Optics and Photonics Journal
Vol.04 No.09(2014), Article ID:50066,8 pages
10.4236/opj.2014.49024
Study on Complex Formation of Fluorescein-p-Sulfonatocalix[4]arene by Spectroscopic Methods
Sharadchandra Gawhale1, Yogita Thakare2, Dipalee Malkhede3*, Gajanan Chaudhari2*
1Department of Applied Chemistry, AISSMS-COE, Pune, India
2Department of Chemistry, Shri.Shivaji Science College, Amravati, India
3Department of Chemistry, University of Pune, Pune, India

*Corresponding authors.
Email: *gnchaudhari@gmail.com, *ddm@chem.unipune.ac.in
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).


Received 10 July 2014; revised 5 August 2014; accepted 29 August 2014

ABSTRACT
The aqueous solution of p-Sulfonatocalix[4]arene-fluorescein complex has been studied based on fluorescence and 1H NMR spectroscopic results. It has been found that the fluorescence intensity quenches regularly upon addition of p-SCX4. The proposed interaction mechanism between p- SCX4 and FL indicates that FL partially goes into the cavity of p-SCX4 with the help of strong electrostatic and π-π* interaction. The quenching constants and stability constants are determined by p-SCX4-FL systems. The proposed inclusion complex is discussed on 1H NMR results. Results are consistent with experimental data obtained from NMR spectroscopy.
Keywords:
p-Sulfonatocalix[4]arene, Fluorescein, Electrostatic Interactions, Hydrophobic Interaction, 1H NMR Analysis

1. Introduction
p-Sulfonatocalix[n]arene is a family of water soluble calixarene derivatives which have gained considerable attention in the fields of molecular recognition and sensing. Aqueous systems are particularly interesting for complexation studies because one can expect hydrophobic forces to play an important role [1] . Calixarenes are known to form inclusion complexes with a variety of guest molecules in solution and in a solid state, since their inherent annular structure exists stable in both phases [2] .
Inclusion complexes are chemical species consisting of two or more associated molecules in which one of the molecules forms a cavity into which it can admit a guest molecule resulting in a stable association without formation of any covalent bonds such as electrostatic interaction, cation-π interactions, hydrogen bonding, van der Waals and hydrophobic interactions [3] . p-Sulfonic calix[n]arenes provided not only hydrophobic environment (benzene rings) with the above favorable properties, but also hydrophilic heads (sulfonates) and form water soluble encapsulated complexes [4] . Many researchers have studied inclusion properties of water soluble calixarenes with organic ions and neutral molecules [5] -[15] . Secondary forces are alone responsible for maintenance of the integrity of all inclusion complexes. The molecular ratio of guest to calixarene is usually found to be 1:1. However this can change depending on the shape and geometry of the guest and calixarene. The minimum requirement for an inclusion complex formation is size compatibility between host and guest molecules, i.e. guest molecules must fit, entirely or at least partially, into the calixarene cavity [16] [17] .
We use FL as a guest molecule because fluorescein derivatives are the most common fluorescent reagents for biological research because of their high absorptivity and excellent fluorescence quantum yield. The present stu- dy investigate the formation for complexation of fluorecscein by water soluble p-Sulfonatocalix[4]arene and discuss about their interaction.
2. Experimental Section
The p-SCX4 is purchased from TCI chemicals with 99.0% purity and used as it is. Fluorescein (FL) was procured from Sigma-Aldrich. All aqueous solutions were prepared with ultrapure water obtained from a Millipore Milli-Q. For all experiments pH was adjusted to 3.5 using dilute HCl.
pH was measured by digital pH meter of Elico LI 120 make with combined glass and calomel electrode. Absorbance spectra were performed on Shimadzu UV-1800 spectrophotometer. The variation in fluorescence intensity for the determination of stability constants were monitored on a Jasco FP-8300 spectrofluorometer using 1 cm × 1 cm quartz cell. The excitation wavelength was set at 511 nm. Emission spectra were collected in the range 290 - 600 nm. The slits for the excitation and emission monochromator were fixed at 2.5 nm. The NMR experiments were performed on a Varian mercury YH-300 spectrometer.
