International Journal of Organic Chemistry
Vol.07 No.01(2017), Article ID:73018,11 pages
10.4236/ijoc.2017.71001
Solid-Phase Aromatic Nitration with Mg(NO3)2 on Silica Gel
Tomoko Matsumoto1, Ayaka Yamauchi2, Jun Ishikawa2, Guan-Hong Jin2, Jin Matsumoto2, Yoshiyuki Fueda3, Masahide Yasuda2*
1Center for Collaborative Research and Community Cooperation, University of Miyazaki, Miyazaki, Japan
2Department of Applied Chemistry, Faculty of Engineering, University of Miyazaki, Miyazaki, Japan
3Fuji Silysia Chemical Ltd., Miyazaki, Japan

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: September 30, 2016; Accepted: December 24, 2016; Published: December 27, 2016
ABSTRACT
Nitroaromatics are usually prepared using a mixed acid of nitric acid with strong acids. However, the use of strong acids caused dangerous work-up and the disposal of large amounts of acid-waste. Therefore, much effort has been made on the improvement of nitration process without strong acids. We examined solid-phase aromatic nitration with Mg(NO3)2 on silica gel in order to establish the nitration process without strong acids. The nitration of 1,2- and 1,3-, 1,4-dimethoxybenzenes and 4-methylanisole with Mg(NO3)2 proceeded by heating on silica gel at 150˚C for 4 - 5 h to produce the nitroaromatics. The nitration of 1,3,5-trimethoxybenzene produced the nitrated dimer, 2,4,6,2’, 4’,6’-hexamethoxy-3-nitrobiphenyl, which was not isolated in other solid-phase nitration. In the cases of naphthalene derivatives, the α-nitrated compounds were obtained. In the cases of p-cresol and 2-naphthol, the esterification occurred at the hydroxyl group to give 4-tolyl nitrate and 2-naphthyl nitrate, respectively. It is synthetic interest to note that nitrate esters were isolated in solid phase. Thus Mg(NO3)2-SiO2 composite was mild reagent for solid-phase nitration. Acidity of Mg(NO3)2-SiO2 composite was determined to be pH 0.96 by the measurement of absorption spectra on a micro spectrophotometer using meso-tetra(p-cyanophenyl)porphyrin as a pH-indicator. Mg(NO3)2-SiO2 composite made acidic conditions. Therefore, it was suggested that Mg(NO3)2 reacted with proton on silica gel to form the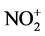 . Thus, electron-rich aromatic hydrocarbons led the efficient nitration through electrophilic attack of
. Thus, electron-rich aromatic hydrocarbons led the efficient nitration through electrophilic attack of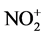 . After the nitration, acidic Mg(NO3)2-SiO2 composite could be turned into neutrality by exposing wet conditions and disposed safely since the composite did not involve harmful elements. Thus the solid-phase nitration using Mg(NO3)2-SiO2 composite will provide safety and environmentally conscious chemical process.
. After the nitration, acidic Mg(NO3)2-SiO2 composite could be turned into neutrality by exposing wet conditions and disposed safely since the composite did not involve harmful elements. Thus the solid-phase nitration using Mg(NO3)2-SiO2 composite will provide safety and environmentally conscious chemical process.
Keywords:
Aromatic Nitration, Silica Gel, Mg(NO3)2, Solid State

1. Introduction
Nitroaromatics are important chemicals which are applicable to dyes, explosives, pharmaceuticals, and the intermediates to prepare amines. Industrial synthesis of nitroaromatics has been achieved using a mixed acid of nitric acid with strong acids (e.g. sulfuric acid). However, the use of strong acids caused dangerous work-up and the disposal of large amounts of acid-waste. Therefore, much effort has been made on the improvement of nitration process without strong acids [1] . Preliminarily in order to avoid the risk of sulfuric acid, sulfuric acid was supported on silica gel to apply to the aromatic nitration with nitric acid (HNO3) [2] . Recently, silica gel which is the most commonly desiccant [3] has been used as a dehydration agent instead of sulfuric acid together with nitration reagents such as Bi(NO3)3 [4] , HNO3 [5] , Ce(NH4)(NO3)5 [6] , and AcONO2 [7] in solid state. Also, montmorillonite and charcoal were used for solid-state aromatic nitration with Bi(NO3)3 [8] and Zn(NO3)2 [9] . Also, Bi(NO3)3 [10] and NaNO2 [11] were used as reagents for indirect nitration in solution phase. Thus solid-phase nitration using nitrate salts such as Bi(NO3)3, Al(NO3)3・9H2O [12] [13] and Zn(NO3)2 on silica gel has been reported so far.
