International Journal of Organic Chemistry
Vol.08 No.01(2018), Article ID:81770,15 pages
10.4236/ijoc.2018.81001
Synthetic Approach for Novel Fluorine Substituted α-Aminophosphonic Acids Containing 1,2,4-Triazin-5-One Moiety as Antioxidant Agents
Mohammed S. T. Makki, Reda M. Abdel-Rahman, Abdulrahman S. Alharbi*
Department of Chemistry, Faculty of Science, King Abdul Aziz University, Jeddah, KSA

Copyright © 2018 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: December 8, 2017; Accepted: January 13, 2018; Published: January 16, 2018
ABSTRACT
Novel fluorine substituted α-amino phosphonic acids containing 1,2,4-triazin- 5-one (6a-f) have been obtained from fluoroacylation of 6-(2ʹ-amino-5ʹ-ni- trophenyl)-3-thioxo-1,2,4-triazin-5(4H)-one (1) followed by ammonilysis to give the corresponding 3-amino-derivative 3. Condensation of compound 3 with nitro/halogenated aromatic aldehydes yielded the Schiff bases 4. The simple addition of diethyl phosphonate to compound 4 produced the α-amino phosphonates 5. Acidic hydrolysis of compound 5 produced the fluorine substituted α-amino acids derivatives 6. Structures of the new compounds have been established with the help of elemental analysis and spectral measurements. Also, the products evaluated as antioxidants, where the fluorinated α-amino phosphonic acids 6 are more active than the other synthesized systems.
Keywords:
Synthetic, Fluorine α-Amino Acids, 1,2,4-Triazin-5-One Moiety, Antioxidants Activity

1. Introduction
Recently, α-amino phosphonic acids and α-amino phosphonates have a vital importance of research chemists [1] [2] [3] , which is due to these family of compounds display, enzymatic inhibitors for HIV protease antagonists [4] and collagenase inhibitors [5] . Also, they use as anticancer [6] , antibacterial [7] , antiviral [8] and antioxidant [9] agents. On the other hands, functionally 1,2,4-triazines have unique properties for biological, medicinal and pharmacological chemistry [10] [11] [12] . Phosphorus compounds bearing and/or containing 1,2,4-triazine moieties exhibit a significant attention due to the specific biological properties [13] [14] [15] [16] [17] . Also, the introduction of fluorine atoms to heterocyclic nitrogen systems, mostly improve their physical, chemical and medical properties [18] [19] [20] [21] . In the present work, we focused on the reactivity of functional 1,2,4-triazines towards different reagents followed by simple addition of diethyl phosphonate to obtain a novel fluorine substituted α-amino phosphonic acids which considered as α-amino acids analog, in view of antioxidant activity.

α-amino phosphonic acids
2. Chemistry
The phosphorylation of amino-organic heterocyclic systems often improves their biological activity because of the P-O bond stores energy for metabolic processes [1] . Also, the chemistry of N-phosphoryl heterocyclic indicates that these compounds form dimensional polymeric chain via intermolecular P-O−・・・・・・+NH hydrogen bond [2] . Moreover, the reactivity of the dipolar ion structures of the tautomeric form of α-amino phosphonates is due to the higher electron-withdrawing properties of two phenoxy and P=O groups. Thus, the α-amino-phosphonate group has a high degree of stability against any reagent attack [3] . To deduce the aims of this work, 6-(2ʹ-Amino-5ʹ-nitrophenyl)- 3-thioxo-1,2,4-triazin-5(2H,4H)-one (1) as a starting material obtained from reflux of 5-nitroisatin with thiosemicarbazide in aq. NaOH (Scheme 1).
Fluoroacylation of compound 1 by boiling with ethyl 2,2,2-trifluoroacetate in THF yielded [22] 2,2,2-trifluoro-N-[2-(5-hydroxy-3-thioxo-2,3-dihydro-1,2,4- triazin-6-yl)-4-nitrophenyl] acetamide (2), which upon ammonilysis by reflux with liquid ammonia in ethanol produced [23] 3-amino-6-[2ʹ-(trifluoroaceta- mido-5ʹʹ-nitrophenyl)]-1,2,4-triazin-5(4H)-one (3) (Scheme 2). Condensation of compound 3 with various nitro and halogenated aromatic aldehydes in boiling ethanol yielded the corresponding Schiff bases 4 (Scheme 2).
The main aims of the present work produce a novel fluorine substituted α-amino acids containing 1,2,4-triazinone moiety. The addition of compounds with phosphorus hydrogen bonds to azomethine (HC=N-Ar) bonds provides an economical method for the synthesis of organophosphorus derivatives. Thus, the addition of diethyl phosphonate to Schiff bases by warm at 80˚C - 100˚C along 6h with a few drops of triethylamine produced [24] α-amino phosphonates 5 which upon acid hydrolysis afforded [25] the novel fluorinated α-amino phosphonic acids 6 as a vital target (Scheme 3). Formation of both compounds 5 & 4
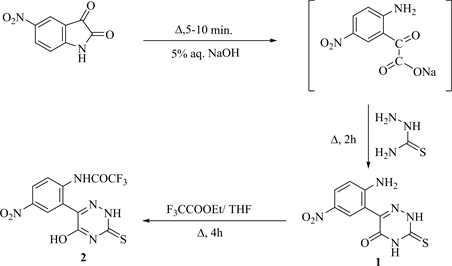
Scheme 1. Synthesis of compounds 1 and 2.

Scheme 2. Synthesis of compounds 3 and 4a-f.
