International Journal of Organic Chemistry
Vol.06 No.02(2016), Article ID:66590,10 pages
10.4236/ijoc.2016.62009
The Synthesis and Cytotoxicity of Novel Thiophene Derivatives Derived from 2-(4-Oxo-4,4-Dihydrothiazol-2-yl) Acetonitrile
Eman M. Samir1*, Amr S. Abouzied1,2, Faten I. Hamed1
1National Organization for Drug Control & Research (NODCAR), Cairo, Egypt
2Department of Pharmaceutical Chemistry, College of Pharmacy, University of Hail, Hail, Kingdom of Saudi Arabia

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 26 March 2016; accepted 16 May 2016; published 19 May 2016
ABSTRACT
The reaction of the 2-(4-oxo-4,4-dihydrothiazol-2-yl)acetonitrile 1 with cyaclopentanone (2) afforded the condensed product 3. The latter underwent a series of heterocyclizations through its reaction with different reagents. Moreover, compound 1 underwent the Gewald’s thiophene to afford compounds 15 and 17. The reaction of either hydrazine hydrate or phenylhydrazine with compound 17 gave the hydrazide derivatives 19a and 19b, respectively. The cytotoxicity of the newly synthesized products was measured towards the three cancer cell lines MCF-7, NCI-H460 and SF-268. The study showed that compounds 3, 5, 9c, 11, 13a, 13c, 17 and 19b were the most active compounds towards the three cancer cell lines.
Keywords:
Thiazole, Cyclopentanone, Thiophene, Hydrazide, Cytotoxicity

1. Introduction
Although the number of drugs is available in the market, the need of discovering the new anti-tumor drugs with better pharmacokinetic profile and lesser toxicity has become the main objective in the field of medicinal chemistry, and it is also due to the fast microbial resistance to the existing molecules [1] - [3] . A large number of compounds containing thiophene system have been investigated because of their broad spectrum of biological activities which include analgesic [4] , antibacterial [5] , antifungal [6] , antiparasitic [7] , antiviral [8] , anti-inflam- matory [9] , anticonvulsant [10] , anti-nociceptive [11] , DNA cleavage [12] , herbicidal [13] , antitubercular [14] , protein kinase inhibition [15] , respiratory syndrome protease inactivation [16] , an active ester in the peptide synthesis and agonists of peroxisome proliferator activated receptors [17] . In the present work, we study the reactivity of compound 3 resulting from reaction of the 2-(4-oxo-4,4-dihydrothiazol-2-yl)acetonitrile (1) with cyclopentanone to produce novel thiophene derivatives together with cytotoxic evaluations of the newly synthesized products towards different cell lines.
2. Chemistry
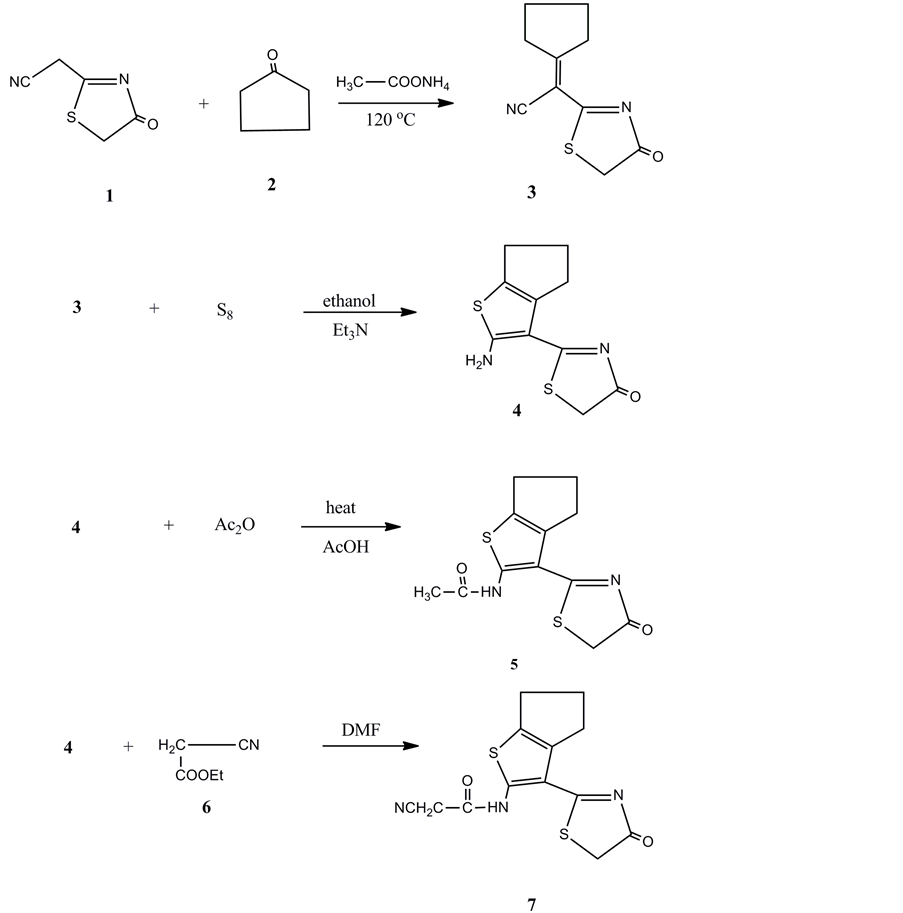
The reaction of the 2-(4-oxo-4,4-dihydrothiazol-2-yl)acetonitrile (1) with cyaclopentanone (2) in the presence of ammonium acetate at 120˚C gave the Knoevenagel condensation compound 3. The structure of the compound 3 was confirmed on the basis of analytical and spectral data. The reaction of compound 3 with elemental sulphur in the presence of ethanol and triethylamine gave the 4,5,6,7-tetrahydrobenzo[b]thiophene derivative 4. The 2-amino group present in compound 4 showed interesting reactivity as primary aromatic amine. Thus, compound 4 reacted with acetic anhydride in presence of acetic acid gave the N-acetyl derivative 5. On the other hand the reaction of compound 4 with ethyl cyanoacetate 6 gave the N-cyanomethylacetamide derivatives 7. The analytical and spectral data are the tools of the structure elucidation of compound 7. Thus, the 1H NMR spectrum showed a multiplet at δ1.18 - 1.69 ppm indicating the cyclopentene three CH2, a singlet at δ2.50 ppm corresponding to the CH2 group, a singlet at δ4.29 ppm for the thiazol CH2, and a singlet at δ8.27 ppm for the NH group (Figure 1).