More than a decade ago, we started to develop cobalt-free humidity indicator for silica gel desiccant using Mg salts and porphyrins [14] . In those days, the desiccant ability of silica gel has been checked by color change of the CoCl2 adsorbed on silica gel (CoCl2-SiO2, silica gel blue). However, considerable caution had to be paid to the CoCl2-SiO2 because CoCl2 was determined to be carcinogenic to humans by International Agency for Research on Cancer [15] . Therefore, it was required to use the humidity indicators instead of CoCl2-SiO2. On the other hand, Gordeeva and co-workers have reported that acidic conditions were made by the reaction of SiO2 with CaCl2 under dry conditions [16] . In order to develop new type of humidity indicator, we mixed MgCl2-SiO2 with pH-sensitive tetraphenylporphyrin and dried under heating to prepare a porphyrin-MgCl2- SiO2 (Indicator FTM, Fuji Silysia), which caused color change from green under dry conditions to pink under wet conditions [17] . During the investigations, we measured the pH of the composites of Mg salts (MgCl2, MgSO4, Mg(NO3)2) with SiO2 using several kinds of tetraarylporphyrins with different basicity [18] . In the case of the combination of Mg(NO3)2-SiO2 and tetra(p-methoxyphenyl) porphyrin, the porphyrin was nitrated. This observation led us to use Mg(NO3)2 for the solid-phase aromatic nitration since there was no report on the nitration using Mg(NO3)2.
Here, we investigated the solid-phase nitration of aromatic hydrocarbons (1) with a composite of Mg(NO3)2 with silica gel (Mg(NO3)2-SiO2) in order to establish the nitration process without strong acids.
2. Experiment
2.1. Instrument
1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were taken with a Bruker AV 400M spectrometer for CDCl3 solution using SiMe4 as an internal standard. High-resolution mass spectra (HRMS) were measured on a Thermo Scientific Q Exactive mass spectrometer equipped with an electrospray ionization source. Almost HRMS spectra were measured at positive mode except for the cases of 4f and 4j which were measured in negative mode. Microscopic spectrophotometry was performed on a confocal laser scanning microscope (CLSM; Olympus FV? 300, Japan) equipped with a spectrophotometer (STFL 250, Seki Technotron, Japan) linked to the CLSM by an optical fiber. Using a 10 folds magnification lens, the measurable area was restricted to the inside of a circle with a 8.56 μm- diameter [18] . Microscopic absorption spectra were taken using a back-light as the light source.
2.2. Nitration of Aromatic Hydrocarbons (1) with Mg(NO3)2 on Silica Gel
General procedure of solid-phase nitration was performed as follows. A MeOH solution (10 mL) containing 1,4-dimethoxybenzene (1a; 3.62 mmol, 500 mg) and Mg(NO3)2・6H2O (504.9 mg, 1.97 mmol, Wako Chemicals, Japan ) was added slowly to silica gel (2.92 g, 48.7 mmol, Fuji Silysia A type, the average diameter = 79 μm) in a flask. After standing for 30 min to adsorb 1a and Mg(NO3)2 on silica gel, the solvent was removed by evaporation. The resulting composite of 1a, Mg(NO3)2, and silica gel was heated at a given temperature under N2 atmosphere for 4-10 h under vigorous magnetic steering. The reacted composite was set on a silica gel column (Fuji Silysia BW 300, 50 mL) and was subjected to chromatography. Starting material (1a) and the nitrated products (2a) were isolated by elution with hexane and CHCl3, respectively. The products were listed in Scheme 1. The 2a-2c, 2e-2i, 3d, 4f, and 4j had the following spectral data.
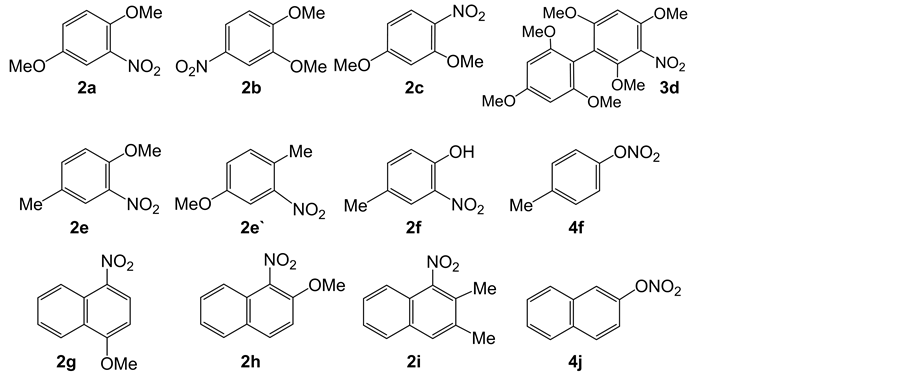
Scheme 1. Products (2a-2i, 3d, and 4f, 4j) of solid-phase nitration. Reaction conditions: 1 (3.62 mmol), Mg(NO3)2 (1.97 mmol), silica gel (2.92 g) under heating at 150˚C.
