Stem Cell Discovery
Vol. 2 No. 3 (2012) , Article ID: 21349 , 7 pages DOI:10.4236/scd.2012.23013
Human dental pulp stem cells differentiate into neural precursors but not into mature functional neurons
![]()
1Neuro Group, Institute of Biomedical Technology, University of Tampere, Tampere, Finland; *Corresponding Author: riikka.aanismaa@uta.fi
2Adult Stem Cell Group, Institute of Biomedical Technology, University of Tampere, Tampere, Finland
3Finnish Student Health Service, Tampere, Finland
Received 7 April 2012; revised 8 May 2012; accepted 10 June 2012
Keywords: Dental Pulp Stem Cell; Neural; Differentiation; Neural Networks
ABSTRACT
Large numbers of neuronal cells are needed for regenerative medicine to treat patients suffering from central nervous system diseases and deficits such as Parkinson’s disease and spinal cord injury. One suggestion has been the utilization of human dental pulp stem cells (hDPSCs) for production of neuronal cells which would offer a patient-specific cell source for these treatments. Neuronal differentiation of hDPSCs has been described previously. Here, we tested the differentiation of DPSCs into neuronal cells with previously reported protocol and characterized the cells according to their morphology, gene and protein expressions and most importantly according to their spontaneous electrical functionality with microelectrode array platform (MEA). Our results showed that even though hDPSCderived neural progenitor stage cells could be produced, these cells did not mature further into functional neuronal cells. Thus, utilization of DPSCs as a cell source for producing grafts to treat neurological deficits requires more efforts before being optimal.
1. INTRODUCTION
Human stem cells have been intensively studied due to the possibility to use them for regenerative purposes for neurological diseases such as Parkinson’s disease, spinal cord injury, and stroke [1]. Both human pluripotent stem cells, i.e. human embryonic [2] and human induced pluripotent [3,4] stem cells, as well as human fetal stem cells [5] have been shown to improve the functional recovery after the disease manifestations in both experimental animals [6] and clinical experiments [7]. The source of cells, however, should be thoroughly considered and assessed due to possible harmful side effects and tissue rejections. Indeed, the most optimal case would be harvesting the stem cells from patient’s own body before culturing and differentiating them further and finally transplanting these cells back to the same patient. Stem cells can be obtained from various sources in the human body such as bone marrow and adipose tissue [8,9]. Further, these stem cells have been successfully used in treating leukaemia [10] or bone deficits [11]. The neural differentiation potential of human mesenchymal stem cells is not, however, extensively and reliably shown [8].
One interesting adult stem cell population is the human dental pulp stem cells (hDPSCs). These cells have been shown to be able to differentiate along several pathways, including mesenchymal and neural cells [12,13]. Thus, if they could be differentiated into neural cells in large quantities in vitro, they would offer a promising patient-specific cell population for transplantation therapies. So far, neural differentiation of hDPSCs has been reported [14-18]. HDPSC-derived neural cells have been characterized with gene and protein expression analysis but the most important aspect, the spontaneous electrical activity of the produced neural cells, has not been studied. Currently, it is noted that neuronal cells derived from any of the available stem cell sources should be able to form spontaneous action potentials and further spontaneously active functional neuronal networks. Indeed, it has been shown with human embryonic stem cells [19] and human cord blood stem cells [20] and should also be shown with hDPSCs. Patch clamp analysis has been reported from hDPSC-derived neuronal cells showing typical voltage-activated sodium and potassium currents [16,21] but no actual action potentials have been shown. The neuronal activity properties can also be investigated at network level using microelectrode array (MEA) platform [22].
Here, we performed neural differentiation of hDPSCs with previously published method [16] and characterized the cells using qPCR, immunocytochemistry, and MEA setup. Human embryonic stem cell (hESC)-derived neuronal cells were used as a positive control.