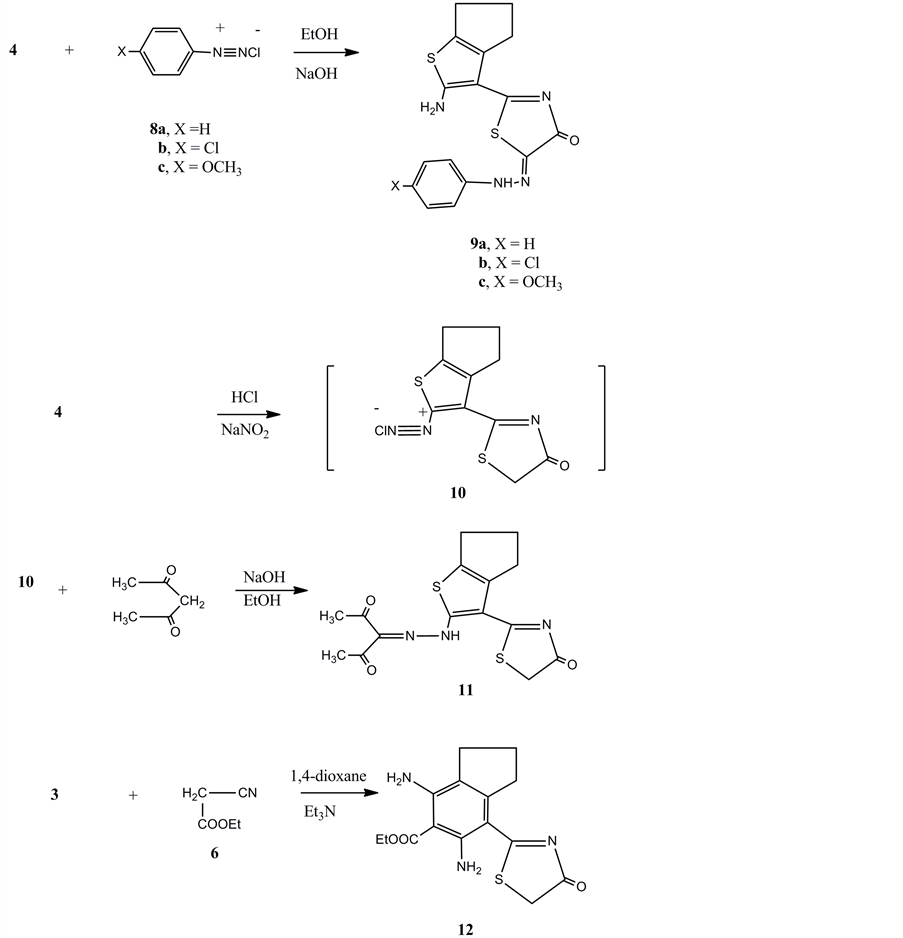
The high yield of compound 4 encouraged us to study its further reactivity towards some chemical reagents. Thus, the reaction of 4 with any of benzene diazoniumchloride 8a, 4-chlorobenzene-diazonium chloride 8b or 4-methoxybenzene-diazonium chloride 8c in the presence of ethanol and sodiumhydroxide gave the arylhydrazonederivatives 9a-c, respectively. The analytical and spectral data of the latter products are consistent with their respective structures. On the other hand, compound 4 is capable for diazotization and coupling. Thus, compound 4 reacted with sodium nitrite in the presence of sodium nitrite and acetic acid at 0˚C - 5˚C gave the non isolablediazonium salt 10. The latter coupled with acetylacetone to give the hydrazoderivative 11.
The reaction of compound 3 with ethyl cyanoacetate 6 in the presence of 1,4-dioxane and triethylamine gave the ethyl 4,6-diamino-7-(4-oxo-4,5-dihydrothiazol-2-yl)-2,3-dihydro-1H-indene-5-carboxylate 12. The structure of compound 12 was confirmed on the basis of analytical and spectral data. Thus the 1H NMR spectrum showed a triplet at δ1.16 ppm for ester CH3, a multiplet at δ1.54 - 1.72 ppm indicating the cyclopentene three CH2, a singlet at δ4.19 ppm corresponding to the NH2 group, a quartet at δ4.24 ppm for ester CH2, a singlet at δ4.97 ppm for the NH2 group, a singlet at δ6.01 ppm for the thiazol CH2 (Figure 2).
