Journal of Cosmetics, Dermatological Sciences and Applications
Vol.06 No.01(2016), Article ID:62532,8 pages
10.4236/jcdsa.2016.61001
A Guide to Cheek Augmentation: Single-Point Deep Injection of Hyaluronic Acid Filler at Midface in Close Proximity to Medial Suborbicularis Oculi Fat (SOOF) Area
Chung-Pin Liang1, Haw-Yueh Thong2*
1Department of Dermatology, Chung-Shan University Hospital, Taiwan
2Department of Dermatology, Shin-Kong Wu Ho-Su Memorial Hospital, Taiwan

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 17 December 2015; accepted 1 January 2016; published 4 January 2016
ABSTRACT
Loss of volume in midface can result in an aged, wasted appearance. Osseous and fat atrophy with aging may further contribute to the loss of soft tissue support and midface ptosis. In the aging of periorbital area and midface, fat atrophy occurs mostly in the suborbicularis oculi fat (SOOF) area. The authors proposed that injection of hyaluronic acid (HA) filler to support the SOOF area could counteract the aging sign due to fat atrophy, restore volume loss and achieve a more youthful appearance. The authors described the treatment of 10 female patients who received CHAP®-particle hyaluronic acid (CHAP®-HA) injections for cheek augmentation, using single-point deep injection technique at midface in close proximity to SOOF area. Such approach provides satisfactory cheek augmentation results without significant complications. The authors discussed a rationale for their choice of dermal filler and provided an injection technique for restoring volume in the midface region with CHAP®-HA. Such technique is relatively quick to perform, have little down time, and result in a high rate of patient satisfaction.
Keywords:
Midface Lift, Cheek Augmentation, Fat Compartment, Suborbicularis Oculi Fat (SOOF), Single-Point Deep Injection, Hyaluronic Acid (HA) Filler, CHAP®-Hyaluronic Acid (Crosslinked Hyaluronic Acid Platform, CHAP®-HA), Hyadermis®

1. Introduction
Apart from amelioration of isolated wrinkles and folds, volume restoration and contour enhancement have become the objectives of advanced injectors [1] -[3] . The value of midfacial volume restoration and enhancement has been well documented in the literature. However, when treating this area, the injector can experience adverse events, such as the significant and long-lasting complication of malar edema, nodules and lumps, visible materials, bruising, erythema, pain, infection, skin necrosis, over- and under-correction, and infraorbital nerve injuries resulting in numbness and dysesthesia have been reported [1] - [4] .
Midface Volume Restoration
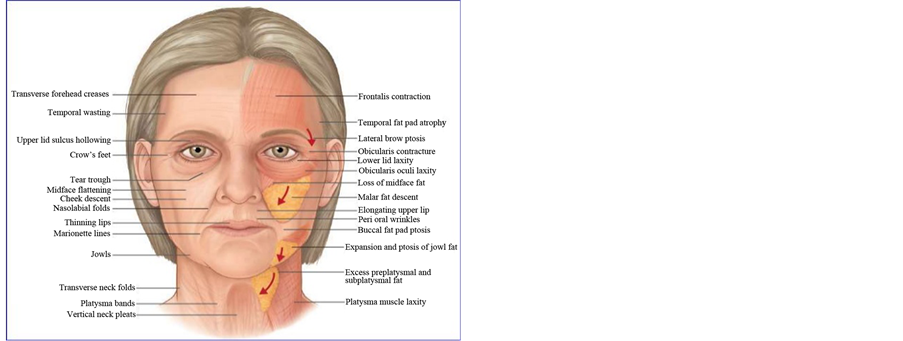
Hyaluronan is a naturally occurring linear polysaccharide which can be found in skin, connective, epithelial, and neural tissues. The amount of naturally occurring hyaluronan in the skin decreases with age, contributing to the development of the aging features and wrinkle formation. The new understanding of the subcutaneous tissue of the face has led to improvements in rejuvenation techniques that focus on the skin and superficial tissue, using fillers and autologous fat [5] . A major advancement that has contributed to the current state of filler injection is the knowledge that the face does not age as one homogenous object but as many dynamic compartments, which need to be evaluated, augmented, and modified as such [6] . Recent advancements in understanding the fat compartments of the face would provide additional insights to dermal filler rejuvenation techniques. By using dermal filler to support distorted fat compartments one would be able to restore a youthful appearance.