An appropriate volume of 1 × 10−6 M FL was taken in cuvette. To this solution 1 × 10−4 M solution of p- SCX4 was added in different volumes. NMR data was collected by adding 1 - 2 M concentrations of p-SCX4 to 2 M FL in D2O.
3. Results and Discussion
The sulfonic acid moieties of p-SCXn hosts are completely dissociated at pH 0.4 and the pKa value of the first dissociation step of the phenolic OH groups is in the range of 3.00 - 3.7 showing slight growth with the ring size. Therefore, all readings were taken at pH 3.5 [18] . A large part of our existing knowledge of noncovalent binding is based on the measurement of equilibrium constants. Equilibrium constants afford the scientist information of the mechanism of the chemical process involved.
Despite multiple reports [19] otherwise, we assume only one ligand may occupy each receptor (or host) site. A scheme for this event may be represented by,
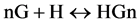
where n signifies the maximum number of binding sites on substrate H and G denotes the ligands that are free to bind to each site.
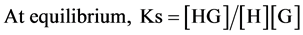
where [H], [G] and [HG] represent the concentration of the host, guest and supramolecular inclusion complex respectively.
3.1. UV-Visible Absorption Spectral Titration
By keeping the concentration of the FL fixed at 1 × 10−6 M and varying the concentration of the host-pSCX4, UV-visible absorption spectrum of FL was recorded. The fall in absorbance is observed showing blue shift with change in maximum absorbance wavelength from 479 nm to 472 nm. Also it is observed that with each addition the first shoulder increasing with decreasing second shoulder indicates transition from n to π* (Figure 1). The stability constant (Ks) which controls the equilibrium between the free ligand and the complex was obtained from the variation of either absorbance or fluorescence intensity at proper observation wavelengths [20] . The stability constant was calculated as 1.95 × 104 M−1 which also indicates strong binding between p-SCX4 and FL (Figure 2).
Figure 1. Absorption spectra of guest, FL (1 × 10−6 M) with the addition of different concentrations of host, p-SCX4 (10 - 1000 μl, 1 × 10−4 M).
Figure 2. Binding constant = 1.95 × 104 M−1.
3.2. Fluorescence Spectral Titrations
To assess the inclusion complexation behavior of p-SCX4-FL system, spectral titrations were performed at 25˚C in aqueous solution by measuring fluorescence measurement. Figure 3 shows fluorescence spectroscopy experiments which were carried out at scan speed 500 nm/min, sensitivity medium, and response time of 1 second. Initially spectral changes were recorded of FL having concentration 1 × 10−6 M with gradual addition of p-SCX4 having concentration 1 × 10−4 M. The stepwise increase of concentration of the host causes significant reduction in fluorescence intensity with no change in peak shape of dye. When FL was excited at 460 nm, the maximum emission wavelength was observed at 511 nm. With the addition of p-SCX4, the maximum emission wavelength was a bit blue-shifted from 508 - 511 nm, indicating the formation of host-guest inclusion complex between p-SCX4-FL (Figure 3). The fluorescence intensity of the first band (510 nm; excitation wavelength 460 nm) decreased markedly with increasing calixarene concentration, owing to the quenching effect of the calixarene ᴨ-system. The possible explanation for decrease in the fluorescence intensity of dye with the addition of p-SCX4 is that the increase in polarity or hydrophilicity around the dye molecules causes much higher fluorescent quenching [21] .
Figure 3. Fluorescence emission spectra (λ ex 511 nm) of FL (1 × 10−6 M) at different concentration of p-SCX4 (10 - 300 μl, 1 × 10−4 M), at pH = 3.5. The inset shows i) Stern- Volmer plot of fluorescence quenching of FL Vs [p-SCX4]−1, Ksv = 1.36 × 10−5 M.
Stern-Volmer analysis was utilized to probe the nature of the quenching process in the complex formation. Stern-Volmer plot is a useful method of presenting data on emission quenching. Plotting relative emission intensities Io/I against quencher concentration yields alinear Stern-Volmer plot for a static quenching process (Figure 4). Slope of the line gives Ksv, static quenching constant. I and Io are the initial and final fluorescence intensities.