2. MATERIALS AND METHODS
2.1. Isolation and Culture of Human Dental Pulp Stem Cells
Normal impact third molars were collected from young adults (n = 4) (21 - 25 years of age) with their informed consent at Finnish Student Health Service, Tampere, Finland. Three of the pulps were from male and one (pulp 1) from a female patient. The donors did not smoke nor had diabetes or asthma. Two pulps (from maxilla and mandible) from one patient were pooled. The pulp tissue was separated from the crown and the root and placed on 2 ml of DMEM/F12 (Gibco Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen), 1% antibiotic-antimycotic (Gentaur, Belgium), and 1% GlutaMax (Invitrogen). Tissue was digested with 3 mg/ml collagenase type I and 4 mg/ml dispase for 1 hour at 37˚C, centrifuged, and resuspended with medium described above. Cell suspensions were filtered with 100 μm cell strainer and cells were seeded in 6-well plates (Nunc, Thermo Fisher Scientific, Rochester, NY, USA). Subconfluent cultures were passaged using trypsin in phosphate buffered saline (PBS). Cells were transferred to T-25 cell culture flasks when wells were confluent. Cells were cultured 3 - 6 weeks after isolation to increase the number of cells for the experiments.
2.2. Neural Differentiation
Neural differentiation was conducted with protocol published previously [16]. Briefly, dental pulp stem cells (DPSCs) at passage 2 were seeded at 20,000 cells/cm2 in 10 µg/ml mouse laminin (Sigma-Aldrich, St Louis, MO, USA)-coated 24- and 48-well plates (Nunc) and 20,000 cells/well in 0.1% polyethyleneimine (PEI) and 20 µg/ml mouse laminin-coated 6-well MEA-plates (MultiChannel Systems, Reutlingen, Germany). The used differentiation protocol consisted of three stages. Epigenetic reprogramming was induced for 48 h with DMEM/F12 supplemented with 10 µM 5-azacytidine (Sigma-Aldrich), 2.5% FBS (Invitrogen), and 10 ng/ml bFGF (R&D Systems, Minneapolis, MN, USA) starting 24 h after cell seeding. Next, neural induction was conducted with DMEM/F12 supplemented with 250 µM IBMX, 50 µM forskolin, 200 nM TPA, 1% ITS (all from Sigma-Aldrich), 1 mM dbcAMP, 10 ng/ml bFGF, 10 ng/ml NGF, and 30 ng/ml NT-3 (all from R&D Systems) for 3 days. Cells were washed with PBS before neural maturation with Neurobasal medium (Gibco Invitrogen) supplemented with 1 mM dbcAMP (Sigma-Aldrich), 1% N2, 1% B27 without vitamin A (both from Gibco Invitrogen), and 30 ng/ml NT-3 for 7 days. All solutions were freshly prepared prior to use. Control cells were maintained in control medium (DMEM/F12 supplemented with 2.5% FBS and 1% penicillin/streptomycin) which was changed as differentiation mediums.
2.3. Quantitative RT-PCR
RNA samples from DPSCs was collected at three time points, at time 0 (before cell plating), after 3 days of neural induction, and after 7 days of neural maturation. Similar samples were also collected from control cells. Total RNA was isolated using NucleoSpin® RNA XS kit (Macherey-Nagel, Germany) according to manufacturer’s instructions. Next, 50 ng of total RNA per sample was used for cDNA synthesis using random primers (High Capacity cDNA Reverse Transcription Kit, Applied Biosystems) in a reaction volume of 20 μl. Next, quantitative real-time PCR was performed with Taqman® gene expression assays (Applied Biosystems) with 3 µl cDNA according to manufacturer’s instructions in a reaction volume of 15 μl for Musashi for neural progenitor cells (Hs01045984_m1), light neurofilament for neuronal cells (NF68, Hs00196245_m1), glial fibrillary acid protein for astrocytes (GFAP, Hs00909236_m1), brain lipid-binding protein for radial glial cells (BLBP, Hs00361426_ m1), and Olig2 for oligodendrocytes (Hs00377820_m1). GAPDH was used as the internal control (4352934E). Quantitative real-time PCR was performed using the following conditions: 50˚C for 2 min, 95˚C for 10 min, and 40 cycles of 95˚C for 15 s and 60˚C for 1 min with ABI 7300. All samples were analyzed as technical triplicates (variation required less than 0.5 CT) and no-template control was used. The data was analyzed with a 7300 System SDS Software (Applied Biosystems). Relative quantification was calculated using 2–ΔΔCT-method [23]. Gained data was normalized to the expression of GAPDH. The data is presented as mean fold change compared to start point.