1,4-Dimethoxy-2-nitrobenzene (2a). Yellow solid. M.p. 69˚C - 70˚C (lit. 72˚C - 73˚C [19] ). 1H NMR δ = 3.82 (s, 3H), 3.92 (s, 3H), 7.04 (d, J = 9.2 Hz, 1H), 7.16 (dd, J = 9.2, 3.1 Hz, 1H), 7.39 (d, J = 3.08 Hz, 1H). 13C NMR δ = 55.03, 56.09, 108.96, 114.12, 119.91, 138.52, 146.37, 151.86. HRMS: m/z calcd for C8H10NO8: ([M + H]+) 184.0604, found 184.0605.
1,2-Dimethoxy-4-nitrobenzene (2b). Yellow solid. M.p. 99˚C (lit 97.4˚C [20] ). 1H NMR δ = 3.90 (3, 3H), 3.92 (s, 3H), 6.85 (d, J = 8.9 Hz, 1H), 7.69 (d, J = 2.6 Hz, 1H), 7.86 (dd, J = 8.9, 2.6 Hz, 1H). 13C NMR δ = 56.47, 56.33, 106.45, 109.86, 117.81, 141.50, 148.87, 154.52. HRMS: m/z calcd for C8H10NO8: ([M + H]+) 184.0604, found 184.0602.
2,4-Dimethoxy-1-nitrobenzene (2c). Brown solid. M.p. 70˚C (lit 72˚C - 76˚C [21] ). 1H NMR δ = 3.89 (s, 3H), 3.95 (s, 3H), 6.51 (d, J = 8.9, 2.5 Hz, 1H), 6.54 (d, J = 2.5 Hz, 1H), 8.01 (d, J = 8.9, 2.5 Hz, 1H). 13C NMR δ = 55.92, 56.48, 99.67, 104.70, 128.53, 129.87, 155.70, 164.80. HRMS: m/z calcd for C8H10NO8: ([M + H]+) 184.0604, found 184.0602.
4-Methyl-2-nitroanisole (2e). M.p. 128˚C. 1H NMR δ = 2.34 (s, 3H), 3.93 (s, 3H), 6.98 (d, J = 8.5 Hz, 1H), 7.34 (dd, J = 8.6, 2.2 Hz, 1H), 7.65 (d, J = 1.8 Hz, 1H). HRMS: m/z calcd for C8H9NO3: ([M + H]+) 168.0655, found 168.0654.
4-Methyl-3-nitroanisole (2e'). 1H NMR δ = 2.44 (s, 3H), 3.89 (s, 3H), 7.01 (d, J = 8.8 Hz, 1H), 7.84 (d, J = 8.8, 1H), 8.13 (s, 1H).
4-Methyl-2-nitrophenol (2f). Oil, 1H NMR δ = 2.77 (s, 3H), 6.98 (d, J = 8.6 Hz, 1H), 7.32 (d, J = 8.8 and 2.0 Hz, 1H), 7.83 (d, J = 2.0 Hz, 1H).
1-Methoxy-4-nitronaphthalene (2g). Yellow solid. M.p. 80.0˚C (lit 81˚C - 83˚C [22] ), 1H NMR δ = 4.11 (s, 3H), 6.82 (d, J = 8.7 Hz, 1H), 7.59 (dd, J = 8.2, 6.9 Hz, 1H), 7.74 (dd, J = 8.5, 6.9 Hz, 1H), 8.37 (d, J = 8.5 Hz, 1H), 8.40 (d, J = 8.7 Hz, 1H), 8.78 (d, J = 8.7 Hz, 1H). 13C NMR δ = 56.29, 101.90, 122.77, 123.50, 125.62, 126.58, 126.88, 127.20, 129.46, 130.07, 160.60. HRMS: m/z calcd for C11H9NO3: ([M + H]+) 204.0655, found 204.0654.
2-Methoxy-1-nitronaphthalene (2h). Green solid. M.p. 126˚C, 1H NMR δ = 4.00 (s, 3H), 7.31 (d, J = 9.2 Hz, 1H), 7.43 (dd, J = 8.2, 6.8 Hz, 1H), 7.58 (dd, J = 8.6, 6.8 Hz, 1H), 7.66 (d, J = 8.6 Hz, 1H), 7.82 (d, J = 8.2 Hz, 1H), 7.92 (d, J = 9.2 Hz, 1H). 13C NMR δ = 57.05, 113.06, 120.39, 125.15, 125.66, 128.07, 128.20 129.14, 132.21, 136.03, 148.62. HRMS: m/z calc. for [M + H], C11H9NO3+: 204.0655; Found: 204.0655.