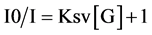
The complex stability constant was calculated using Valeur’s method [22] . The quantity Io/Io-I is plotted against [p-SCX4] with the stability constant given by the ratio of intercept/slop [23] . Stability constants for complexation of p-SCX4-FL are approximately the same calculated by two methods, spectrophotometric and spectrofluorometric.
3.3. Inclusion Mechanism
To explore the possible inclusion model between p-SXC4 and FL (Figure 5), 1H
Figure 4. The plot of nonlinear least-squares fitting used for the association constant. I0/I0-I vs. [p-SCX4]−1, Binding constant = 3.89 × 104 M−1.
Figure 5. Protons shown in Fluoroescein and p-SCX4.
Table 1. (a) 1H chemical shift values of SCX4; (b) 1H chemical shift values of Fluorescein.







Figure 6. (a-g): 1H NMR spectra of (a) FL, (b) p-SCX4 and (c-g) mixture of FL+ p-SCX4. (a) SCX4, (b) Fluorescein, (c) p-SCX4-Fluorescein-1, (d) p-SCX4-Fluorescein-2, (e) p-SCX4-Fluorescein-3, (f) p-SCX4-Fluorescein-4, (g) p-SCX4- Fluorescein-5.
Figure 7. The proposed inclusion pattern of FL-p-SCX4 complex.
4. Conclusion
The formation constant of the p-Sulfonatocalix[4]arene with FL system has been performed by spectrophotometric and spectrofluometric titrations in water. It has been shown from NMR data that FL is inserted partially into the cavity of p-SCX4 due to favorable formation constant of π-π interactions with benzene rings and electrostatic interactions with the 
Acknowledgements
STG and DDM acknowledge financial support from University Grants Commission, New Delhi, India.
References
- Shinkai, S., Araki, K., Mastsuda, T., Nishijama, N., Ikeda, H., Takasuand, I. and Iwamoto, M. (1990) NMR and Crystallographic Studies of a p-Sulfonatocalixarene-Guest Complex. Journal of the American Chemical Society, 112, 9053-9058. http://dx.doi.org/10.1021/ja00181a004
- Lakowicz, J.R. (2006) Principles of Fluorescence Spectroscopy. 3rd Edition, Springer, Berlin. http://dx.doi.org/10.1007/978-0-387-46312-4
- Shinkai, S. (1993) Calixarenes—The Third Generation of Supramolecules. Tetrahydron, 49, 8933-8968.
- Alexis, M., Alberto, S., Gerhard, A. and Enrique, M. (2011) Host-Guest Interactions between Calixarenes and Cp2NbCl2. Journal of Organometallic Chemistry, 696, 2419-2527. http://dx.doi.org/10.1016/j.jorganchem.2011.03.021
- Kunsági-Mátéa, S., Szabó, K., Lemlia, B., Bitter, I., Nagy, G. and Kollár, L. (2005) Host-Guest Interaction between Water-Soluble Calixarene Hexasulfonate and p-Nitrophenol. Thermochimica Acta, 425, 121-126. http://dx.doi.org/10.1016/j.tca.2004.06.015
- Zhou, Y.Y., Ding, X.P., Fang, X.L., Li, T., Tang, D.B. and Lu, Q. (2011) Studies on the Inclusion Behavior of Amphiphilic p-Sulfonatocalixarene with Ascorbic Acid by Spectrofluorometric Titrations. Optics and Photonic Journal, 1, 59-64. http://dx.doi.org/10.4236/opj.2011.12009
- Zhou, Y.Y., Lu, Q., Liu, C., She, S. and Wang, L. (2006) Study on the Inclusion Behavior of p-Sulphonatocalixarene with 9-Amino-Acridine by Spectrofluorometric Titrations. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 63, 423-426. http://dx.doi.org/10.1016/j.saa.2005.04.056
- da Sliva, D.L., do Couto Tavares, E., de Souza Conegero, L., de Fatima, A., Pilli, R.A. and Fernandes, S.A. (2011) NMR Studies of Inclusion Complexation of the Pyrrolizidine Alkaloid Retronecine and p-Sulfonic Acid Calixarene. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 69, 149-155. http://dx.doi.org/10.1007/s10847-010-9825-1
- Wang, K., Guo, D.S., Zhang, H.Q., Li, D., Zheng, X.L. and Liu, Y. (2009) Highly Effective Binding of Viologens by p-Sulfonatocalixarenes for the Treatment of Viologen Poisoning. Journal of Medicinal Chemistry, 52, 6402- 6412.