2.4. Immunocytochemistry
Samples for immunocytochemistry were collected after neural induction and neural maturation stages. Differentiating DPSCs were fixed with 4% PFA in PBS for 20 min in room temperature (RT). Blocking was conducted with 10% normal donkey serum (NDS), 0.1% TritonX-100, and 1% bovine serum albumin (BSA) in PBS for 45 min at RT. Cells were washed once with 1% NDS, 0.1% TritonX-100, and 1% BSA in PBS and incubated with primary antibodies diluted to the same solution at 4˚C overnight. Antibodies used were mouse anti-nestin (1:100, Chemicon, Temecula, CA, USA) for neural progenitor cells, mouse anti-β-tubulin3 (1:1200, Sigma-Aldrich) and rabbit anti-microtubule associated protein 2 (MAP-2, 1:600, Chemicon) for neuronal cells, sheep anti-GFAP (1:600, R&D Systems) for astrocytes, and mouse anti-GalC (1:200, Chemicon) for oligodendrocytes. All solutions for GalC stainings were made without TritonX-100. After washing three times with 1% BSA in PBS cells were incubated for 1 hour with Alexa Fluor-488 and/or -568 conjugated with anti-mouse, antirabbit, and anti-sheep antibodies (1:400, Molecular Probes/ Invitrogen, Carlsbad, CA, USA). After washing with PBS and phosphate buffer the cells were mounted with Vectashield containing DAPI (4’,6-diamidino-2-phenylindole) (Vector Laboratories Inc., Burlingame, CA, USA). Stained cells were imaged with a phase contrast microscope with fluorescence optics (Olympus IX51, Olympus, Finland) and Olympus DP30BW camera. Images were edited with Adobe Photoshop CS2.
2.5. Microelectrode Arrays
HDPSCs were also cultured on planar microelectrode array (MEA) 6-well plates (6 × 9 electrode layout, electrode diameter 30 µm, inter-electrode distance 200 µm, MultiChannel Systems, Reutlingen, Germany) during neural differentiation (n = 8 wells/differentiating cells/pulp and n = 4 wells/control cells/pulp) as previously described for hESC-derived neurons [8]. Measurements were performed 1 - 2 times/week. Measurements were performed with MEA 1060-Inv-BC-amplifier with integrated TPC Temperature controller adjusted to +37˚C and data was recorded with MC_Rack software (all from Multichannel systems). Prior to measurements, MEA plates were sealed with PDMS discs in laminar hood to keep the cultures sterile for repeated measurements. After executing the differentiation protocol, the cells were maintained on MEA plates for additional 1 - 2 weeks to prolong the measurement period to 4 weeks.
2.6. Positive Control
As a positive control we used hESC-derived neuronal cells differentiated with previously published protocol [24]. Briefly, the cells were fixed and stained as described above after 8 weeks of differentiation as neurospheres and then 3 days on mouse laminin-coated 24- wells. 8 weeks old neurospheres were also plated on PEI and mouse laminin-coated MEA-plates and measured 1 - 2 times/week for 4 weeks.
3. RESULTS
3.1. Isolation and Culture of hDPSCs
The culture time before actual neural differentiation experiment varied from 3 to 6 weeks depending on the cell number after isolation. Morphology of dental pulp stem cells in general varied from spindle-shaped fibroblasts to flat cells as previously shown [25].