2,3-Dimethyl-1-nitronaphthalene (2i). Yellow solid. M.p. 86.0˚C - 88.0˚C, 1H NMR δ = 2.33 (s, 3H), 2.43 (s, 3H), 7.45-7.60 (m, 2H), 7.58 (d, J = 7.6 Hz), 7.68 (s, 1H), 7.75 (d, J = 7.2 Hz, 1H). 13C NMR δ = 14.84, 20.47, 120.95, 126.66, 127.19, 127.50, 128.02, 129.76, 132.03, 133.70, 135.23, 148.51. HRMS: m/z calcd for C12H12NO2: ([M + H]+) 202.0863, found 202.0862.
2,4,6,2',4',6'-Hexamethoxy-3-nitrobiphenyl (3d). Yellow solid. M.p. 69.0˚C - 70.0˚C, 1H NMR δ = 3.46 (s, 3H), 3.72 (s, 6H), 3.76 (s, 3H), 3.92 (s, 3H), 3.86 (s, 3H), 6.21 (s, 2H), 6.34 (s, 1H). 13C NMR δ = 55.31, 55.95, 56.32, 56.36, 61.70, 90.87, 91.54, 102.73, 110.34, 130.87, 151.94, 152.52, 159.07, 160.24, 161.54. HRMS: m/z calcd for C18H22NO8: ([M + H]+) 380.1340, found 380.1337.
4-Tolyl nitrate (4f). Oil, 1H NMR δ = 2.24 (s, 3H), 6.72 (d, J = 8.4 Hz, 2H), 7.00 (dd, J = 8.4 Hz, 1H). 13C NMR δ = 19.46, 114.12, 128.93, 129.04, 152.08. HRMS: m/z calcd for C7H7NO3: ([M − H]−) 152.0353, found 152.0353.
2-Naphtyl nitrate (4j). Black solid. M.p. 150.0˚C, 1H NMR δ = 7.09 (dd, J = 8.8, 2.5 Hz, 1H), 7.14 (d, J = 2.5 Hz, 1H), 7.31 (dd, J = 8.1, 6.9 Hz, 1H), 7.42 (dd, J = 8.1, 6.9 Hz, 1H), 7.66 (d, J = 8.1, 1H), 7.73 (d, J = 8.8 Hz, 1H), 7.75 (d, J = 8.1 Hz, 1H). 13C NMR δ = 109.48, 117.78, 123.59, 126.37, 126.52, 127.77, 129.84, 131.46, 134.60, 153.42. HRMS: m/z calcd for C10H7NO3: ([M − H]−) 188.0353, found 188.0353.
2.3. Preparation of Meso-Tetra(p-Cyanophenyl)Porphyrin
As pH-indicator, meso-tetra(p-cyanophenyl)porphyrin (H2tcp) was prepared according to the reported method as follows [23] . BF3・OEt2 (0.1 mL) were added to CHCl3 solution (500 mL) of p-cyanobenzaldehyde (1048 mg; 8.0 mmol). A CHCl3 solution (300 mL) of pyrrole (0.56 mL; 8.0 mmol) was then added. After the solution turned from pale orange to red-violet, it was confirmed that the Soret band appeared at 410 nm. NEt3 (0.2 mL; 1.43 mmol) and chloranil (2.2 g; 9.0 mmol) were added to the solution and then heated at 60˚C for 1 h under dark conditions. After evaporation, the condensed solution was filtrated and washed with CHCl3. The crude H2tcp was purified by a column chromatography on silica gel (Fuji Silysia BW 300) using CHCl3-MeOH (50:1) as eluent.
meso-Tetra(p-cyanophenyl)porphyrin. Yield 1.2%. 1H NMR δ = 8.10 (d, J = 8.6 Hz, 8H), 8.33 (d, J = 8.3 Hz, 8H), 8.60 (s, 8H). HRMS: m/z calcd for C48H27N8: ([M + H]+) 715.2359, found 715.2350.