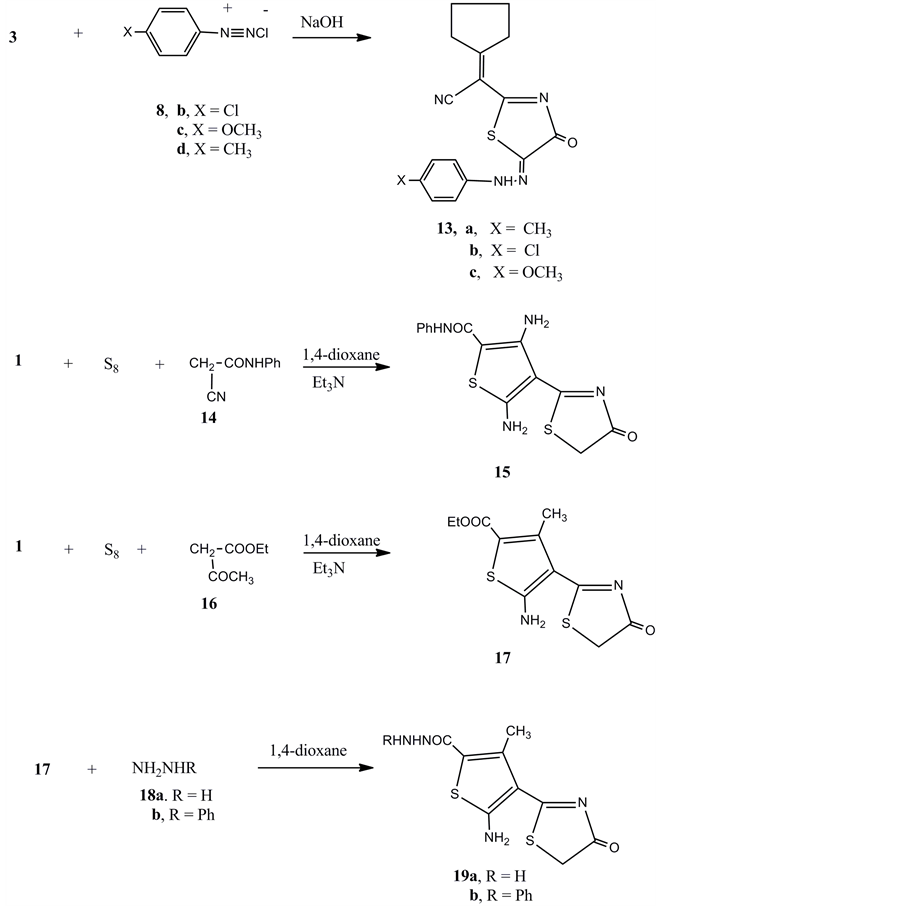
Moreover, compound 3 was coupled with any of 4-chlorobenzenediazonium chloride 8b, 4-methoxybenzene- diazonium chloride 8c or 4-methyl benzene diazonium chloride 8d in the presence of ethanol and sodium hydroxide gave the arylhydrazone derivatives 13a-c, respectively. Compound 1 reacted with elemental sulphur and cyanoacetanilide (14) in 1,4-dioxane and the presence of triethylamine to give the ethyl 3,5-diamino-4- (4-oxo-4,5- dihydrothiazol-2-yl)thiophene-2-carboxylate 15.
Similarly the reaction of compound 1 with elemental sulphur and ethyl acetoacetate 16 gave the ethyl 5-ami- no-3-methyl-4-(4-oxo-4,5-dihydrothiazol-2-yl)thiophene-2-carboxylate (17). Compound 17 reacted with either hydrazinehydrate or phenylhydrazine to give the hydrazide derivatives 19a and 19b, respectively (Figure 3).
3. Cytotoxicity
3.1. Antitumor and Normal Cell Line Activity Tests
The cytotoxicity of the synthesized compounds was tested for Three human tumor cell lines, MCF-7 (breast adenocarcinoma), NCI-H460 (non-small cell lung cancer), and SF-268 (CNS cancer) were used. MCF-7 was obtained from the European Collection of Cell Cultures (ECACC, Salisbury, UK), NCI-H460, SF-268 and normal fibroblast cells (WI 38) were kindly provided by the National Cancer Institute (NCI, Cairo, Egypt). They grow as monolayer and routinely maintained in RPMI-1640 medium supplemented with 5% heat inactivated FBS, 2 mM glutamine and antibiotics (penicillin 100 U/mL, streptomycin 100 µg/mL), at 37˚C in a humidified
Figure 1. Synthesis of compounds 3, 4, 5 and 7.
atmosphere containing 5% CO2. Exponentially growing cells were obtained by plating 1.5 × 105 cells/mL for MCF-7 and SF-268 and 0.75 × 104 cells/mL for NCI-H460, followed by 24 h of incubation. The effect of the vehicle solvent (DMSO) on the growth of these cell lines was evaluated in all the experiments by exposing untreated control cells to the maximum concentration (0.5%) of DMSO used in each assay.
The effects of synthesized compounds on the in vitro growth of human tumor cell lines were evaluated according to the procedure adopted by the National Cancer Institute (NCI, USA) in the “In Vitro Anticancer Drug Discovery Screen” that uses the protein-binding dye sulforhodamine B to assess cell growth (12). Briefly, exponentially, cells growing in 96-wellplates were then exposed for 48 h to five serial concentrations of each compound, starting from a maximum concentration of 150 μM. Following this exposure period adherent cells were fixed, washed, and stained. The bound stain was solubilized and the absorbance was measured at 492 nm in a plate reader (Bio-Tek Instruments Inc., Powerwave XS, Wincoski, USA). For each test compound and cell line, a dose-response curve was obtained and the growth inhibition of 50% (IC50), corresponding to the concentration of the compounds that inhibited 50% of the net cell growth was calculated as described elsewhere. Doxorubicin was used as a positive control and tested in the same manner.
Figure 2. Synthesis of compounds 9a-c, 11 and 12.
3.2. Structure Activity Relationship
It is clear from Table 1 that compounds 3, 5, 9c, 11, 13a, 13c, 17 and 19b were the most active compounds towards the three cancer cell lines with IC50’s against MCF-7 cell line (as an example) 0.02, 0.2, 0.01 1.6, 0.6, 0.4, 0.01 and 0.20, respectively. On the other hand, compounds 12, 13b and 19a were of moderate activities with IC50’s against MCF-7 6.1, 11.1 and 10.6, respectively. The rest of compounds showed low activity. Consider compounds 9a-c it is clear that compound 9c showed the highest activity among the three compounds which is attributed to the presence of the OCH3 group. Considering compounds 13a, b it is clear that compound 13a with the 4-CH3 group showed higher activity than 13b with the 4-Cl group. For the hydrazide derivatives 19a, b it is obvious that compound 19b with the phenyl moiety is more potent than compound 19a.