The malar fat pad is a discrete, triangular shaped area of thickened subcutaneous fat, based at the nasolabial fold with its apex at the malar eminence in the youthful face [3] . It is attached to the overlying skin and is supported by multiple fibrous septae that extend from the superficial musculoaponeurotic system (SMAS) and into the dermis. Loss of skin elasticity and weakening of these septae, as well as volume loss within the deep medial cheek fat [4] , leads to a downward and forward descent of the skin and malar fat pad until it bulges against the fixed nasolabial fold. These sequelae of aging result in deepening of the nasolabial folds, progressive hollowing of the cheeks, and loss of prominence of the malar eminences. The lower eyelid lengthens, increasing the visibility of orbicularis oculi muscle, coupled with the formation of teartrough and a crescent or “V”-shaped deformity along the maxilla and zygoma, in addition to the recession of the nasal alar cheek junction. Individual fat pockets become discernable as separate entities rather than the smooth transitions from convexities to concavities seen in youth [4] . Rohrich et al. [7] demonstrated that when saline was injected into the deep medial fat compartment, as opposed to superficially in the area of the nasolabial fold, numerous changes occurred, including an increase in anterior projection of the midface, flattening of the nasojugal and nasolabial creases, and an overall improved appearance of the malar region. These changes could all be brought about by manipulating one fat compartment. Therefore, we postulate that the sole correction of midcheek would be an effective way for rejuvenation [4] . Based on Rohrich’s proposed concept, we hypothesized that injecting dermal filler into fat compartment would provide a more youthful appearance in an aging face. The aim of this work is to evaluate the efficacy and safety of single-point deep injection of Hyaluronic acid (HA) fillers in midface in close proximity to medial SOOF for cheek augmentation.
2. Methods
2.1. Material
The Hyadermis® product line (SciVision Biotech Inc., Taiwan ROC), including Hyadermis® Blink, Hyadermis® Kiss, Hyadermis® Smile, Hyadermis® Chic, has been marketed in European Union since 2005 and Taiwan since 2010. Hyadermis® consisted of 20 mg/ml bacterium-derived non-animal stabilized HA. These HA hydrogels are developed using CHAP® (Crosslinked Hyaluronic Acid Platform, CHAP®) technology and have a high gel-to- fluid ratio which accounts for its longevity in performance. Hyadermis® Blink is mainly used to improve fine lines and as skin boosters ; Hyadermis ® Kiss is used for correcting teartrough and lip augmentation, whereas Hyadermis® Smile and Hyadermis® Chic are favorable for contouring facial structure, volumizing medium depth facial wrinkles and lifting tissues. Hyadermis® is one of the most popular dermal filler in Taiwan. In our study, 2 syringes of 1 ml Hyadermis® Smile were used (1ml for each side) for cheek augmentation.
2.2. Subjects
Ten healthy non-pregnant female individuals with mild to moderate midface ptosis, who have never received dermal filler injection received single-point deep injection of Hyadermis® smile at midface in close proximity to medial SOOF area. Treatment area with tattoos, scars, dermatitis, or open wounds and patients with a history of major diseases, diabetes mellitus, HIV infection, connective tissue disease, and malignant disease were excluded. Those with recent (within 3 months) or aesthetic laser/chemical peeling treatment and concurrent use of medication that could disrupt coagulation (e.g., aspirin, NSAIDs, warfarin) were also excluded. All patients provided written informed consent.
2.3. Preparation and Treatment
Before treatment, a thorough evaluation of the patient’s medical history was conducted. Injection point on each side of cheek was chosen based on the following guidelines: gross anatomy which would be in close proximity to medial SOOF area (Figure 1(a) & Figure 1(b)) and four clinical testing, namely pushing test, smiling test, laxity evaluation and snap test (Figure 2), to identify the point with most volume depletion, where dermal filler could be injected with supposedly satisfactory results. Photographic imaging was performed before and after treatment.
2.4. Treatment Protocol
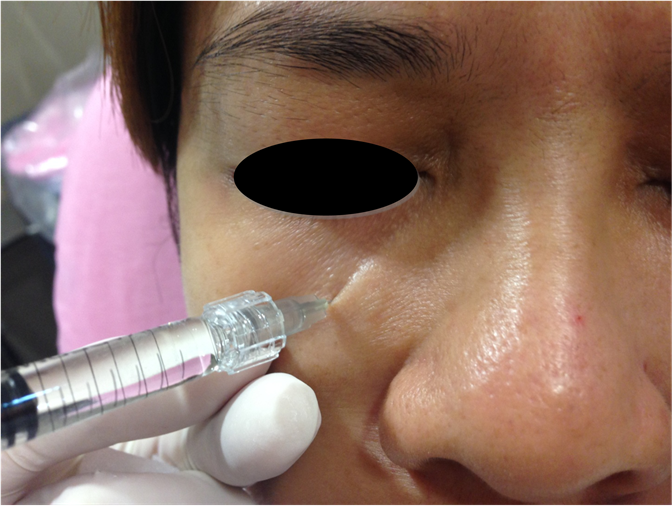
The treatment area was first cleansed with a mild cleanser prior to treatment. All patients were photographed prior to injection. Local anesthetics were applied for 10 minutes. Prior to injection, ultrasound imaging was performed (Philips ultrasound iU22, with L 12-5 MHz probe) to briefly evaluate the presence of blood vessels on the intended injection point. After the confirmation of an absence of major blood vessels, single point injection was performed on both cheek using either sharp needle or micro-cannula, depending on the preference of the subjects (Figure 3). After injection, ultrasound imaging was performed to confirm the depth of injection (Figure 4).