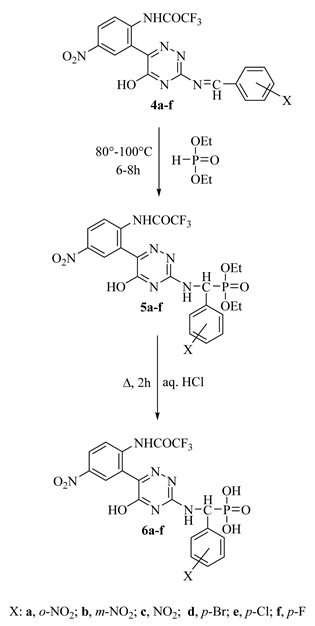
Scheme 3. Synthesis of compounds 5a-f and 6a-f.
may as they shown in Figure 1.
3. Result and Discussion
The former structures of novel fluorinated α-amino phosphonic acids have been deduced from correct their elemental analysis and spectral measurements. IR spectra of both the compounds 1-6 recorded the absorption bands ῡ at 1530, 1350 cm−1for asymmetric and symmetric NO2 groups, 3200 - 3100 and 1660 cm−1 for NH and C=O of 1,2,4-triazine, also ῡ at 1630 cm−1 attribute to CONH group and 1250 cm−1 of C-F functional groups. IR of compound 3 showed ῡ at 3300 and 1610 cm−1 stretching and bending of NH2 group, which lacks in all the compounds 4-6. Also, the presence of ῡ at 1600 - 1580 cm−1 for the exocyclic
Figure 1. Formation of compound 5 from 4.
CH=N group in the compound 4. New functional groups at ῡ 1220 - 1215 (P=O) and 1050 (P-O-R) cm−1 observed in the spectrum of 5. In addition, showed ῡ at 2900 - 2880 and 1480 - 1440 cm−1 for stretching and bending of CH3 & CH2. On the other hand, IR spectrum of 6 showed ῡ at 2730, 2680 cm−1 attribute for two hydroxy groups bonded to the phosphorus atom. All the fluorinated 1,2,4-tri- azinones 2 - 6 showed a stable true hydroxy group at the position-5 of 1,2,4-tri- azines at ῡ 3500 - 3450 cm−1. Presence of these hydroxy groups may be due to a large withdrawing from both NO2, CF3 groups via a type of H-bonding.
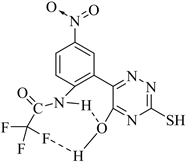
The H-bonding form’s of compound 2.
1H NMR spectra of the novel fluorinated α-amino phosphonic acids give us a good indication of what those structures. Thus, 1H NMR spectra of compound 1 exhibit δ at 3.5, 13.0, 11.8 ppm for NH2, NH, NH of 1,2,4-triazinone, in addition to δ 8.8, 8.2, 7.9 ppm for aromatic protons. 1H NMR spectra of 2 & 4 recorded a lack’s of NH2 protons while showed δ at 9.22 ppm for methin proton (-CH=N-) in compound 4. Also, 1H NMR spectrum of compound 5 showed a new resonated signal at δ 1.2(J = 6.8 Hz) and large signal 4.0 - 3.9 ppm for OCH2CH3 protons, with a broad signal at 3.0 - 2.95, 2.7, 2.5 and 1.05, 1.03 ppm for CH2 & CH3 protons. 1H NMR spectrum of compound 6f showed lacks both NH protons which is due to a type of F….H bond while reporting the signal at 4.95 ppm attribute to OH proton. 1H NMR spectra of all new synthetic compounds 1 - 6 recorded the δ at 11.8 ppm for internal NH of 1,2,4-triazine at position-4, and 8.55 ppm for NHCO protons. 31P NMR (DMSO) of the 6f exhibit resonated signals at δ 14.0 (O=P-OH) and 20.5 (P-CH) ppm. Also, 1H NMR spectra of 6 recorded the P-CHAr proton at tow doublets at 4.55 (JPCH = 21 Hz) and 4.64 (JPCH = 18 Hz) ppm while that showed the P-OH protons at 3.00 ppm which supported the existence of that structures. 13C NMR spectrum of compound 5 supported their structure due to the presence of the characteristic carbon atoms at δ 16, 60.1, 45.2 and 177.5 ppm attributed to CH3, CH2, CH-P and C=O of 1,2,4-tri- azines. In addition, signals at δ 155 and 130 - 127 ppm for CONH and aromatic carbons. The aliphatic carbons CH2, CH3 of compound 5 disappeared in that of compound 6. Finally, mass spectrometry study of novel fluorinated α-amino phosphonic acids, for example, 6band 6f recorded a molecular ion fragments at low intensity, with base peaks at m/e 231 for (6b) and m/e 95 for (6f) attribute to α-amino phosphonic radicals (Figure 2 & Figure 3).
The higher stability of their base peak may be due to the tautomeric forms present and the free delocalization from HN to P=O centers (Figure 4).
4. Experimental
The melting point recorded on Stuart scientific SMP3 (Bibby, UK) melting point
Figure 2. Mass fragmentation pattern of compound 6b.
Figure 3. Mass fragmentation pattern of compound 6f.
Figure 4. The stability of α-amino phosphonic acids 6b.
apparatus and reported as uncorrected. A Perkin Elmer (Lambda EZ-2101) double beam spectrophotometer (190 - 1100 nm) used for recording the electronic spectra. A Perkin Elmer model RXI-FT-IR 55,529 cm−1 used for recording the IR spectra. A Brucker advance DPX 400 MHz using TMS as an internal standard for recording the 1H/13C NMR spectra in deuterated DMSO (δ in ppm). AGC-MS-QP 1000 Ex model used for recording the mass spectra. Hexafluorobenzene used as an external standard for 19FNMR at 84.25 MHz and 31P (in CDCl3, 101.25 MHZ). Elemental analysis performed on Micro Analytical Center of National Reaches Center-Dokki, Cairo, Egypt.