3.2. Morphology of hDPSCs during Neural Differentiation
After seeding the cells mostly resembled fibroblasts. There were no differences in the morphology after the epigenetic reprogramming. The control cells had, however, proliferated notably more efficiently. After initiating the neural induction stage, the morphology of the cells of three hDPSC lines over went a change; the cells became more round-shaped and some processes were developed. During the following three days the morphology changed further. Many processes could be detected in the cell populations as well as branched cells with round soma resembling astrocytes, neuronal cells, or oligodendrocytes. Differences between hDPSC lines could be observed. Figure 1(a) represents the induction stages of all 4 hDPSC lines. In induction stage, according to morphology, pulp 2 derived populations resembled mostly neuronal cells. Pulp 4 was clearly different from the others as no clear neuronal morphologies were detected. The neuronal cell morphologies were detected only during neural induction stage. In the neural maturation stage the cell morphology changed again more into fibroblast-like as the number of processes decreased, as represented in Figure 1(b) with pulps 1 and 2.
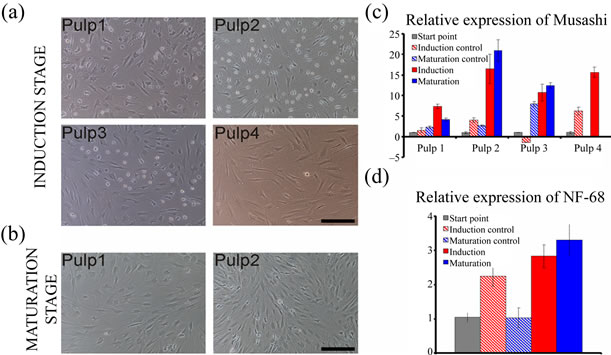
Figure 1. Morphology and gene expression of differentiating DPSC lines. At induction stage: (a) Cells resembling neuronal cells could be detected among pulp 1, 2, and 3 cells but pulp 4 did not seem to be differentiating towards neuronal phenotypes. At maturation stage; (b) The cells of any of the DPSC lines did not seem neuronal whereas more fibroblast-like cells. Increasing Musashi expression; (c) Could be detected with all DPSC lines during neural differentiation protocol whereas NF-68 expression; (d) Could be detected only with pulp 2 cells. Scale in A and B 100 µm.
3.3. Quantitative RT-PCR
The neural progenitor cell marker Musashi was detectable already in non-differentiated cells and the expression increased with all 4 hDPSC lines during differentiation experiment (Figure 1(c)). With three hDPSC lines (pulps 2, 3, and 4) the increment in expression of Musashi was at least 10-fold. In line with hDPSC line morphologies during differentiation experiment, the expression of NF68 was not detected in any of the DPSC lines expect with pulp 2. In this hDPSC line NF68 was expressed already before the differentiation protocol was started and a 3-fold increase in expression could be detected at the maturation stage (Figure 1(d)). The expression of GFAP, Olig2, or BLBP was not detected in any of the hDPSC lines at any timepoints.
3.4. Neuronal Cells Were Not Detected with Immunostaining
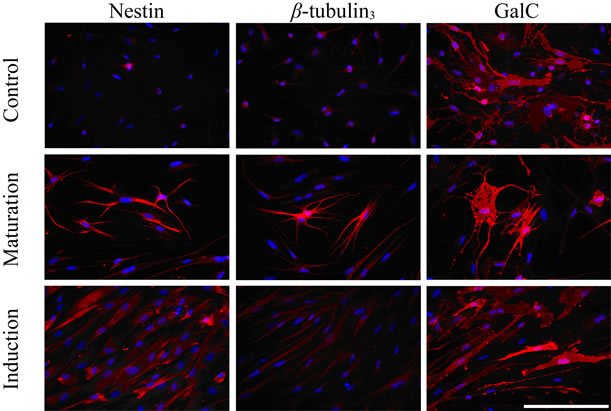
Variation between hDPSC lines was also detected in protein expression. Neural precursor marker nestin could be detected in cultures after maturation stage with all the studied pulps. Also, control cultures were positive for nestin but the morphology of the cells was different (Figure 2(a)). Neuronal marker β-tubulin3 was detected only with pulp 2 after maturation stage but the morphology did not resemble that of neurons (Figure 2(a)). MAP-2 staining was negative in all the cultures. Oligodendrocyte marker GalC stained cells after neural induction and maturation stages in pulp 2 as well as in control cultures (Figure 2(a)). GFAP-positive astrocytes were not detected among any of the differentiated DPSC lines nor control cultures. As a positive control for immunostaining we used hESC-derived neuronal cells which stained positive for both β-tubulin3 and MAP-2 (Figure 2(c)).