3. Assessments and Analysis
All of the patients underwent single-point deep HA filler injection at the midface region. Photographic documentation of the treated area was recorded prior to and after injection. Ultrasound imaging was also performed prior to and after injection. Efficacy was determined by photographic evaluation, while safety was determined by clinical findings and patients’ report of any discomfort. Adverse events, including evaluation of patient’s response and local skin reaction in the treatment area, were recorded throughout the study. A questionnaire on the pain experience and subjective satisfaction was also performed.
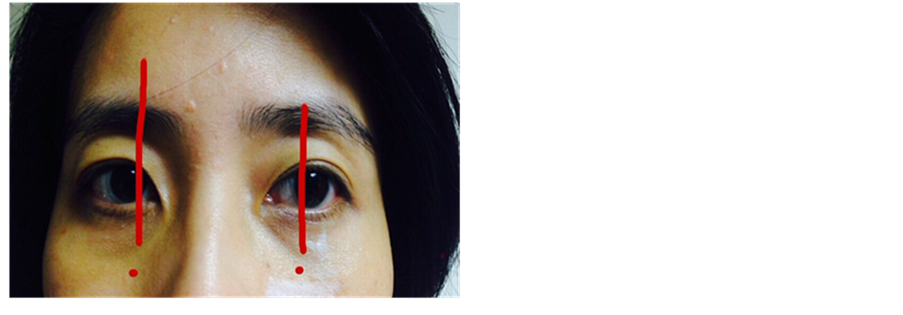
Figure 1. (a) Midface aging due to loss of midface fat and malar fat descent. *SOOF (http://www.slideshare.net/subhakantamohapatra71/facelift-surgery-36146703); (b) Injection points grossly in close proxi- mity to medial SOOF area.
Figure 2. Pushing Test, Smiling Test, Laxity Evaluation and Snap Test are the 4 tests which could be used to identify the point with most volume depletion, where dermal fillers could be injected.
Figure 3. Single point deep injection of HA filler on treatment area using sharp needle on case 5.
4. Results
10 female subjects completed the treatments. All subjects were Taiwanese, with a mean age of 39.5 ± 6.8 years (Table 1). All subjects had mild to moderate midface ptosis, with or without eyebag or teartrough. All subjects received 1 ml each of Hyadermis® Smile with lidocaine, single-point injection using sharp needle or micro- cannula at their preferences, at the midface region grossly in close proximity to medial SOOF area. 6 subjects chose to be injected with sharp needle, whereas 4 subjects decided to be injected with micro-cannula.
Figure 4. Ultrasound imaging of HA filler after injection to confirm depth of filler placement. Arrow = HA filler, * = periosteum.
Table 1. The basic demographics of the subjects and patient satisfaction.
All patients noted satisfactory cheek augmentation immediately after injection, as represented in Table 1 and Figure 5. Treated subjects also noted an improvement in tear trough and eyebag (Figure 6). All subjects were satisfied with the treatment outcome immediately after treatment and at 4-month follow up with phone interview (Table 1). There was no clinically noticeable difference in the cheek augmentation effect and the degree of edema between sharp needle and micro-cannula injections.
Side effects were transient, with the most common side effect being pain and tenderness, and edema on the treatment area which typically resolved within 48 hours post treatment. Minimal bruising was noted with both sharp needle or micro-cannula injections. Pain was generally tolerable. One subject noted intermittent tingling sensation on the left cheek, which resolved within 3 days. This particular subject was injected with sharp needle. No long term complication was noted. All subjects reported to have satisfactory results with no side effects on the 4-month follow up phone interview (Table 1).
Figure 5. Before (upper) and immediately after (lower) single point deep injection of HA filler (1ml on each side) for cheek augmentation using 27 G sharp needle. Satisfactory results were noted with minimal bruising. Left: Case 2, Right: Case 7.
Figure 6. Case 3, before (upper) and immediately after (lower) single point deep injection of HA filler (1 ml on each side) for cheek augmentation using 27 G micro-cannula. Teartrough and eyebag could also be improved with such technique.
5. Discussion
Soft tissue augmentation with temporary dermal fillers is a continuously growing field, supported by the ongoing development and advances in technology and biocompatibility of the products marketed. We hereby presented a new technique of midface lift using a novel CHAP®-HA. Our results showed that such material is safe, and could provide satisfactory midface lifting results.