6-(2ʹ-Amino-5ʹ-nitrophenyl)-3-thioxo-1,2,4-triazin-5(2H,4H)one (1) [26]
A mixture of 5-nitroisatin (0.1 mol, in 100 ml of 5% aq. NaOH) and thiosemicarbazide (0.1 mol, in 10 ml hot water) refluxed for 2 h, cooled then poured onto ice-AcOH. The solid produced filtered off, and crystallization from MeOH to give compound 1 as reddish brown solid, yield 85%, m.p. 290˚C - 291˚C. IR spectrum ῡ(cm−1): 3255(NH), 3138(NH), 3082(NH), 3020(aromatic CH), 1608 (binding NH2), 1595(C=N), 1509, 1310(asym. & sym. NO2), 1173(C=S), 837, 817, 750 (aromatic CH). Calculated C9H7N5O3S(M+ 265): C, 40.75; H, 2.66; N, 26.40; S, 12.09%. Found: C, 40.34; H, 2.60; N, 26.31; S, 11.99%.
2,2,2-Trifluoro-N-[2-(5-hydroxy-3-thioxo-2,3-dihydro-1,2,4-triazin-6-yl)- 4-nitrophenyl]acetamide (2)
Equimolar amounts of compound 1 and ethyl 2,2,2-trifluoroacetate in THF (100 ml) refluxed for 4h, cooled. The solid obtained filtered off and crystallized from EtOH to give compound 2 as deep green solid, yield 76%, m.p: 270˚C - 272˚C. IR spectrum ῡ(cm−1): 3448(OH), 3324, 3191(NH), 3080(aromatic CH), 1703(COCF3), 1615(C=N), 1519, 1321(asym. & sym. NO2), 1242(C-F), 850, 810, 780(aromatic CH). 1H NMR(400 MHz, DMSO-d6) δ(ppm): 14.64(s, 1H, OH), 13.72(s, 1H, NH), 12.45(s, 1H, NH), 9.1, 8.9, 8.5, 8.3(m, 4H, aromatic protons), 3.56(s, OH).13C NMR(100 MHz, DMSO-d6) δ(ppm): 173.32(C=S), 153.61, 153.75(2C=O), 145.7, 143.10(C-F), 135.07(NCN), 128.0-121(aromatic carbons), 118.47, 114.54, 113.52(C5, C6 of 1,2,4-triazine). Calculated C11H6F3N5O4S(M+ 361): C, 36.57; H, 1.67; F, 15.78; N, 19.39; S, 8.87%. Found: C, 36.40; H, 1.61; F, 15.49; N, 19.15; S, 8.75%.
3-Amino-6-[2ʹ-(trifluoroacetamido-5ʹʹ-nitrophenyl)]-1,2,4-triazin-5(4H) one (3)
A mixture of 2(0.1 mol) and a liquid NH3(20 ml, 39%), with ethanol (100 ml), refluxed 6 h, cooled then poured onto ice-drops AcOH. The resulting solid, filtered off and crystallized from EtOH to give light green solid, yield 86%, m.p: 305˚C - 307˚C. IR spectrum ῡ(cm−1): 3448(OH), 3323(NH), 3090(NH), 1702 (C=O), 1615(deformation NH2), 1557(C=N), 1531, 1312(asym. & sym. NO2), 1241(C-F), 988, 830, 749(aromatic CH), 607(C-F). 1H NMR(400 MHz, DMSO-d6) δ(ppm): 13.59, 12.41(each s, 2NH), 9.1(1H, OH-triazine), 8.95 - 8.60, 8.53 - 8.35 (each d, d, 2H, aromatic adjacent of NO2), 8.27 - 8.01, 7.99 - 6.76(d, d 2H, aromatic), 3.44(s, 2H, NH2), protons. 13C NMR(100 MHz, DMSO-d6) δ(ppm): 173.3(C=O), 153, 152(C-O), 145(C-F), 153(NCN), 128 - 121(aromatic carbons), 114, 113(triazine). Calculated C11H7F3N6O4(M+ 344): C, 38.38; H, 2.05; F, 16.56; N, 24.42%. Found: C, 38.18; H, 1.99; F, 16.36; N, 24.21%.
Schiff bases 4a-f
A mixture of 3 (0.01 mol) and (o-, m-, p-nitrobenzaldehydes, p-bromo, p-chloro, and p-fluoro benzaldehydes) (0.01 ml) refluxed in AcOH (50 ml) for 1h, cooled then poured onto ice. The yielded solids filtered off and crystallized from a suitable solvent (EtOH, MeOH & Isopropyl alcohol) to give 4a-f.
4a: Brown green solid, yield 83%, m.p: 270˚C - 272˚C. IR spectrum ῡ(cm−1): 3447(OH), 3323(NH), 3080(aromatic CH), 1703(C=O), 1615(C=C), 1557(C=N), 1479(exo CH=N), 1519, 1322(asym., sym. NO2), 1270(C-F), 927, 831, 749(aromatic CH), 607(C-F). Calculated C18H10F3N7O6(M+ 477): C, 45.29; H, 2.11; F, 11.94; N, 20.54%. Found: C, 44.78; H, 2.08; F, 11.79; N, 20.27%.