2.4. Measurement of Acidity of the Mg(NO3)2-SiO2 Composite
According to the reported method [18] , the acidity of the Mg(NO3)2-SiO2 composite was measured as follows. At first, absorption spectra of H2tcp were measured in CHCl3-MeOH (1:2, v/v) under different pHs which were adjusted by HClO4. The absorptions were observed at 514 and 645 nm due to purple free base porphyrin (H2tcp) and the greenish protonated porphyrin (H4tcp2+), respectively. The absorbances (AP and AG) were measured at 514 nm and 645 nm, respectively. Fraction (F = AP/(AG + AP)) was calculated at every pH and plotted against the pH to make the pH-profile of F values (Figure 1). The pH-profile was fitted by sigmoid curves (Equation (1)) which was presented by three parameters, Fmax, pKa, and S, which denotes maximum F values, acid dissociation constant of H4tcp2+, and slope of the fitting curve at pKa, respectively. The relative standard deviation (RSD) was 0.9996. Each value of Fmax, pKa, and S for H2tcp were determined to be 0.875, 1.376, and 6.220, respectively.
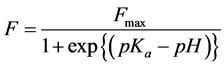 (1)
(1)
When the aromatic hydrocarbon was not added the Mg(NO3)2-SiO2 composite, acidity of the Mg(NO3)2-SiO2 composite was measured in a solid state using the pH-profile as follows. A CHCl3 solution (10 mL) of H2tcp (0.7 μmol) and an
Figure 1. The pH-dependence of F values of H2tcp in CHCl3-MeOH. The pH was adjusted by HClO4.
aqueous solution (2 mL) of Mg(NO3)2 (173 mg) were mixed with SiO2 (1.0 g, Fuji silysia A type, 1.8 - 5.0 mm). Here, large sizes of silica gel were used for CLSM analysis. After standing for 2 h until almost all of the H2tcp had been adsorbed on Mg(NO3)2-SiO2 composite, the solvent was evaporated and the H2tcp-Mg(NO3)2-SiO2 composite was dried under reduced pressure. Figure 2 shows the absorption spectra of H2tcp adsorbed on a Mg(NO3)2-SiO2 composite which was measured for five beads on CLSM. The F-values were determined to be 0.06 ± 0.04 by averaging five spectra. Using fitting curves, F-values were converted to pH, which was determined to be 0.96 for the Mg(NO3)2-SiO2 composite under dry conditions.
3. Results and Discussion
3.1. Solid-Phase Nitration of Aromatic Hydrocarbons (1) with Mg(NO3)2 on Silica Gel
Solid-phase nitration of aromatic hydrocarbons (1) was performed by heating a mixture of 1 (3.62 mmol), Mg(NO3)2・6H2O (504.9 mg, 1.97 mmol), and silica gel (2.92 g, 48.7 mmol) at a given temperature under N2 atmosphere under magnetic steering. The solid-phase nitration of 1,4-dimethoxybenzene (1a) with Mg(NO3)2 on silica gel produced 1,4-dimethoxy-2-nitrobenzene (2a). Figure 3 showed the time- conversion plots of 2a at various temperatures. From the plots, the optimized temperature was determined to be 150˚C. Therefore, the reaction temperature was fixed at 150˚C in the solid-phase nitration of other aromatics (1b-1j). The results are summarized in Table 1.
The solid-phase nitration of 1,2- and 1,3-dimethoxybenzenes (1b and 1c) and 4-methylanisole (1e) gave the nitroaromatics (2b and 2c, 2e and 2e’) but yields were low. In the cases of naphthalene derivatives (1g-1i), the α-nitrated compounds (2g-2j) were obtained. Moreover, it was noteworthy that the nitration of 1,3,5-trimethoxybenzene (1d) produced 2,4,6,2’,4’,6’-hexamethoxy-3-nitrobi-
Figure 2. Measurement of absorption spectra of five beads of the H2tcp-adsorbed Mg(NO3)2-SiO2 under dry conditions using CLSM.
Figure 3. Time-conversion of 2a in solid-phase nitration of 1a with Mg(NO3)2 on silica gel: Reaction temperature = 70 (△), 90 (▲), 110 (◇), 130 (◆), 150 (○), and 170˚C (●).
phenyl (3d) which was the nitrated dimer of 1d. However, 1,3,5-trimethoxy-2- nitrobenzene was not formed, though it was reported that the reaction of 1d with HNO3 in the presence of HClO4 gave 1,3,5-trimethoxy-2-nitrobenzene [24] . The structure of 3d was undoubtedly confirmed by MS and NMR spectra. In the cases of p-cresol (1f) and 2-naphthol (1j), the nitration occurred at the hydroxyl group to give 4-tolyl nitrate (4f) and 2-naphthyl nitrate (4j), respectively. Usual nitration of 4f and 4j in solution occurred at aromatic ring [25] . Recently it was reported that the efficient nitration of phenol and phenol derivatives with Al(NO3)3・9H2O [12] [13] and Bi(NO3)3・5H2O [26] on silica gel occurred at aromatic ring. Therefore, it was suggested that the nitration ability of SiO2-Mg(NO3)2 was not so strong, because the nitration was restricted to the electron rich substrates. In the case of phenol derivatives, silica gel supported the dehydration between OH group and HNO3 to give Ar-ONO2. Since aromatic nitrate had been recognized to be unstable [27] , the present synthesis had synthetic worthy.