3.5. Functional Neuronal Networks Could Not Be Detected
Neuronal network signaling was not detected during 4 weeks follow-up on MEA among hDPSC-derived control or differentiated cultures (Figure 2(b)). Neuronal cells derived from hESCs as a positive control formed functional neuronal networks after 2 weeks of culturing on MEA dishes which could be detected as spikes (Figure 2(c)).
4. DISCUSSION
In the present study we differentiated hDPSCs towards neural phenotype with a previously described protocol [16]. We show that, despite of detecting neural gene expression within the differentiating DPSC lines, protein expression of neural or neuronal markers could not
 (a)
(a)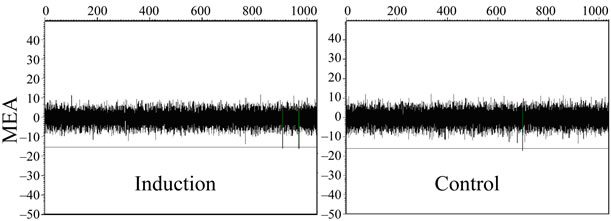 (b)
(b)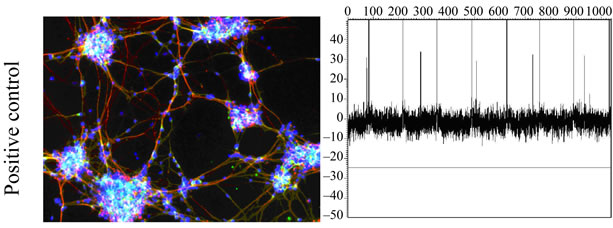 (c)
(c)
Figure 2. (a) Immunostaining of pulp 2-derived cells during neural differentiation. At neural induction stage no cells stained positive for nestin or ß-tubulin3 but some cells stained positive with GalC. At maturation stage some cells stained positive with nestin, ß-tubulin3 and GalC. Positive staining could also be detected in control (undifferentiated) cells. Scale 100 µm; (b) No signals could be detected with MEA thus functional neuronal networks were not forming in differentiated or control hDPSCs; (c) HESC-derived neuronal cells were used as a positive control. These cells stained positive with neuronal markers MAP-2 (green) and ß-tubulin3 (red) and action potentials could be detected in MEA platform.
reliably be observed and the cells did not form electrically active functional neuronal networks.
The efficient neural differentiation of hDPSCs could be clinically relevant due to opening up possibilities to produce patient-specific cells for treating many neurological deficits. Indeed, some groups have already published the production of neural cells from hDPSCs [14-18,26]. The pulp stem cells hold multipotent nature [27] hence neural differentiation should, in principle, be attainable. The solid production of neuronal cells, however, relies on the cells’ electrophysiological properties: capability of forming spontaneous action potentials and further functional neuronal networks. This aspect remains unanswered in previously published articles.
In this study, the DPSCs did not resemble neural cells morphologically prior to differentiation but neuronal phenotypes could be detected at neural induction phase with 3 hDPSC lines, one (pulp 2) being particularly promising. We could detect increasing expression of Musashi in all hDPSC lines during the neural differentiation protocol using quantitative RT-PCR. Musashi, however, was also expressed in DPSCs prior to the onset of the differentiation and thus the validity of Musashi as a neural precursor marker is somewhat questionable. One major challenge, indeed, is the basal expression of neural genes in undifferentiated hDPSCs. For example, a few of previous studies have shown that expression of another neural progenitor marker nestin is negative but the expression of medium sized neurofilament is clearly detectable [14,16] while another study discusses expression of nestin and GFAP in ex vivo-expanded hDPSCs [28]. In contrasts, we could not detect GFAP expression at all. Here, NF-68 was considered as an important gene to study due to its unequivocal presence in neuronal cells. Indeed, increment in NF-68 expression was detected with pulp 2 indicating the presence of neuronal cells. With other pulps, however, expression of this gene was not detected. We studied 4 pulps separately whereas other groups have pooled the pulps collected from several adults [15-18]. To our opinion, the pulp-to-pulp variation should be taken into consideration when collecting cells from human patients.