4b: Deep brown solid, yield 76%, m.p: 268˚C - 270˚C. IR spectrum ῡ(cm−1): 3446(OH), 3322.55(NH), 3083(aromatic CH), 1702.56(C=O), 1615.21(C=N), 1531, 1307(asym., sym. NO2), 1478(CH=N), 1453, 1427(deformation CH=N), 1270(C-F), 1239(C-F), 987, 928, 830, 749(aromatic CH), 607(C-F). 1H NMR(400 MHz, DMSO-d6) δ(ppm): 10.13(s, 1H, NH), 12.42 - 12.23(NHCO), 10.13(s, 1H, CH=N), 9.2 - 8.5, 8.4 - 6.75(each d, d, 8H, aromatic protons), 3.48(s, 1H, OH). 13C NMR(100 MHz, DMSO-d6) δ(ppm): 173(C=O), 153(CONH), 145(C-F), 137(CH=N), 130-123(aromatic carbons), 114, 113(carbons of 1,2,4-triazine). Calculated C18H10F3N7O6(M+ 477): C, 45.29; H, 2.11; F, 11.94; N, 20.54%. Found: C, 44.89; H, 2.00; F, 11.81; N, 20.39%.
4c: Brown green solid, yield 88%, m.p: 274˚C - 276˚C IR spectrum ῡ(cm−1): 3447(OH), 3324(NH), 3084(aromatic CH), 1703(C=O), 1615(C=C), 1557(C=N), 1479(exo CH=N), 1531, 1322(asym., sym. NO2), 1270(C-F), 900, 880, 850 (aromatic CH), 610(C-F). Calculated C18H10F3N7O6(M+ 477): C, 45.29; H, 2.11; F, 11.94; N, 20.54%. Found: C, 44.71; H, 2.01; F, 11.64; N, 20.12%.
4d: Brown solid, yield 79%, m.p: 260˚C - 262˚C. IR spectrum ῡ(cm−1): 3448 (OH), 3324(NH), 3085(NH), 1704(C=O), 1616(C=C),1558(C=N), 1480(exo CH=N), 1515, 1324(asym. & sym. NO2), 1272(C-F), 988, 929, 749(aromatic CH), 690(C-Br), 640(C-F). Calculated C18H10BrF3N6O4(M+ 509): C, 42.29; H, 1.97; Br, 15.63; F, 11.15; N, 16.44%. Found: C, 41.97; H, 1.71; Br, 15.54; F, 10.99; N, 16.24%.
4e: Brownish yellow solid, yield 77%, m.p: 267˚C - 269˚C. IR spectrum ῡ(cm−1): 3447(OH), 3320(NH), 3089(NH), 1701(C=O), 1614(C=C), 1553(C=N), 1477(exo CH=N), 1516, 1322(asym. & sym. NO2), 1269.9(C-F), 986, 929, 749.7(aromatic CH), 688(C-Cl), 646(C-F). Calculated C18H10ClF3N6O4(M+ 466): C, 46.32; H, 2.16; Cl, 7.59; F, 12.21; N, 18.01%. Found: C, 45.89; H, 2.13; Cl, 7.28; F, 11.96; N, 17.71%.
4f: Reddish brown solid, yield 84%, m.p: 271˚C - 273˚C. IR spectrum ῡ(cm−1): 3446(OH), 3321(NH), 3081(NH), 1702(C=O), 1557(C=N), 1517, 1320(asym. & sym. NO2), 1239(C-F), 928, 931, 749(aromatic CH), 647(C-F). 1H NMR(400 MHz, DMSO-d6) δ(ppm): 13.44(s, 1H, NH), 9.52(s, 1H, CH=N), 8.35 - 7.99 & 7.01 - 6.75(each d, d 7H, aromatic protons), 3.41(s, 1H, OH of C5-1,2,4-triazine). 13C NMR(100 MHz, DMSO-d6) δ(ppm): 173(C=O), 153, 152(C-OR), 145.7(C-F), 135(NCN of 1,2,4-triazine), 128-126(aromatic carbons), 114, 113(C5, C6 of 1,2,4-triazine). Calculated C18H10F4N6O4(M+ 450): C, 48.01; H, 2.24; F, 16.88; N, 18.66%. Found: C, 47.83; H, 2.19; F, 16.66; N, 18.46%.
Diethyl [6-(2ʹ-trifluoroacetamido-5ʹ-nitrophenyl)-5-hydroxy-1,2,4-triazin- 3-yl]-amino-(aryl) methyl phosphonates (5a-f)
A mixture of 4a-e and/ or 4f (0.01 mol) and diethyl phosphonate (0.01 mol) in few drops of TEA, fused at 80˚C - 100˚C for 6 - 8 h, cooled the treated with dioxan. The solid obtained crystallized from a suitable solvent to give 5a-f.
5a: Deep brown solid, yield 82%, m.p: 263˚C - 265˚C. IR spectrum ῡ(cm−1): 3447(OH), 3321(NH), 3085(aromatic CH), 2970(aliphatic CH), 1701(C=O), 1615(C=N), 1532, 1309(asym., sym. NO2), 1481, 1428(deformation CH2, CH3), 1237(C-F), 1159(P=O), 1100(O-P-O-R), 840, 805(aromatic CH), 610(C-F). Calculated C22H21F3N7O9P(M+ 615): C, 42.94; H, 3.44; F, 9.26; N, 15.93; P, 5.03%. Found: C, 42.59; H, 3.38; F, 9.15; N, 15.70; P, 4.93%.
