013-7 - 12. Hou, H., Ge, J.J., Zeng, J., Li, Q., Reneker, D.H. and Greiner, A. (2005) Electrospun Polyacrylonitrile Nanofibers Containing a High Concentration of Well-Aligned Multiwall Carbon Nanotubes. Chemistry of Materials, 17, 967-973.
https://doi.org/10.1021/cm0484955 - 13. Barick, A.K. and Tripathy, D.K. (2010) Effect of Nanofiber on Material Properties of vapor-Grown Carbon Nanofiber Reinforced Thermoplastic Polyurethane (TPU/CNF) Nanocomposites Prepared by Melt Compounding. Composites: Part A, 41, 1471-1482.
https://doi.org/10.1016/j.compositesa.2010.06.009 - 14. Travati, G., Sanches, E.A., Neto, S.C., Mascarenhas, Y.P. and Chierice, G.O. (2010) Characterization of Polyurethane Resins by FTIR, TGA, and XRD. Journal of Applied Polymer Science, 115, 263-268.
https://doi.org/10.1002/app.31096 - 15. Beltran, M., Garcia, J.C. and Marcilla, A. (1997) Infrared Spectral Changes in PVC and Plasticized PVC during Gelation and Fusion. European Polymer Journal, 33, 453-462.
https://doi.org/10.1016/S0014-3057(96)00213-3 - 16. Ramesh, S. and Yi, L.J. (2009) FTIR Spectra of Plasticized High Molecular Weight PVC-LiCF3SO3 Electrolytes. Ionic, 15, 413-420.
https://doi.org/10.1007/s11581-008-0279-z - 17. Elashmawi, I.S. and Hakeem, N.A. (2008) PMMA Addition on Characterization and morphology of the PVDF. Polymer Engineering & Science, 48, 895-901.
https://doi.org/10.1002/pen.21032 - 18. Kramadhati, S. and Thyagarajan, K. (2013) Optical Properties of Pure and Doped (Kno3 & Mgcl2) Polyvinyl Alcohol Polymer Thin Films. International Journal of Engineering Research and Development, 6, 15-18.
- 19. Ramirez, R.J., Arellano, C., Varia, J.C. and Martinez, S.S. (2015) Visible Light-Induced Photocatalytic Elimination of Organic Pollutants by TiO2: A Review. Current Organic Chemistry, 19, 540-555.
https://doi.org/10.2174/138527281906150417094736 - 20. Coats, A.W. and Redfern, J.P. (1964) Kinetic Parameters from Thermogravimetric Data. Nature, 201, 68-69.
https://doi.org/10.1038/201068a0 - 21. Broido, A. (1969) A Simple, Sensitive Graphical Method of Treating Thermogravimetric Analysis Data. Journal of Polymer Science Part A, 7, 1761-1773.
https://doi.org/10.1002/pol.1969.160071012