3.3. Conclusion
We have reported a convenient synthesis of variety compounds from compound 1 to 19b derivatives. The cyto-
Figure 3. Synthesis of compounds 13a-c, 15, 17 and 19a, b.
toxicity of some derivatives towards three types of cancer cell lines were studied most of the synthesized compounds were found to be cytotoxic and hence deserve further pharmacological investigation. The results of these investigation will be published in due time.
4. Experimental
All melting points determined on an electrothermal digital melting point apparatus and are uncorrected. IR spectra (KBr discs) were recorded on a FTIR plus 460 or Pyeunicam SP-1000 spectrophotometer. 1H-NMR spectra were recorded with varian Gemini 200 (200 MHz) (cairo university) instrument in DMSO-d6 as solvent using TMS as internal standard and chemical shifts are expressed as δ ppm. The mass spectra were recorded with Hewlett Packard 5988 A GC/MS system and GCMS-QP 1000 Ex shimadzu instruments. Analytical data were obtained from the microanalytical data unit at cairo university and were performed on vario El III Elemental CHNS analyzer.
2-Cyclopentylidene-2-(4-oxo-4,5-dihydrothiazol-2-yl)acetonitrile (3)
To a solution of compound 1 (1.40, 0.01 mol) cyclopentanone 2 (0.84 g, 0.01 mol) was added and the reaction
Table 1. Effect of the synthesized compounds on the growth of three human tumor cell lines.
Results are given in concentrations that were able to cause 50 % of cell growth inhibition (GI50) after a continuous exposure of 48 h and show means ± SEM of three-independent experiments performed in duplicate.
mixture was heated under fusion with ammonium acetate to 120˚C for 1hr, then cooled and then poured onto ice/water mixture and crystallized from 1,4-dioxane Yellow crystals, yield 1.75 g (85%), m.p. 120˚C - 122˚C; IR (KBr) (υ-cm−1): 2928 - 2385 (CH2), 2200 - 2195 (2CN), 1604 (C=O), 1580 (C=C). 1H-NMR (DMSO-d6, δ ppm): 1.55 - 1.75 (m, 8H, 4CH2), 4.97 (s, 2H, thiazole CH2). MS m/e = 206 (M+, 12); Anal. Calcd. for C10H10N2OS: C, 58.23; H, 4.89; N, 13.58; S, 15.55%. Found, C, 58.21; H, 5.01; N, 13.63; S, 15.33%.
2-(2-Amino-5,6-dihydro-4H-cyclopenta[b]thiophen-3-yl)thiazol-4(5H)-one (4)
To a solution of 3 (2.06 g, 0.01 mol) in ethanol (35 ml) containing triethylamine (1.00 ml) solid sulfur (0.32 g, 0.01 mol) was added, the reaction mixture was then heated under reflux for 30 min then cooled and neutralized by pouring onto ice/water mixture containing few drops of hydrochloric acid, the solid product formed was collected by filtration and crystallized from 1,4-dioxane Yellow crystals, (1,4-dioxane) yield 1.90 g (80%), m.p. 93˚C - 95˚C; IR (KBr) (υ-cm−1): 3400, 3320 (NH2), 2930 (CH2), 2220 (CN), 1686 (C=O), 1620 (C=C). 1H-NMR (DMSO-d6) δ ppm: 1.62 - 2.55 (m, 6H, 3CH2), 4.21 (s, 2H, NH2), 5.73 (s, 2H, thiazole CH2). MS m/e = 238 (M+, 18); Anal. Calcd. for C10H10N2OS2: C, 50.40; H, 4.23; N, 11.75; S, 26.91%. Found, C, 50.22; H, 4.31; N, 11.49; S, 26.94%.
N-(3-(4-Oxo-4,5-dihydrothiazol-2-yl)-5,6-dihydro-4H-cyclopenta[b]thiophen-2-yl)acetamide (5)
To a solution of 4 (2.38 g, 0.01 mol), acetic acid/acetic anhydride (10:3 ml) was added, the reaction mixture was heated under reflux 1 hr, the solid product formed upon pouring onto ice/water mixture, collected by filtration then washed with water and crystallized. The solid product formed was collected by filtration and crystallized from 1,4-dioxane. Pale yellow crystals, yield 2.24 g (80%), m.p. 133˚C - 135˚C; IR (KBr) (υ-cm−1): 3853, 3480 (NH), 2858 - 2430 (CH3, CH2), 2221 (CN), 1692 (2C=O), 1589 (C=C). 1H-NMR (DMSO-d6) δ ppm: 1.56 - 1.89 (m, 6H, 3CH2), 2.66 (s, 3H, CH3), 4.97 (s, 2H, thiazol CH2), 12.1 (s, 1H, NH). MS m/e = 280 (M+, 25); Anal. Calcd. for C12H12N2O2S2: C, 51.41; H, 4.31; N, 9.99; S, 22.87%. Found, C, 51.70; H, 4.55; N, 9.72; S, 22.90%.
2-Cyano-N-(3-(4-oxo-4,5-dihydrothiazol-2-yl)-5,6-dihydro-4H-cyclopenta-[b]thiohen-2-yl)acetamide (7)
To a solution of compound 4 (2.38 g, 0.01 mol) in DMF (20 ml) containing triethylamine (1.00 ml) ethylcyanoacetate (1.13 g, 0.01 mol) was added, the reaction mixture was heated under reflux 30 mins, then cooled and neutralized by pouring onto ice/water mixture containing few drops of hydrochloric acid, the solid product formed in each case was collected by filtration and crystallized from 1,4-dioxane. Brown crystals, (1,4-dioxane) yield 2.44 g (80%), m.p. 164˚C - 166˚C; IR (KBr) (υ-cm−1): 3458 - 3320 (NH), 2884 (CH2), 2227, 2220 (2CN), 1683, 1679 (2C=O), 1610 (C=C). 1H-NMR (DMSO-d6) δ ppm: 1.18 - 1.69 (m, 6H, cyclopentene 3CH2), 2.50 (s, 2H, CH2), 4.29 (s, 2H, thiazol CH2), 8.27 (s, 1H, NH). MS m/e =305 (M+, 84.54); Analy. Calcd. for C13H11N3O2S2: C, 51.13; H, 3.63; N, 13.76; S, 21.00%. Found, C, 50.86; H, 3.79; N, 13.83; S, 21.26%.