5b: Deep brown solid, yield 78%, m.p: 260˚C - 262˚C. IR spectrum ῡ(cm−1): 3433(OH), 3316(NH), 3083(aromatic CH), 2969(aliphatic CH), 1698(C=O), 1616(C=N), 1532, 1311(asym., sym. NO2), 1481, 1428(deformation CH2, CH3), 1245(C-F), 1159(P=O), 1098(O-P-O-R), 860, 810(aromatic CH), 600(C-F). 1H NMR(400 MHz, DMSO-d6) δ(ppm): 13.59(NH), 8.37(CH-NH), 8.36 - 8.0, 7.99 - 6.76(each d, d, aromatic protons), 3.82(s, 1H, OH), 3.76 - 3.44(b, NH), 2.89, 2.53 & 1.05, 1.03(each s, 2 CH2 & CH3). 13C NMR(100 MHz, DMSO-d6) δ(ppm): 173.3(C=O), 153, 152(C-OR), 145.80(C-F), 135(NCN), 128 - 126(aromatic carbons), 114, 113.59(C5, C6 of 1,2,4-triazine), 38.30(carbons of CH2, CH3). Calculated C22H21F3N7O9P(M+ 615): C, 42.94; H, 3.44; F, 9.26; N, 15.93; P, 5.03%. Found: C, 42.66; H, 3.25; F, 9.11; N, 15.78; P, 4.85%.
5c: Brown sold, yield 87%, m.p: 270˚C - 272˚C. IR spectrum ῡ(cm−1): 3446(OH), 3320(NH), 3082(aromatic CH), 2970(aliphatic CH), 1700(C=O), 1614(C=N), 1528, 1310(asym., sym. NO2), 1477, 1427(deformation CH2, CH3), 1238(C-F), 1159(P=O), 1104(O-P-O-R), 850, 810(aromatic CH), 608(C-F). Calculated C22H21F3N7O9P(M+ 615): C, 42.94; H, 3.44; F, 9.26; N, 15.93; P, 5.03%. Found: C, 42.69; H, 3.44; F, 9.26; N, 15.93; P, 5.03%.
5d: Deep brown solid, yield 92%, m.p: 302˚C - 305˚C. IR spectrum ῡ(cm−1): 3434(OH), 3317(NH), 3081(aromatic CH), 2970(aliphatic CH), 1700(C=O), 1553(C=N), 1532, 1311(asym. & sym. NO2), 1479, 1427(deformation aliphatic), 1242(C-F), 1223(P=O), 1100(P-O-Et) 987, 780, 749(aromatic CH), 700(C-Br), 605(C-F). Calculated, C22H21BrF3N6O7P(M+ 649): C, 40.70; H, 3.26; Br, 12.31; F, 8.78; N, 12.94; P, 4.77%. Found: C, 40.39; H, 3.11; Br, 12.16; F, 8.61; N, 12.80; P, 4.66%.
5e: Black brown solid, yield 86%, m.p: 296˚C - 298˚C. IR spectrum ῡ(cm−1): 3434(OH), 3317(NH), 3084(aromatic CH), 2970(aliphatic CH), 1700(C=O), 1553(C=N), 1532, 1311(asym. & sym. NO2), 1480, 1427(deformation aliphatic), 1242(C-F), 1210(P=O), 1099(P-O-Et) 987, 780, 749(aromatic CH), 730(C-Cl), 600(C-F). Calculated C22H21ClF3N6O7P(M+ 604): C, 43.69; H, 3.50; Cl, 5.86; F, 9.42; N, 13.89; P, 5.12%. Found: C, 43.48; H, 3.43; Cl, 5.57; F, 9.22; N, 13.69; P, 4.98%.
5f: Light brown solid, yield 78%, m.p: 285˚C - 287˚C. IR spectrum ῡ(cm−1): 3447(OH), 3284(NH), 3198(NH), 3084(aromatic CH), 2970(aliphatic CH), 1695(C=O), 1556(C=N), 1516, 1304(asym. & sym. NO2), 1478, 1427(deformation aliphatic), 1240(C-F), 1222(P=O), 1070(P-O-Et) 983, 787, 749(aromatic CH), 608(C-F). 1H NMR(400 MHz, DMSO-d6) δ(ppm): 12.25(s, 1H, NH), 10.76(s, 1H, OH), 9.9(s, 1H, CH-N), 9.05 - 8.01 & 7.99 - 6.54(each d, d 7H, aromatic protons), 3.44(s, 1H, OH), 2.52, 1.24(each m, 10H, O-CH2CH3).13C NMR(100 MHz, DMSO-d6) δ(ppm): 173(C=O), 152(C-OH), 150(C-NH), 147(C-F), 145(C6 of 1,2,4-triazine), 142(C-NO2), 137(C3 of 1,2,4-triazine)130 - 126(aromatic carbons), 116(Ar-CH-P), 113(CH2-O), 40(CH3-CH2). Calculated C22H21F4N6O7P(M+ 588): C, 44.91; H, 3.60; F, 12.92; N, 14.28 P, 5.26%. Found: C, 44.66; H, 3.58; F, 12.71; N, 14.11 P, 5.01%.
[((6-(5ʹ-nitro-2ʹ-(2ʹʹ,2ʹʹ,2ʹʹ-trifluoroacetamido)phenyl)-5-oxo-2,5-dihydro-1,2,4-triazin-3-yl)amino)(phenyl)methyl]phosphonic acids(6a-f)
A mixture of 5 (0.01 mol) and dil. HCl (10 ml, 5%) refluxed for 2 h, cooled, then neutralized with diluted NaHCO3. The solid poured, filtered off and crystallized from suitable solvents to give (6a-f).
6a: Brown solid, yield 85%, m.p: 282˚C - 284˚C. IR spectrum ῡ(cm−1): 3446(OH), 3321(NH), 3080(aromatic CH), 2970(aliphatic CH), 1701(C=O), 1614(C=N), 1557, 1305(asym., sym. NO2), 1269(C-F), 1160(P=O), 1090(O-P-O), 980, 840(aromatic CH), 610(C-F). Calculated C18H13F3N7O9P(M+ 559): C, 38.65; H, 2.34; F, 10.19; N, 17.53; P, 5.54%. Found: C, 38.41; H, 2.08; F, 9.99; N, 17.30; P, 5.28%.