As proposed by Sandoval et al., the new conceptual understanding of the facial fat will lead to new and improved methods of autologous fat injection [5] . These fat compartments may serve as the “GPS” for the injection of facial fillers. Our study has demonstrated the effect of the placement of fillers in close proximity to single fat compartment and the visual changes created by their augmentation. The safety and efficacy of such single-point deep injections has also been studied. Regrettably, with current real time imaging technology, we were unable to be certain that the filler was injected directly and solely into the SOOF area, but a placement of filler at its close proximity, with adequate injection depth as suggested by ultrasound imaging, was sufficient enough to achieve clinically satisfactory cheek augmentation. There is minimal skin texture change, irregularity, discoloration, ecchymosis, bruising and edema, allowing the subjects to resume their normal activities immediately after treatment. In the present study, adverse cutaneous events are few and range from transient tenderness to intermittent tingling sensation of the cheek possibly due to edema and irritation around the infraorbital nerve. Such complications were mild and self-limiting.
In terms of injection technique, injection with sharp needle is more precise, and less intimidating to the patients, but may have higher chance of vessel and nerve injury; whereas injection with micro-cannula is generally safer but is more technique-dependent to achieve precision. There is no difference between sharp needle and micro-cannula injection in the total amount of HA filler needed to achieve comparable cosmetic results.
6. Conclusion
Single-point deep injection of HA filler at midface in close proximity to medial Suborbicularis Oculi Fat (SOOF) area for cheek augmentation is a technique which is relatively quick and easy to perform, has little down time, and may result in a high rate of patient satisfaction. Adverse events of filler injections include intra-vascular injection, infection, nodules or granuloma formation, hypersensitivity to products and could be avoided by injecting with micro-cannula, proper injection techniques, aseptic techniques, proper plane of injection, and the selection of safe products. Ultrasound evaluation before injection could be a helpful tool to avoid inadvertent intravascular injection and could also be used to confirm the depth of injection. Proper patient selection and communication can lessen over-expectation and are paramount to optimize treatment outcome and patient satisfaction. This pilot study was aimed to elaborate on a simple technique for midface rejuvenation, and was limited by its few case number and hence the inability for statistic analysis. Further prospective studies may be needed to compare the efficacy, safety, and longevity of this technique to other commonly used techniques for the injection of HA fillers in this area of the face.
Disclosures
The authors have no conflict of interest to disclose. There are no funding sources for this work.
Cite this paper
Chung-PinLiang,Haw-YuehThong, (2016) A Guide to Cheek Augmentation: Single-Point Deep Injection of Hyaluronic Acid Filler at Midface in Close Proximity to Medial Suborbicularis Oculi Fat (SOOF) Area. Journal of Cosmetics, Dermatological Sciences and Applications,06,1-8. doi: 10.4236/jcdsa.2016.61001
References
- 1. Werschler, W.P. (2007) Treating the Aging Face: A Multidisciplinary Approach with Calcium Hydroxylapatite and Other Fillers, Part 2. Cosmetic Dermatology, 20, 791-796.
- 2. Busso, M. and Karlsberg, P.L. (2006) Cheek Augmentation and Rejuvenation Using Injectable Calcium Hydroxylapatite (Radiesse R). Cosmetic Dermatology, 19, 583-588.
- 3. Pessa, J.E. and Garza, J.R. (1997) The Malar Septum: The Anatomic Basis of Malar Mounds and Malar Edema. Aesthetic Surgery Journal, 17, 11-17.
http://dx.doi.org/10.1016/S1090-820X(97)70001-3 - 4. Funt, D.K. (2011) Avoiding Malar Edema during Midface/Cheek Augmentation with Dermal Fillers. Journal of Clinical and Aesthetic Dermatology, 4, 32-36.
- 5. Sandoval, S.E., Cox, J.A., Koshy, J.C., Hatef, D.A. and Hollier Jr., L.H. (2009) Facial Fat Compartments: A Guide to Filler Placement. Seminars in Plastic Surgery, 23, 283-287.
http://dx.doi.org/10.1055/s-0029-1242181 - 6. Donofrio, L.M. (2000) Fat Distribution: A Morphologic Study of the Aging Face. Dermatologic Surgery, 26, 1107-1112.
http://dx.doi.org/10.1046/j.1524-4725.2000.00270.x - 7. Rohrich, R.J., Pessa, J.E. and Ristow, B. (2008) The Youthful Cheek and the Deep Medial Fat Compartment. Plastic and Reconstructive Surgery, 121, 2107-2112.
http://dx.doi.org/10.1097/PRS.0b013e31817123c6
NOTES
*Corresponding author.