Synthesis of diazotized 2-(2-amino-4,5,6,7-tetrahydrobebzo[b]thiophen-3-yl)thiazol-4 (5H)-one derivatives (9a-c).
General procedure:
To a cold (0˚C - 5˚C) solution of compound 4 (2.38 g, 0.01 mol) in ethanol (20 mL) containing sodium hydroxide (1.00 g) an equivalent amount of either benzenediazonium chloride, 4-chlorobenzenediazonium chloride, or 4-methoxybenzenediazonium chloride [which was prepared by adding NaNO2 (0.70 g, 0.01 mol) solution to a cold solution of either aniline (1.0 g, 0.01 mol) in HCl (6 mL) or 4-chloroaniline (0.01 mol) or 4-methoxyaniline (0.01 mol)] was gradually added while stirring, the solid product formed upon cooling in an ice bath, collected by filtration and then washed with water.
2-(2-Amino-5,6-dihydro-4H-cyclopenta[b]thiophen-3-yl)-5-(2-phenylhydrazono)thiazol-4(5H)-one (9a)
Orange crystals (1,4-dioxane), yield 2.91 g (85%), m.p. 144˚C - 147˚C; IR (KBr) (υ-cm−1): 3490 - 3320 (NH, NH2), 3056 (CH aromatic), 2872 (CH2), 1686 (C=O), 1620 (C=N), 1580 (C=C). 1H-NMR (DMSO-d6) δ ppm: 1.26 - 1.59 (m, 6H, 3CH2), 4.8 (s, 2H, NH2), 6.83 - 7.46 (m, 5H, C6H5), 8.12 (s, 1H, NH). MS m/e = 342 (M+, 18); Analy. Calcd. for: C16H14N4OS2: C, 56.12; H, 4.12; N, 16.36; S, 18.73%. Found, C, 56.23; H, 4.29; N, 16.47; S, 18.63%.
2-(2-Amino-5,6-dihydro-4H-cyclopenta[b]thiophen-3-yl)-5-(2-(4-chlorophenyl)hydrazono)thiazol-4(5H)-one (9b)
Orange crystals (1,4-dioxane), yield 3.20 g (85%), m.p. 188˚C - 190˚C; IR (KBr) (υ-cm−1): 3478 - 3320 (NH, NH2), 3054 (CH aromatic), 2872 (CH2), 2200 (CN), 1690 (C=O), 1580 (C=C), 1530 (=N-NH). 1H-NMR (DMSO-d6) δ ppm: 1.61 - 1.72 (m, 6H, 3CH2), 2.59 (s, 2H, NH2), 7.38 - 7.42 (m, 4H, C6H4), 8.27 (s, 1H, NH), MS m/e = 376 (M+, 60); Analy. Calcd. For: C16H13ClN4OS2: C, 50.99; H, 3.48; N, 14.87; S, 17.02%. Found, C, 50.72; H, 3.62; N, 14.62; S, 17.22%.
2-(2-Amino-5,6-dihydro-4H-cyclopenta[b`]thiophen-3-yl)-5-(2-(4-methoxyphenyl)hydrazono)thiazol-4(5H)-one (9c)
Brown crystals (1,4-dioxane) yield: 2.44 g (85%), m.p. 180˚C - 183˚C; IR (KBr) (υ-cm−1): 3478 - 3328 (NH, NH2), 3053 (CH aromatic), 2873 (CH2), 2220 (CN), 1690 - 1685 (2C=O), 1603 (C=N), 1588 (C=C). 1H-NMR (DMSO-d6) δ ppm: 1.56 - 1.69 (m, 6H, cyclopentene, 3CH2), 2.28 (s, 3H, CH3), 4.58 (s, 2H, NH2), 8.29 (s, 1H, NH), 7.28 - 7.43 (m, 4H, C6H4) MS m/e = 372 (M+, 40); Analy. Calcd. for C17H16N4O2S2: C, 54.82; H, 4.33; N, 15.04; S, 17.22%. Found, C, 54.66; H, 4.53; N, 15.42; S, 17.42%.
3-(2-(3-(4-Oxo-4,5-dihydrothiazol-2-yl)-5,6-dihydro-4H-cyclopenta[b]thiophen-2-yl)hydrazono)pentane-2,4-dione (11)
To a cold solution (0˚C - 5˚C) of acetyl acetone (1 mL) in ethanol (20 ml) containing sodium hydroxide (1.00 g) the diazotized 2-(2-amino-4,5,6,7-tetrahydrobenzo[b]thiophen-3-yl)thiazol-4-(5H)-one [which was prepared by adding NaNO2 (0.70 g, 0.01 mol) solution to a cold solution of compound 4 (2.38 g, 0.01 mol) in acetic acid (20 mL), HCl (6 mL)] was gradually added while stirring, the solid product formed upon cooling in an ice bath, collected by filtration then washed with water and crystallized from acetic acid. Red crystals (1,4-dioxane), yield 2.79 g (80%), m.p. 199˚C - 202˚C; IR (KBr) (υ-cm−1): 3498 - 3329 (NH), 2978 - 2850 (CH3, CH2), 2221 (CN), 1690 - 1669 (3C=O). 1H-NMR (DMSO-d6) δ ppm: 1.49 - 1.63 (m, 6H, cyclopentene, 3 CH2), 2.62, 2.80 (2s, 6H, 2 CH3), 5.08 (s, 2H, CH2), 8.25 (s,1H, NH). MS m/e = 349 (M+, 18); Anal. Calcd. for C15H15N3O3S2: C, 51.56; H, 4.33; N, 12.03; S, 18.35%. Found, C, 51.42; H, 5.51; N, 12.27; S, 18.50%.