6b: Brown solid, yield 94%, m.p: 289˚C - 291˚C. IR spectrum ῡ(cm−1): 3447(OH), 3323(NH), 1702(C=O), 1615(C=N), 1531, 1308(asym., sym. NO2), 1270(C-F), 1159(P=O), 1090(O-P-O), 987, 850(aromatic CH), 606(C-F). 1H NMR(400 MHz, DMSO-d6) δ(ppm): 13.5, 13.19, 12.24(each s, 3NH), 9.0(s, 1H, CH-P), 8.61 - 8.14, 7.98 - 6.75(each d, d, 7H, aromatic protons), 5.95(s, 1H, P-OH). 13C NMR(100 MHz, DMSO-d6) δ(ppm): 173.3(C=O), 153, 152(C-O), 145.80(C-F), 135.1(NCN), 128.02 - 126.68(aromatic carbons), 114.48, 113.48 (carbons of 1,2,4-triazine). Calculated C18H13F3N7O9P(M+ 559): C, 38.65; H, 2.34; F, 10.19; N, 17.53; P, 5.54%. Found: C, 38.38; H, 2.11; F, 9.98; N, 17.35; P, 5.39%. M/S(Int.%): 555(M+3, 1.00), 234(11.11), 231(100), 136(18.2), 69(5.00).
6c: Deep brown solid, yield 88%, m.p: 273˚C - 275˚C. IR spectrum ῡ(cm−1): 3446(OH), 3323(NH), 3078(aromatic CH), 2970(aliphatic CH), 1704(C=O), 1615(C=N), 1555, 1307(asym., sym. NO2), 1271(C-F), 1158(P=O), 1093(O-P-O), 970, 825(aromatic CH), 604(C-F). Calculated C18H13F3N7O9P(M+ 559): C, 38.65; H, 2.34; F, 10.19; N, 17.53; P, 5.54%. Found: C, 38.40; H, 2.13; F, 10.01; N, 17.40; P, 5.33%.
6d: Deep brown solid, yield 78%, m.p: 251˚C - 253˚C. IR spectrum ῡ(cm−1): 3447(OH), 3323(NH), 3080(aromatic CH), 2970(aliphatic CH), 1702(C=O), 1614(C=N), 1557, 1304(asym. & sym. NO2), 1269(C-F), 1218(C-P=O), 1060 (P-O-H) 975, 900, 880, 790(aromatic CH), 729(C-Br), 600(C-F). Calculated C18H13BrF3N6O7P(M+ 593): C, 36.45; H, 2.21; Br, 13.47; F, 9.61; N, 14.17; P, 5.22%. Found: C, 36.15; H, 2.15; Br, 13.47; F, 9.39; N, 14.05; P, 5.11%.
6e: Deep brown solid, yield 74%, m.p: 263˚C - 265˚C. IR spectrum ῡ(cm−1): 3446(OH), 3321(NH), 3080(aromatic CH), 2970(aliphatic CH), 1702(C=O), 1615(C=N), 1557, 1307(asym. & sym. NO2), 1269(C-F), 1219(C-P=O), 1060 (P-O-H) 980, 900, 870, (aromatic CH), 748(C-Cl), 605(C-F). Calculated C18H13ClF3N6O7P(M+ 548): C, 39.40; H, 2.39; Cl, 6.46; F, 10.39; N, 15.32; P, 5.64%. Found: C, 39.15; H, 2.19; Cl, 6.31; F, 10.15; N, 15.20; P, 5.45%.
6f: Brown solid, yield 83%, m.p: 245˚C - 247˚C. IR spectrum ῡ(cm−1): 3448(OH), 3325(NH), 3088(NH), 1703(C=O), 1616(C=N), 1517, 1324(asym. & sym. NO2), 1240(C-F), 1216(C-P=O), 1060(P-O-H) 988, 910, 880, 790(aromatic CH), 607(C-F). 1H NMR(400 MHz, DMSO-d6) δ(ppm): 8.35(s, 1H, CH-N), 8.04 - 7.48 & 7.47 - 6.75(each d, d, 7H, aromatic protons), 4.98(s, 1H, OH), 4.95(s, 1H, OH), 4.011(s, 1H, OH). 13C NMR(100 MHz, DMSO-d6) δ(ppm): 173.44 (C=O), 153, 152(C-O), 145.81(C-F), 135.12(NCN), 129.28 - 126.47(aromatic carbons), 114.68, 113.67(C5, C6 of 1,2,4-triazine). Calculated C18H13F4N6O7P(M+ 532): C, 40.62; H, 2.46; F, 14.28; N, 15.79; P, 5.82%. Found: C, 40.45; H, 2.30; F, 14.12; N, 15.56; P, 5.69%. M/S(Int.%): 534(M+2, 5.18), 234(43.1), 204(80.33), 136(22.15), 121(8.9), 96(12.15), 95(100).
5. The Antioxidant Evaluation
1,1-Diphenyl-2-picrylhydrazyl (DPPH) use to produce and reduce the odd electron stable-free radical which showed a strong UV-absorption maximum at λ = 517 nm. The new systems obtained dissolved in DMSO/EtOH at 150 & 300 μmol∙L−1 added to DPPH at 100 μmol∙L−1. The tube kept at room temperature for 20 minutes and the absorption measured at λ 517 nm. The difference between the test and the control taken as the percent scavenging of the DPPH radical by use the formula: % inhibition =
where AB: absorption of blank; AA: absorption of the tested compound. The radical scavenging activity of ascorbic acid also measured and compared with that of the different synthesized compounds [27] . The observed data on the antioxidant-activated of the compounds and control shown in Table 1.