In immunostaining we could detect nestin positive cells in all pulps after the neural maturation stage which goes hand-in-hand with the musashi expression. ß-tubulin3 could be detected only with pulp 2 after maturation but the morphology of the positive cells was not typical to neuronal cells. We have been differentiating human pluripotent stem cells to neural lineages routinely [24,29] and are familiar with accurate neural and neuronal morphologies and valid staining with neural, neuronal, and glial markers [24]. To support the lack of neuronal cells we could not detect positive MAP-2 staining with any of the hDPSC lines studied. No GFAP-positive astrocytes were detected either. Interestingly, a few cells from pulp 2 stained positive for oligodendrocytic marker GalC whereas gene expression for Olig2 was absent. Whether GalC stains other cell populations in addition to oligodendrocytes remains as an open question as the morphology of the cells was not typical to oligodendrocytes [8,16]. Other published studies do not describe staining with oligodendrocytical markers [14,16,26]. On the other hand, very recent publication shows in vivo differentiation of hDPSC to oligodendrocytes after transplantation in to spinal cord lesion [30] which suggests that hDPSCs have potential to differentiate into this particular neural lineage.
None of the differentiated cultures formed spontaneously active neuronal networks. Previous studies have reported the presence of voltage-activated sodium and potassium currents with patch clamp technique [14,16,21] but no evidence of spontaneous action potentials have been shown. Neuron is considered as a neuron by its capability to form action potentials, thus it is the key aspect to show. Further, single action potential forming cells should be able to form electrically active neuronal networks. We used MEA to detect the neuronal network forming properties of differentiating hDPSCs and could not detect any activity within these cultures. Thus, even though neural progenitor gene expression was detected, expression of neuronal proteins and proper neuronal functionality remained undetected. This indicates that neural progenitor stage cells could be produced but they were not maturing further into neuronal cells. The protocol used here has been previously published reporting neuronal cell differentiation from hDPSCs, but the proper functionality was not shown in that study either [16]. Thus, even though Kiraly and co-workers supplemented their maturation medium with B27 containing vitamin-A [16] whereas our B27 supplement did not contain vitamin-A it is highly unlikely that vitamin-A had effects on the end phenotype of the cells. The pulp-to-pulp variation between the patients is another aspect that should be more thoroughly considered when investigating neural differentiation of hDPSCs.
In this study we show that production of functional neuronal cells from dental pulp stem cells is not as straightforward as suggested in previous studies. Even though neural progenitor cells could be produced as indicated by gene expression, their further maturation into functional neuronal cells was unsuccessful. In our study and in previously published articles the amount of detected neuronal cells has not been great and no one has been able to prove the neuronal functionality. For clinical trials the amount of cells needed is millions (ReNeuron www.reneuron.com, StemCells Inc. www.stemcellsinc.com) and thus it seems that for the time-being the most optimal way for large scale production of human neuronal cells remains with the utilization of human pluripotent stem cells.
5. ACKNOWLEDGEMENTS
Use the personnel of IBT Stem cell unit is acknowledged for the support in stem cell research. The study was funded by TEKES Stem-In-Clin project.
REFERENCES
- Lindvall, O. and Kokaia, Z. (2010) Stem cells in human neurodegenerative disorders: Time for clinical translation? The Journal of Clinical Investigation, 120, 29-40. doi:10.1172/JCI40543
- Thomson, J.A., Itskovitz-Eldor, J., Shapiro, S.S., Waknitz, M.A., Swiergiel, J.J., Marshall, V.S. and Jones, J.M. (1998) Embryonic stem cell lines derived from human blastocysts. Science, 282, 1145-1147. doi:10.1126/science.282.5391.1145
- Takahashi, K., Okita, K., Nakagawa, M. and Yamanaka, S. (2007) Induction of pluripotent stem cells from fibroblast cultures. Nature Protocols, 2, 3081-3089. doi:10.1038/nprot.2007.418
- Yu, J., Vodyanik, M.A., Smuga-Otto, K., AntosiewiczBourget, J., Frane, J.L., Tian, S., Nie, J., Jonsdottir, G.A., Ruotti, V., Stewart, R., Slukvin, I.I. and Thomson, J.A. (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science, 318, 1917-1920.