Ethyl 4,6-diamino-7-(4-oxo-4,5-dihydrothiazol-2-yl)-2,3-dihydro-1H-indene-5-carboxylate (12)
To a solution of compound 3 (2.06 g, 0.01 mol) in 1,4 dioxane (35 ml) containing triethylamine (1.00 ml), ethylcyanoacetate 6 (1.13 g, 0.01 mol) was added, the reaction mixture was heated under reflux for 45 mins, then cooled and neutralized by pouring onto ice/water mixture. Solid product formed was collected by filteration and crystallized from 1,4-dioxane. Yellow crystals (1,4-dioxane) yield 3.19 g (65%), m.p. 120˚C - 122˚C; IR (KBr) (υ-cm−1): 3477 - 3329 (2NH2), 2969 (CH2), 2220 (CN), 1989-1687 (3C=O), 1610 (C=C). 1H-NMR (DMSO-d6)δ ppm: 1.16 (t, 3H, ester CH3), 1.54 - 1.72 (m, 6H, 3CH2), 4.19 (s, 2H, NH2), 4.24 (q, 2H, ester CH2), 4.97 (s, 2H, NH2), 6.01 (s, 2H, thiazole CH2). MS m/e = 319 (M+, 30); Anal. Calcd. for C15H17N3O3S: C, 56.41; H, 5.37; N, 13.16; S, 10.04%. Found, C, 56.32; H, 5.42; N, 12.94; S, 9.88%.
Synthesis of diazotized 2-cyclohexylidene-2-(4-oxo-4,5-dihydrothiazol-2-yl) acetonitrile derivatives (13a-c)
General procedure:
To a cold solution of compound 3 (2.06 g, 0.01 mol) in ethanol (20 mL) containing sodium hydroxide (1.00 g), either of the diazo-4-methylaniline, diazo-4-chloroaniline ordiazo-4-methoxyaniline [which was prepared by adding NaNO2 (0.70 g, 0.01 mol) solution to a cold solution of 4-methylaniline (1.07 g, 0.01 mol) 4-chloroani- line (1.27 g, 0.01 mol) or 4-methoxyaniline (1.23 g, 0.01 mol) in concentrated hydrochloric acid (6 ml)] was gradually added while stirring, the solid product formed upon cooling in an ice bath was collected by filtration, washed by water.
2-Cyclopentylidene-2-(4-oxo-5-(2-phenylhydrazono)-4,5-dihydrothiazol-2-yl)acetonitrile (13a)
Red crystals (1,4-dioxane), yield 2.05 g (66%), m.p. 177˚C - 179˚C; IR (KBr) (υ-cm−1): 3488 - 3346 (NH), 3054 (CH aromatic) 2979 (CH2), 2227 ? 2220 (3CN), 1690 (C=O), 1620 (C=N), 1596 (C=C). 1H- NMR (DMSO-d6) δ ppm: 1.59 - 1.75 (m, 8H, 4 CH2), 3.11 (s, 3H, CH3), 7.29 - 7.38 (m, 4H, C6H4), 8.28 (s, 1H, NH). MS m/e = 324 (M+, 28); Anal. Calcd. for C17H16N4OS: C, 62.94; H, 4.97; N, 12.27; S, 9.88%. Found, C, 62.29; H, 4.86; N, 12.93; S, 10.02%.
2-(5-(2-(4-Chlorophenyl)hydrazono)-4-oxo-4,5-dihydrothiazol-2-yl)-2-cyclopentylideneacetonitrile (13b)
Brown crystals (1,4-dioxane), yield 2.24 g (65%), m.p. 210˚C - 212˚C; IR (KBr) (υ-cm−1): 3498 - 3326 (NH), 3056 (CH aromatic), 2920 (CH2), 2228 - 2220 (3CN), 1687 (C=O), 1610 (C=N), 1580 (C=C). 1H-NMR (DMSO-d6) δ ppm: 1.53 - 1.82 (m, 8H, 4CH2), 6.29 - 7.38 (m, 4H, C6H4), 8.29 (s, 1H, NH); MS m/e = 344 (M+, 28); Analy. Calcd. for C16H13ClN4OS: C, 55.73; H, 3.80; N, 16.25; S, 9.30%. Found, C, 55.60; H, 3.72; N, 16.32; S, 9.26%.
2-Cyclopentylidene-2-(5-(2-(4-methoxyphenyl)hydrazono)-4-oxo-4,5-dihydrothiazol-2-yl)acetonitrile (13c)
Orange crystals (1,4-dioxane), yield 2.62 g (77%), m.p. 167˚C - 170˚C; IR (KBr) (υ-cm−1): 3388 - 3340 (NH), 3050 (CH aromatic), 2850 - 2430 (CH3, CH2), 2200 (3CN), 1690 (C=O), 1580 (C=C), 1530 (C=N). 1H- NMR (DMSO-d6) δ ppm: 1.48 - 1.72 (m, 8H, 4CH2), 3.11 (s, 3H, OCH3), 7.28 - 7.39 (m, 4H, C6H4), 3.45 (s, 1H, NH). MS m/e = 340 (M+, 18); Anal. Calcd. for C17H16N4O2S: C, 59.98; H, 4.74; N, 16.46; S, 9.42%. Found, C, 60.18; H, 4.62; N, 16.59; S, 9.33%.
General procedure for the synthesis of thiophene derivatives 15 and 17
To solution of compound 1 (1.40 g, 0.01 mol) in 1,4 dioxane (25 mL) containing triethylamine (1.0 mL), either cyanoacetanilide (1.60 g, 0.01 mol) or ethyl acetoacetate (1.30 g, 0.01 mol) was added followed by elemental sulfur (0.32 g, 0.01 mol) and the reaction mixture was heated under reflux for 2 h then poured onto ice/water mixture containing few drops of hydrochloric acid. The formed solid product, in each case, was then collected by filtration and crystallized from 1,4dioxane.