From the results obtained (Table 1) we can conclude that:
Presence of CF3 and NO2 of 6-aryl-1,2,4-triazinone and α-amino phosphonate bearing 3-substituted amino-1,2,4-triazinones deployed a good to perfect scavenging activities. The ordering activity is 6f > 6e > 6d > 6b > 6a > 6c, which mainly attribute to the presence of C-halogen and C-NO2 of aryl-amino derivatives. The activity of α-amino phosphonic acids 6 is higher than the activity of α-amino phosphonates 5. Also, high activity of 6f comparing with the other systems is may be due to a rich of fluorine atoms bonded to a 1,2,4-triazinone moiety, and the phosphonate groups are the potent antioxidant agent.
6. Conclusion
In the search for new antioxidant agents, the present work reports a simple route to synthetic novel fluorine substituted nitroaryl-1,2,4-triazine bearing α-amino phosphonic acids. Presence of rich aliphatic/aromatic fluorine atoms and nitro
Table 1. The DPPH radical scavenging activity of the novel fluorinated α-amino phosphonates and the related α-amino phosphonic acids at 150 and 300 μmol∙L−1.
groups bonded to 1,2,4-triazinone bearing α-amino phosphonic acids enhance the antioxidant activities, which may use the feature of medicinal treatments.
Cite this paper
Makki, M.S.T., Abdel-Rahman, R.M. and Alharbi, A.S. (2018) Synthetic Approach for Novel Fluorine Substituted α-Aminophosphonic Acids Containing 1,2,4-Triazin-5-One Moiety as Antioxidant Agents. International Journal of Organic Chemistry, 8, 1-15. https://doi.org/10.4236/ijoc.2018.81001
References
- 1. Abdel-Rahman, R.M., Ali, T.E. and Abdel-Kariem, S.M. (2016) Methods for Synthesis of N-Hetrocyclyl/Hetroaryl-α-Aminophosphonates and α-(Azahetrocyclyl) Phosphonates. ARKIVOC, 1, 183-211.
- 2. Gholivand, K., Shariatinia, Z., Mahzouni, H.R. and Amiri, S. (2007) Phosphorus Heterocycles: Synthesis, Spectroscopic Study and X-Ray Crystallography of Some New Diazaphosphorinanes. Structural Chemistry, 18, 653-660. https://doi.org/10.1007/s11224-007-9197-3
- 3. Abdel-Rahman, R.M. and Ali, T.E. (2013) Synthesis and Biological Evaluation of Some New Polyfluorinated 4-Thiazolidinone and α-Aminophosphonic Acid Derivatives. Monatsheftefür Chemie—Chemical Monthly, 144, 1243-1252. https://doi.org/10.1007/s00706-013-0934-6
- 4. Peyman, A., Budt, K.H., Spanig, J., Stowasser, B. and Ruppert, D. (1992) C2-Symmetric Phosphinic Acid Inhibitors of HIV Protease. Tetrahedron Letters, 33, 4549-4552. https://doi.org/10.1016/S0040-4039(00)61309-6
- 5. Markwell, R. (2000) Aminophosphonic and Aminophosphinic Acid Derivatives as Inhibitors of Human Collagenase. In: Kukhar, V.P. and Hudson, H.R., Eds., Amino-phosphonic and Aminophosphinic Acids: Chemistry and Biological Activity, Wiley, Chichester, 579-621.
- 6. Kenawy, E.-R.S., Azaam, M.M. and Saad-Allah, K.M. (2015) Synthesis and Antimicrobial Activity of α-Aminophosphonates Containing Chitosan Moiety. Arabian Journal of Chemistry, 8, 427-432. https://doi.org/10.1016/j.arabjc.2013.12.029
- 7. Reddy, Y.H., Kumar, B.S., Reddy, G.C., Dadapeer, E. and Reddy, K.S. (2012) Synthesis and Bioassay of α-Aminophosphonates. Der Chemica Sinica, 3, 817-823.
- 8. Zhang, Y., Bai, S., Song, B., Bhadury, P.S., Hu, D., Yang, S., Zhang, X., Fan, H. and Lu, P. (2010) Enantioseparation and Plant Virucidal Bioactivity of New Quinazoline Derivatives with α-Aminophosphonate Moiety. Journal of Chromatography B, 878, 1285-1289. https://doi.org/10.1016/j.jchromb.2009.11.024
- 9. Ali, T.E., Abdel-Aziz, S.A., Somaya, M., Mohamed, E.H.A. and Abdel-Kariem, S.M. (2013) Synthesis and Biological Evaluations of a Series of Novelazolyl, Azinyl and Azepinyl Phosphonates. Heterocycles, 87, 2513-2522.https://doi.org/10.3987/COM-13-12836
- 10. Abdel-Rahman, R.M., Makki, M.S., Ali, T.E. and Ibrahim, M.A. (2015) 1,2,4-Triazine Chemistry Part IV: Synthesis and Chemical Behavior of 3-Functionalized 5,6-Diphenyl-1,2,4-Triazines towards Some Nucleophilic and Electrophilic Reagents. Journal of Heterocyclic Chemistry, 52, 1595-1607.https://doi.org/10.1002/jhet.2014
- 11. Abdel-Rahman, R.M., Makki, M.S.T., Ali, T.E. and Ibrahim, M.A. (2013) 1,2, 4-Triazine Chemistry Part III: Synthetic Strategies to Functionalized Bridgehead Nitrogen Heteroannulated 1,2,4-Triazine Systems and Their Regiospecific and Pharmacological Properties. Current Organic Synthesis, 10, 136-160.