- Buc-Caron, M.H. (1995) Neuroepithelial progenitor cells explanted from human fetal brain proliferate and differentiate in vitro. Neurobiology of Disease, 2, 37-47. doi:10.1006/nbdi.1995.0004
- Daadi, M.M., Maag, A.L. and Steinberg, G.K. (2008) Adherent self-renewable human embryonic stem cell-derived neural stem cell line: Functional engraftment in experimental stroke model. PLoS ONE, 3, e1644.
- Schwarz, S.C. and Schwarz, J. (2010) Translation of stem cell therapy for neurological diseases. Translational Research, 156, 155-160. doi:10.1016/j.trsl.2010.07.002
- Joyce, N., Annett, G., Wirthlin, L., Olson, S., Bauer, G. and Nolta, J.A. (2010) Mesenchymal stem cells for the treatment of neurodegenerative disease. Regenerative Medicine, 5, 933-946. doi:10.2217/rme.10.72
- Gimble, J.M., Katz, A.J. and Bunnell, B.A. (2007) Adipose-derived stem cells for regenerative medicine. Circulation Research, 100, 1249-1260. doi:10.1161/01.RES.0000265074.83288.09
- Brignier, A.C. and Gewirtz, A.M. (2010) Embryonic and adult stem cell therapy. The Journal of Allergy and Clinical Immunology, 125, S336-S344. doi:10.1016/j.jaci.2009.09.032
- Mesimäki, K., Lindroos, B., Törnwall, J., Mauno, J., Lindqvist, C., Kontio, R., Miettinen, S. and Suuronen, R. (2009) Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. International Journal of Oral and Maxillofacial Surgery, 38, 201-209. doi:10.1016/j.ijom.2009.01.001
- D’Aquino, R., Graziano, A., Sampaolesi, M., Laino, G., Pirozzi, G., De Rosa, A. and Papaccio, G. (2007) Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: A pivotal synergy leading to adult bone tissue formation. Cell Death and Differentiation, 14, 1162-1171. doi:10.1038/sj.cdd.4402121
- Nosrat, I.V., Smith, C.A., Mullally, P., Olson, L. and Nosrat, C.A. (2004) Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. European Journal of Neuroscience, 19, 2388-2398. doi:10.1111/j.0953-816X.2004.03314.x
- Arthur, A., Rychkov, G., Shi, S., Koblar, S.A. and Gronthos, S. (2008) Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells, 26, 1787-1795. doi:10.1634/stemcells.2007-0979
- Ryu, J.S., Ko, K., Lee, J.W., Park, S.B., Byun, S.J., Jeong, E.J., Ko, K. and Choo, Y.K. (2009) Gangliosides are involved in neural differentiation of human dental pulp-derived stem cells. Biochemical and Biophysical Research Communications, 387, 266-271. doi:10.1016/j.bbrc.2009.07.005
- Kiraly, M., Porcsalmy, B., Pataki, A., Kadar, K., Jelitai, M., Molnar, B., Hermann, P., Gera, I., Grimm, W.D., Ganss, B., Zsembery, A. and Varga, G. (2009) Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochemistry International, 55, 323-332. doi:10.1016/j.neuint.2009.03.017
- Karaöz, E., Demircan, P.C., Saglam, O., Aksoy, A., Kaymaz, F. and Duruksu, G. (2011) Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochemistry and Cell Biology, 136, 455-473. doi:10.1007/s00418-011-0858-3
- Nourbakhsh, N., Soleimani, M., Taghipour, Z., Karbalaie, K., Mousavi, S.B., Talebi, A., Nadali, F., Tanhaei, S., Kiyani, G.A., Nematollahi, M., Rabiei, F., Mardani, M., Bahramiyan, H., Torabinejad, M., Nasr-Esfahani, M.H. and Baharvand, H. (2011) Induced in vitro differentiation of neural-like cells from human exfoliated deciduous teethderived stem cells. The International Journal of Development Biology, 55, 189-195. doi:10.1387/ijdb.103090nn