3.2. Reaction Pathways
The silica gel is constructed by Si-O bonds such as Si-O-Si, Si=O, and Si-OH. It is well-known that the Si-OH group remained on the surface under heating below 300˚C [7] . We previously elucidated that the Si-OH on silica gel could react with MgCl2 and MgSO4 under dry conditions to release proton [18] . Their acidities were determined by the colorimeter analysis using meso-tetraarylporphyrin as a pH-indicator. In the present study, the reaction of Mg(NO3)2 with silica gel generated HNO3 along with the adsorption of Mg2+ ion on silica gel (Equation (2)). The acidity (pH) of Mg(NO3)2-SiO2 composite was determined to be 0.96, which was more acidic compared with MgCl2-SiO2 and MgSO4-SiO2 composites whose pH were 1.73 and 1.61, respectively. The presence of excess water made the pH of Mg(NO3)2-SiO2 neutral. Aromatic nitration usually occurs under acidic conditions. It was suggested that HNO3 reacted with the proton to form the 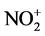 (Equation (3)). More electron-rich aromatic hydrocarbons (1a and 1d) were allowed the efficient nitration. Therefore, the nitration proceeded through electrophilic attack of
(Equation (3)). More electron-rich aromatic hydrocarbons (1a and 1d) were allowed the efficient nitration. Therefore, the nitration proceeded through electrophilic attack of 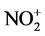 to the aromatic ring (Equation (4)). Mg(NO3)2 and silica gel played as nitration reagent and desiccant, respectively.
to the aromatic ring (Equation (4)). Mg(NO3)2 and silica gel played as nitration reagent and desiccant, respectively.
Generation of H+
 (2)
(2)
Generation of 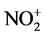
 (3)
(3)
Nitration
 (4)
(4)
In order to elucidate the mechanism of the formation of 3d, acid-catalyzed dimerization of 1d was attempted by the heating of 1d on silica gel at 150˚C for 7 h with MgCl2 which can release proton but has no electrophilicity. The dimerization of 1d did not occur. It is well known that the dimerization of aromatic hydrocarbons in the presence of electrophiles has been reported [28] . The electrophilic attack of NO2+ to 1d gave intermediates 5A and/or 5B. The 5A is less stable and more reactive than 5B, leading to the reaction of 5Awith another 1d at ortho and/or para positions (Friedel-Crafts acylation) to give the dimer which allowed the nitration to form 3d (Scheme 2).
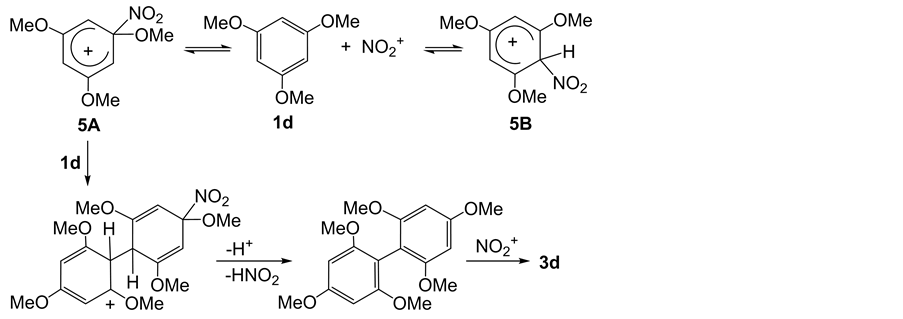
Scheme 2. Possible mechanism of the formation of 3d.
Table 1. The solid-phase nitration of benzene (1a-1f) and naphthalene derivatives (1g-1j) with Mg(NO3)2 on solid state of silica gela.
a) The solid-phase nitration was performed by heating 1a-1j (3.62 mmol) with Mg(NO3)2 (1.97 mmol) on silica gel (2.92 g) at 70˚C - 170˚C; b) Conversion of 1; c) Isolated yields based on the 1 used.
4. Conclusion
In conclusion, the solid phase nitration of electron-rich aromatic hydrocarbons such as 1,4-dimethoxybenzene (1a) proceeded using Mg(NO3)2 on silica gel. Unique nitration occurred in 1,3,5-trimethoxybenzene (1d) which afforded nitrated dimer. In the cases of p-cresol (1f) and 2-naphthol (1j), esterification occurred to give aromatic nitrates. Thus the combination of Mg(NO3)2 with silica gel can eliminate the use of sulfuric acid from the aromatic nitration. Moreover, the acidic Mg(NO3)2-SiO2 composite could be turned into neutrality by only exposing wet conditions and disposed safely since the composite did not involve harmful elements. Thus the solid-phase nitration using Mg(NO3)2-SiO2 composite will provide safety and environmentally conscious chemical process.