3,5-Diamino-4-(4-oxo-4,5-dihydrothiazol-2-yl)-N-phenylthiophene-2-carboxamide (15)
Dark crystals (1,4-dioxane), yield 2.26 g (70%), m.p. 223˚C - 226˚C; IR (KBr) (υ-cm−1): 3428 - 3400 (2NH2), 3056 (CH aromatic), 2978 (CH2), 1723 - 1682 (2 C=O), 1577 (C=C). 1H- NMR (DMSO-d6) δ ppm: 4.35, 5.15 (2s, 4H, 2NH2), 6.06 (s, 2H, thiazole CH2), 7.28 - 7.43 (m, 5H, C6H5), 8.20 (s, 1H, NH). MS m/e = 332 (M+, 20); Anal. Calcd. for C14H12N4O2S2: C, 50.59; H, 3.64; N, 16.86; S, 19.29%. Found, C, 50.88; H, 3.83; N, 16.73; S, 19.32%.
Ethyl 5-amino-3-methyl-4-(4-oxo-4,5-dihydrothiazol-2-yl)thiophene-2-carboxylate (17)
Yellow crystals (1,4-dioxane), yield 1.99 g (70%), m.p. 166˚C - 168˚C; IR (KBr) (υ-cm−1): 3490, 3341(NH2), 2980, 2880 (CH3, CH2), 1683, 1672 (2C=O). 1H-NMR (DMSO-d6) δ ppm: 1.13 (t, 3H, J = 6.97 Hz, CH3), 2.61 (s, 3H, CH3), 4.21 (q, 2H, J = 6.67 Hz, CH2), 5.15 (s, 2H, NH2), 6.18 (s, 2H, thaizole CH2). MS m/e = 284(M+, 20); Anal. Calcd. for C11H12N2O3S2: C, 46.46; H, 4.25; N, 9.86; S, 22.55%. Found, C, 46.70; H, 4.28; N, 9.68; S, 22.71%.
General procedure for the synthesis of the hydrazide derivatives 19a, b
To a solution of compound 17 (2.84 g, 0.01 mol) in 1,4-dioxane (40 mL) either hydrazine hydrate (0.50 g, 0.01 mol) or phenylhydrazine (1.08 g, 0.01 mol) was added. The reaction mixture in each case was heated under reflux for 4 h then poured onto ice/water containing few drops of hydrochloric acid and the formed solid product was collected by filtration and crystallized from 1,4-dioxane.
5-Amino-3-methyl-4-(4-oxo-4,5-dihydrothiazol-2-yl)thiophene-2-carbohydrazide (19a)
Yellow crystals (1,4-dioxane), yield 0.43 g (16%), m.p. 122˚C - 125˚C; IR (KBr) (υ-cm−1): 3490 - 3348 (2NH2, NH), 2974, 2883 (CH3, CH2), 1689, 1682 (2C=O). 1H-NMR (DMSO-d6) δ ppm: 2.61 (s, 3H, CH3), 4.56, 5.11 (2s, 4H, 2NH2), 6.18 (s, 2H, thaizole CH2), 8.15 (s, 1H, NH). MS m/e = 270(M+, 16); Anal. Calcd. for C9H10N4O2S2: C, 39.99; H, 3.73; N, 20.73; S, 23.72%. Found, C, 40.21; H, 3.82; N, 20.81; S, 24.01%.
5-Amino-3-methyl-4-(4-oxo-4,5-dihydrothiazol-2-yl)-N'-phenylthiophene-2-carbohydrazide (19b)
Yellow crystals (1,4-dioxane), yield 2.28 g (66%), m.p. 142˚C - 145˚C; IR (KBr) (υ-cm−1): 3484 - 3328 (NH2, 2NH), 2980, 2881 (CH3, CH2), 1688, 1684 (2C=O), 1651 (C=N). 1H-NMR (DMSO-d6) δ ppm: 2.67 (s, 3H, CH3), 4.58 (s, 2H, NH2), 6.16 (s, 2H, thaizole CH2), 7.35 - 7.38 (m, 5H, C6H5), 8.20, 8.29 (2s, 2H, 2NH). MS m/e = 346 (M+, 28); Anal. Calcd. for C15H14N4O2S2: C, 52.01; H, 4.07; N, 16.17; S, 18.51%. Found, C, 51.99; H, 3.93; N, 15.86; S, 18.44%.
5. Conclusion
We have reported a convenient synthesis of variety compounds from compound 1 to 19b derivatives. The cytotoxicity of some derivatives towards three types of cancer cell lines was studied. Most of the synthesized compounds were found to be cytotoxic and hence deserve further pharmacological investigation. Compounds 3, 5, 9c, 11, 13a, 13c, 17 and 19b were the most active compounds towards the three cancer cell lines. The results of these investigations will be published in due time.