- Heikkilä, T.J., Ylä-Outinen, L., Tanskanen, J.M., Lappalainen, R.S., Skottman, H., Suuronen, R., Mikkonen, J.E., Hyttinen, J.A. and Narkilahti, S. (2009) Human embryonic stem cell-derived neuronal cells form spontaneously active neuronal networks in vitro. Experimental Neurology, 218, 109-116. doi:10.1016/j.expneurol.2009.04.011
- Buzanska, L., Habich, A., Jurga, M., Sypecka, J. and Domanska-Janik, K. (2005) Human cord blood-derived neural stem cell line: Possible implementation in studying neurotoxicity. Toxicology in Vitro, 19, 991-999. doi:10.1016/j.tiv.2005.06.036
- Király, M., Kádár, K., Horváthy, D.B., Nardai, P., Rácz, G.Z., Lacza, Z., Varga, G. and Gerber, G. (2011) Integration of neuronally predifferentiated human dental pulp stem cells into rat brain in vivo. Neurochemistry International, 59, 371-381. doi:10.1016/j.neuint.2011.01.006
- Pine, J. (1980) Recording action potentials from cultured neurons with extracellular microcircuit electrodes. Journal of Neuroscience Methods, 2, 19-31. doi:10.1016/0165-0270(80)90042-4
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods, 4, 402-408. doi:10.1006/meth.2001.1262
- Lappalainen, R.S., Salomaki, M., Yla-Outinen, L., Heikkila, T.J., Hyttinen, J.A., Pihlajamaki, H., Suuronen, R., Skottman, H. and Narkilahti, S. (2010) Similarly derived and cultured hESC lines show variation in their developmental potential towards neuronal cells in long-term culture. Regenerative Medicine, 5, 749-762. doi:10.2217/rme.10.58
- Khanna-Jain, R., Vuorinen, A., Sandor, G.K., Suuronen, R. and Miettinen, S. (2010) Vitamin D3 metabolites induce osteogenic differentiation in human dental pulp and human dental follicle cells. The Journal of Steroid Biochemistry and Molecular Biology, 122, 133-141. doi:10.1016/j.jsbmb.2010.08.001
- Takeyasu, M., Nozaki, T. and Daito, M. (2006) Differentiation of dental pulp stem cells into a neural lineage. Pediatric Dental Journal, 16, 154-162.
- Lindroos, B., Maenpaa, K., Ylikomi, T., Oja, H., Suuronen, R. and Miettinen, S. (2008) Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochemical and Biophysical Research Communication, 368, 329-335. doi:10.1016/j.bbrc.2008.01.081
- Nesti, C., Pardini, C., Barachini, S., D’Alessandro, D., Siciliano, G., Murri, L., Petrini, M. and Vaglini, F. (2011) Human dental pulp stem cells protect mouse dopaminergic neurons against MPP+ or rotenone. Brain Research, 1369, 94-102. doi:10.1016/j.brainres.2010.09.042
- Sundberg, M., Skottman, H., Suuronen, R. and Narkilahti, S. (2010) Production and isolation of NG2+ oligodendrocyte precursors from human embryonic stem cells in defined serum-free medium. Stem Cell Research, 5, 91-103. doi:10.1016/j.scr.2010.04.005
- Sakai, K., Yamamoto, A., Matsubara, K., Nakamura, S., Naruse, M., Yamagata, M., Sakamoto, K., Tauchi, R., Wakao, N., Imagama, S., Hibi, H., Kadomatsu, K., Ishiguro, N. and Ueda, M. (2012) Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. Journal of Clinical Investigation, 122, 80-90.