Cite this paper
Matsumoto, T., Yamauchi, A., Ishikawa, J., Jin, G.-H., Matsumoto, J., Fueda, Y. and Yasuda, M. (2017) Solid-Phase Aromatic Nitration with Mg(NO3)2 on Silica Gel. International Journal of Organic Chemistry, 7, 1-11. http://dx.doi.org/10.4236/ijoc.2017.71001
References
- 1. Yan, G. and Yang, M. (2013) Recent Advances in the Synthesis of Aromatic Nitro Compounds. Organic & Biomolecular Chemistry, 11, 2554-2566.
https://doi.org/10.1039/c3ob27354g - 2. Riego, J.M., Sedin, Z., Zaldívar, J.M., Marziano, N.C. and Tortato, C. (1996) Sulfuric Acid on Silica-Gel: An Inexpensive Catalyst for Aromatic Nitration. Tetrahedron Letters, 37, 513-516.
https://doi.org/10.1016/0040-4039(95)02174-4 - 3. Iler, R.K. (1979) The Chemistry of Silica. John Wiley & Sons, New York.
- 4. Badgujar, D.M., Taiwar, M.B., Asthana, S.N. and Mahulikar, P.P. (2007) Environmentally Benign Synthesis of Aromatic Nitro Compounds Using Silica Supported Inorganic Nitrates. Journal of Scientific and Industrial Research, 66, 250-251.
- 5. Hajipour, A.R. and Ruoho, A.E. (2005) Nitric Acid in the Presence of P2O5 Supported on Silica Gel—A Useful Reagent for Nitration of Aromatic Compounds under Solvent-Free Conditions. Tetrahedron Letters, 46, 8307-8310.
https://doi.org/10.1016/j.tetlet.2005.09.178 - 6. Grenier, J.-L., Catteau, J.-P. and Cotelle, P. (1999) Nitration of Electron-Rich Aromatic Compounds by Cerium Ammonium Nitrate Coated on Silica. Synthetic Communications, 29, 1201-1208.
https://doi.org/10.1080/00397919908086091 - 7. Augusto, J., Rodrigues, R., De Oliveira Filho, A.P., Moran, P.J.S. and Custódio, R. (1999) Regioselectivity of the Nitration of Phenol by Acetyl Nitrate Adsorbed on Silica Gel. Tetrahedron Letters, 55, 6733-6738.
- 8. Samajdar, S., Becker, F.F. and Banik, B.K. (2000) Surface-Mediated Highly Efficient Regioselective Nitration of Aromatic Compounds by Bismuth Nitrate. Tetrahedron Letters, 41, 8017-8020.
https://doi.org/10.1016/S0040-4039(00)01397-6 - 9. Iranpoor, N., Firouzabadi, H., Heydari, R. and Shiri, M. (2005) Nitration of Aromatic Compounds by Zn(NO3)2· 2N2O4 and Its Charcoal-Supported System. Synthetic Communications, 35, 263-270.
https://doi.org/10.1081/SCC-200048450 - 10. Al-Masum, M. and Welch, R.L. (2014) Catalyst Free, Base Free Microwave Irradiated Synthesis of Aryl Nitrites from Potassium Aryltrifluoroborates and Bismuth Nitrate. Tetrahedron Letters, 55, 1726-1728.
https://doi.org/10.1016/j.tetlet.2014.01.102 - 11. Al-Masum, M., Saleh, N. and Islam, T. (2013) A Novel Route to Organonitrites by Pd-Catalyzed Cross-Coupling of Sodium Nitrite and Potassium Organotrifluoroborates. Tetrahedron Letters, 54, 1141-1144.
https://doi.org/10.1016/j.tetlet.2012.12.047 - 12. Patil, M.R., Mohite, P.H., Shisodia, S. and Keri, R.S. (2015) Regioselective Nitration of Phenols and Phenyl Ethers Using Aluminium Nitrate on Silica as a Nitrating System. Letters in Organic Chemistry, 12, 129-135.
https://doi.org/10.2174/1570178612666150108000402 - 13. Ghorbani-Choghamarani, A., Goudarziafshar, H., Nikoorazm, M. and Yousefi, S. (2009) Aluminum Nitrate and Silica Sulfuric Acid as Efficient Nitrating Media for the Mononitration of Phenols under Mild and Heterogeneous Conditions. Canadian Journal of Chemistry, 87, 1144-1147.
https://doi.org/10.1139/V09-081 - 14. Fueda, Y., Matsumoto, J., Shiragami, T., Nobuhara, K. and Yasuda, M. (2007) Porphyrin/MgCl2/Silica Gel Composite as a Cobalt-Free Humidity Indicator. Chemistry Letters, 36, 1246-1247.
https://doi.org/10.1246/cl.2007.1246 - 15. Consolidated Version of Directive EU/67/548/EEC.