- 12. Abdel-Rahman, R.M. and Saad, H.A. (2016) Synthesis and Chemical Behavior of 1,2,4-Triazine Derivatives Bearing Phosphorus Amides as Donor-Acceptors: A Review. Current Organic Synthesis, 13, 408-425.https://doi.org/10.2174/1570179412666150905001956
- 13. Al-Romaizan, A.N., Makki, M.S.T. and Abdel-Rahman, R.M. (2014) Synthesis of New Fluorine/Phosphorus Substituted 6-(2'-Amino Phenyl)-3-Thioxo-1,2,4-Triazin-5(2H, 4H)One and Their Related Alkylated Systems as Molluscicidal Agent as against the Snails Responsible for Bilharziasis Diseases. International Journal of Organic Chemistry, 4, 154-168.
- 14. Ali, T.E., Abdel-Rahman, R.M., Hanafy, F.I. and El-Edfawy, S.M. (2008) Synthesis and Molluscicidal Activity of Phosphorus-Containing Heterocyclic Compounds Derived from 5,6-Bis (4-Bromophenyl)-3-Hydrazino-1,2,4-Triazine. Phosphorus, Sulfur, and Silicon and the Related Elements, 183, 2565-2577.https://doi.org/10.1080/10426500801967864
- 15. Abdel-Rahman, R.M., Ibrahim, M.A. and Ali, T.E. (2010) 1,2,4-Triazine Chemistry Part II: Synthetic Approaches for Phosphorus Containing 1,2,4-Triazine Derivatives. European Journal of Chemistry, 1, 388-396.https://doi.org/10.5155/eurjchem.1.4.388-396.154
- 16. Ali, T.E. (2009) Synthesis and Antibacterial Activity of Some New Thiadiaza/Triazaphospholes, Thiadiaza/Triaza/Tetrazaphosphinines and Thiadiaza/Tetrazaphosphepines Containing 1,2,4-Triazinone Moiety. European Journal of Medicinal Chemistry, 44, 4539-4546. https://doi.org/10.1016/j.ejmech.2009.06.022
- 17. Abdel-Rahman, R.M. (2002) Chemoselectiveheterocyclization of Pharmacological Activities of New Heterocycles—A Review: Synthesis of New Phosphaheterobicyclic Systems Containing 1,2,4-triazine Moiety Part IX: Straightforward Synthesis of New Fluorine Bearing 5-Phospha-1,2,4-triazin/1,2,4-triazepine-3 thiones. Trends in Heterocyclic Chemistry, 8, 187-195.
- 18. Makki, M.S.T., Abdel-Rahman, R.M. and AbuAli, O.A. (2015) Synthesis of New Fluorinated 1,2,4-Triazino [3,4-b] [1,3,4]thiadiazolones as Antiviral Probes-Part II-Reactivities of Fluorinated 3-Aminophenyl-1,2,4-triazinothiadiazolon. International Journal of Organic Chemistry, 5, 153-165. https://doi.org/10.4236/ijoc.2015.53017
- 19. Makki, M.S.T., Abdel-Rahman, R.M. and Khan, K.A. (2014) Fluorine Substituted 1,2,4-triazinones as Potential anti-HIV-1 and CDK2 Inhibitors. Journal of Chemistry, 2014, Article ID: 430573.
- 20. Abdel-Rahman, R.M., Makki, M.S.T. and Al-Romaizan, A.N. (2014) Synthesis of Novel Fluorine Substituted Isolated and Fused Heterobicyclic Nitrogen Systems Bearing 6-(2’-Phosphorylanilido)-1,2,4-Triazin-5-One Moiety as Potential Inhibitor towards HIV-1 Activity. International Journal of Organic Chemistry, 4, 247-268. https://doi.org/10.4236/ijoc.2014.44028
- 21. Abdel-Rahman, R.M., Makki, M.S.T. and Bawazir, W.A. (2011) Synthesis of Some More Fluorine Heterocyclic Nitrogen Systems Derived From Sulfa Drugs as Photochemical Probe Agents for Inhibition of Vitiligo Disease—Part I. E-Journal of Chemistry, 8, 405-414. https://doi.org/10.1155/2011/586063
- 22. Abdel-Rahman, R.M. (1991) Synthesis and Anti-Human Immune Virus Some New Fluorine-Containing Substituted-3-Thioxo-1,2,4-Triazin-5-Ones. IL Farmaco, 42, 379-389.
- 23. Abdel-Rahman, R.M. (2001) Chemistry of Uncondensed 1,2,4-Triazines, Part IV. Synthesis and Chemistry of Bioactive 3-Amino-1,2,4-Triazines and Related Com-pounds—An Overview. Pharmazie, 56, 275-286.
- 24. Ali, T.E. (2009) Synthesis of Some New 1,3,2-Oxazaphosphinine, 1,3,2-Diazaphos-phinine, Acyclic, and/or Cyclic α-Aminophosphonate Derivatives Containing the Chromone Moiety. Phosphorus, Sulfur, and Silicon and the Related Elements, 185, 88-96. https://doi.org/10.1080/10426500802713309
- 25. Ali, T.E. and Halacheva, S.S. (2009) Synthetic Approach for Novel Bis(α-aminophos-phornic Acid) Derivatives of Chromone Containing 1,2,4,3-Triazaphosphole Moieties. Heteroatom Chemistry, 20, 117-122. https://doi.org/10.1002/hc.20520
- 26. Pandeya, S.N., Kumar, R., Pathak, A.K. and Nath, G. (2010) Synthesis and Biological Evaluation of Triazine Derivatives. Der Pharma Chemica, 2, 257-266.
- 27. Siddhuraju, P. and Becker, K. (2007) The Antioxidant and Free Radical Scavenging Activities of Processed Cowpea (Vignaunguiculata (L.) Walp.) Seed Extracts. Food Chemistry, 101, 10-19. https://doi.org/10.1016/j.foodchem.2006.01.004