- Mareeswaran, P.M., Babu, E., Sathish, V., Kim, B., Woo, S.I. and Rajagopa, S. (2014) P-Sulfonatocalixarene as a Carrier for Curcumin. New Journal of Chemistry, 38, 1336-1345. http://dx.doi.org/10.1039/c3nj00935a
- Miskolczy, Z. and Biczok L. (2009) Inclusion Complex Formation of Ionic Liquids with 4-Sulfonatocalixarenes Studied by Competitive Binding of Berberine Alkaloid Fluorescent Probe. Chemical Physics Letters, 477, 80-84.
- Liu, Y., Han, B.-H. and Chen, Y.-T. (2002) Molecular Recognition and Complexation Thermodynamics of Dye Guest Molecules by Modified Cyclodextrins and Calixarenesulfonates. The Journal of Physical Chemistry B, 106, 4678- 4687. http://dx.doi.org/10.1021/jp015603r
- Liu, Y., Han, B.-H. and Chen, Y.-T. (2000) Inclusion Complexation of Acridine Red Dye by Calixarenesulfonates and Cyclodextrins: Opposite Fluorescent Behavior. The Journal of Organic Chemistry, 65, 6227-6230. http://dx.doi.org/10.1021/jo991654x
- Fei, X.N., Zhang, Y., Zhu, S., Liu, L.J. and Yu, L. (2013) Spectral Study and Protein Labeling of Inclusion Complex between Dye and Calixarene Sulfonate. Applied Spectroscopy, 67, 520-525.
- Arena, G., Contino, A., Gulino, F.G., Magri, A., Sciotto, D. and Ungaro, R. (2000) Complexation of Small Neutral Organic Molecules by Water Soluble Calixarenes. Tetrahedron Letters, 41, 9327-9330. http://dx.doi.org/10.1016/S0040-4039(00)01687-7
- Steed, J.W. and Atwood, J.L. (2009) Supramolecular Chemistry. Wiley Publication, Hoboken.
- Sliwa, W. and Kozlowski, C. (2009) Calixarenes and Resorcinarenes. Wiley Publication, Hoboken.
- Gutsche, D.C. (2008) Calixarene: An Introduction. Royal Society of Chemistry, London.
- Brayant, W.S., Guzei, I.A., Rheigold, A.L., Merola, J.S. and Gibson, H.W. (1998) A Study of the Complexation of Bis(m-Phenylene) Crown Ethers and Secondary Ammonium Ions. The Journal of Organic Chemistry, 63, 7634-7639.
- Sahin, O. and Yilmaz, M. (2011) Synthesis and Fluorescence Sensing Properties of Novel Pyrene-Armed Calix- arene Derivatives. Tetrahedron, 67, 3501-3508. http://dx.doi.org/10.1016/j.tet.2011.03.035
- Zhang, Y.L., Pham, T.H., Pena, M.S., Agbaria, R.A. and Warner, I.M. (1998) Spectroscopic Studies of Brilliant Cresyl Blue/Water-Soluble Sulfonated Calixarene Complex. Applied Spectroscopy, 52, 952-957. http://dx.doi.org/10.1366/0003702981944760
- Bourson, J. and Valeur, B. (1989) Ion-Responsive Fluorescent Compounds. 2. Cation-Steered Intramolecular Charge Transfer in a Crowned Merocyanine. The Journal of Physical Chemistry, 93, 3871-3876. http://dx.doi.org/10.1021/j100346a099
- Valeur, B. (2001) Molecular Fluorescence Principles and Applications. Wiley-VCH, Weinheim. http://dx.doi.org/10.1002/3527600248
- Asfari, M.-Z., Böhmer, V., Harrowfield, J. and Jacques, V. (2001) Calixarene 2001. Springer, Berlin.