- 16. Gordeeva, L.G., Glaznev, I.S., Savchenko, E.V., Malakhov, V.V. and Aristov, Y.I. (2006) Impact of Phase Composition on Water Adsorption on Inorganic Hybrids “Salt/Silica”. Journal of Colloid and Interface Science, 301, 685-691.
https://doi.org/10.1016/j.jcis.2006.05.009 - 17. Matsumoto, T., Mitsumura, Y., Miyamoto, M., Matsumoto, J., Shiragami, T., Fueda, Y., Nobuhara, K. and Yasuda, M. (2011) Quantitative Analysis for a Color-Change of Humidity Indicator by Microscopic Absorption Spectrometry. Analytical Sciences, 27, 623-628.
- 18. Matsumoto, T., Hirose, D., Yamauchi, A., Matsumoto, J., Shiragami, T., Fueda, Y. and Yasuda, M. (2012) Measurement of Acidity of Magnesium Salts-Silica Gel Composites by Microscopic Absorption Spectrometry Using Porphyrin as pH-In- dicator. Bunseki Kagaku, 61, 851-856.
- 19. Tanemura, K., Suzuki, T., Nishida, Y., Satsumabayashi, K. and Horaguchi, T. (2003) A Mild and Efficient Method for the Mononitration of Aromatic Compounds by Cerium (III) Ammonium Nitrate in Acetic Anhydride. Journal of Chemical Research, 2003, 497-499.
https://doi.org/10.3184/030823403103174696 - 20. Clinton, R.O. and Page, D.F. (1963) US Patent 3,076,845.
- 21. Chemical Synthesis Database.
http://www.chemsynthesis.com/ - 22. Mellor, J.M., Mittoo, S., Parkes, R. and Millar, R.W. (2000) Improved Nitrations Using Metal Nitrate-Sulfuric Acid Systems. Tetrahedron, 56, 8019-8024.
https://doi.org/10.1016/S0040-4020(00)00720-1 - 23. Matsumoto, J., Matsumoto, T., Senda, Y., Shiragami, T. and Yasuda, M. (2008) Preparation and Characterization of Porphyrin Chromophores Immobilized on Micro-Silica Gel Beads. Journal of Photochemistry and Photobiology A: Chemistry, 197, 101-109.
https://doi.org/10.1016/j.jphotochem.2007.12.010 - 24. Moodie, R.B., Schofield, K. and Thomas, P.N. (1978) Electrophilic Aromatic Substitution. Part 19. The Nitration of Some Reactive Aromatic Compounds in Perchloric Acid. Journal of the Chemical Society, Perkin Transactions, 2, 318-323.
https://doi.org/10.1039/p29780000318 - 25. Rajanna, K.C., Chary, V.S., Kumar, M.S., Krishnaiah, G., Srinivas, P., Venkanna, P., Venkateswarlu, M., Ramesh, K., Reddy, K.R. and Suresh, B. (2015) Ultrasonic and Microwave Effects in Polyethylene Glycol-Bound Metal Nitrate Initiated Nitration of Aromatic Compounds under Acid Free Conditions. Green Chemistry Letters and Reviews, 8, 50-55.
https://doi.org/10.1080/17518253.2015.1105309 - 26. Sun, H.-B., Hua, R. and Yin, Y. (2005) Highly Efficient Nitration of Phenolic Compounds in Solid Phase or Solution Using Bi(NO3)3·5H2O as Nitrating Reagent. The Journal of Organic Chemistry, 70, 9071-9073.
https://doi.org/10.1021/jo0514669 - 27. Crivello, J.V. (1981) Nitrations and Oxidations with Inorganic Nitrate Salts in Trifluoroacetic Anhydride. The Journal of Organic Chemistry, 46, 3056-3060.
https://doi.org/10.1021/jo00328a013 - 28. Puskas, I. and Fields, E.K. (1966) Synthesis of Nitropolyalkylbiphenyls by Nitrative Coupling of Di- and Trialkylbenzenes. The Journal of Organic Chemistry, 31, 4204- 4210.
https://doi.org/10.1021/jo01350a077