Cite this paper
Eman M. Samir,Amr S. Abouzied,Faten I. Hamed, (2016) The Synthesis and Cytotoxicity of Novel Thiophene Derivatives Derived from 2-(4-Oxo-4,4-Dihydrothiazol-2-yl) Acetonitrile. International Journal of Organic Chemistry,06,85-94. doi: 10.4236/ijoc.2016.62009
References
- 1. Atassi, G. and Tagn, H.J. (1975) A New Antitumor Drug I. Effect on Experimental Tumors and Factors Influencing Effectiveness. European Journal of Cancer and Clinical Oncology, 11, 599-607. http://dx.doi:10.1016/0014-2964(75)90092-4
- 2. Ye, L., He, J., Hu, Z., Dong, Q., Wang, H., Fu, F. and Tian, J. (2013) Antitumor Effect and Toxicity of Lipusu in Rat Ovarian Cancer Xenografts. Food & Chemical Toxicology, 52, 200-206. http://doi:10.1016/j.fct.2012.11.004
- 3. Hama, S., Utsumi, S., Fukuda, Y., Nakayama, K., Okamura, Y., Tsuchiya, H., Fukuzawa, K., Harashima, H. and Kogure, K. (2012) Development of a Novel Drug Delivery System Consisting of an Anti-tumor Agent Tocopheryl Succinate. Journal of Controlled Release, 161, 843-851. http://doi:10.1016/j.jconrel.2012.05.031
- 4. Fakhr, I.M., Radwan, M.A., El-Batran, S., Abd El-Salam, O.M. and El-Shenawy, S.M. (2009) Synthesis and Pharmacological Evaluation of 2-Substituted Benzo[b]Thiophenes as Anti-Inflammatory and Analgesic Agents. European Journal of Medicinal Chemistry, 44, 1718-1725. http://doi:10.1016/j.ejmech.2008.02.034
- 5. Bondock, S., Fadaly, W. and Metwally, M.A. (2010) Synthesis and Antimicrobial Activity of Some New Thiazole, Thiophene and Pyrazolederivatives Containing Benzothiazole Moiety. European Journal of Medicinal Chemistry, 45, 3692-3701. http://dx.doi.org/10.1016/j.ejmech.2010.05.018
- 6. Ryu, C.K., Lee, S.K., Han, J.Y., Jung, O.J., Lee, J.Y. and Jeong, S.H. (2005) Synthesis and Antifungal Activity of 5-Arylamino-4,7-Dioxobenzo[b]Thiophenes. Bioorganic & Medicinal Chemistry Letters, 15, 2617-2620. http://doi.org/10.1016/j.bmcl.2005.03.042
- 7. Athri, P., Wenzler, T., Ruiz, P., Brun, R., Boykin, D.W., Tidwell, R. and Wilson, W.D. (2006) 3D QSAR on a Library of Heterocyclic Diamidine Derivatives with Antiparasitic Activity. Bioorganic & Medicinal Chemistry, 14, 3144-3152.
- 8. Hudson, J.B., Graham, E.A., Miki, N., Towers, G.H.N., Hudson, L.L., Rossi, R., Carpita, A. and Neri, D. (1989) Photoactive Antiviral and Cytotoxic Activities of Synthetic Thiophenes and Their Acetylenic Derivatives. Chemosphere, 19, 1329-1343. http://dx.doi.org/10.1016/0045-6535(89)90080-5
- 9. Molvi, K.I., Vasu, K.K., Yerande, S.G., Sudarsanam, V. and Haque, N. (2007) Syntheses of New Tetrasubstitutedthiophenes as Novel Anti-Inflammatory Agents. European Journal of Medicinal Chemistry, 42, 1049-1058. http://dx.doi.org/10.1016/j.ejmech.2007.01.007
- 10. Kulandasamy, R., Adhikari, A.V. and Stables, J.P. (2009) A New Class of Anticonvulsants Possessing 6 Hz Activity: 3,4-Dialkyloxy Thiophenebishydrazones. European Journal of Medicinal Chemistry, 44, 4376-4384. http://dx.doi.org/10.1016/j.ejmech.2009.05.026
- 11. Jung, H.J., Song, Y.S., Lim, C.J. and Park, E.H. (2009) Anti-Inflammatory, Anti-Angiogenic and Anti-Nociceptive Activities of an Ethanol Extract of Salvia plebeian. R. Brown. Journal of Ethnopharmacology, 126, 355-360. http://dx.doi.org/10.1016/j.jep.2009.08.031
- 12. Chi, W.O. and Chang, L.H. (1986) Antitumor Antibiotics Drug Design. Part II. Synthesis of 4-Ethylamido [5,(2’- Thienyl)-2-Thiophene] Imidazole Iron(II) Complex, a New N2S2-Metallocycle with a “Built in” Intercalating Moiety Which Causes DNA Scissioning in Vitro. Inorganic Chimica Acta, 125, 203-206. http://dx.doi.org/10.1016/S0020-1693(00)81212-8
- 13. Friedman, D.C.S. and Friedman, P. (1995) A Theoretical Study of 2,2’;5’,2”-Terthiophene (α-T) and Its Analogs. Part 1. Correlation of Electronic Structure and Energies with Herbicidal Phototoxicity. Journal of Molecular Structure: THEOCHEM, 333, 71-78. http://dx.doi.org/10.1016/0166-1280(94)03930-J
- 14. Parai, M.K., Panda, G., Chaturvedi, V., Manju, Y.K. and Sinha, S. (2008) Thiophene Containing Triarylmethanes as Antitubercular Agents. Bioorganic & Medicinal Chemistry Letters, 18, 289-292. http://dx.doi.org/10.1016/j.bmcl.2007.10.083
- 15. Caridha, D., Kathcart, A.K., Jirage, D. and Waters, N.C. (2010) Activity of Substituted Thiophene Sulfonamides against Malarial and Mammalian Cyclin Dependent Protein Kinases. Bioorganic & Medicinal Chemistry Letters, 20, 3863-3867. http://dx.doi:10.1016/j.bmcl.2010.05.039
- 16. Fear, G., Komarnytsky, S. and Raskin, I. (2007) Protease Inhibitors and Their Peptidomimetic Derivatives as Potential Drugs. Pharmacology & Therapeutics, 113, 354-368. http://dx.doi:10.1016/j.pharmthera.2006.09.001
- 17. Al-Najjar, B.O., Wahab, H.A., Muhammad, T.S.T., Shu-Chien, A.C., Noruddin, N.A.A. and Taha, M.O. (2011) Discovery of New Nanomolar Peroxisome Proliferator-Activated Receptor γ Activators via Elaborate Ligand-Based Modelling. European Journal of Medicinal Chemistry, 46, 2513-2529. http://dx.doi:10.1016/j.ejmech.2011.03.040
NOTES
*Corresponding author.