Journal of Crystallization Process and Technology
Vol.05 No.03(2015), Article ID:57744,4 pages
10.4236/jcpt.2015.53006
Dispersion and Polar Component of Specific Surface Free Energy of NaCl(100), KCl(100), and KBr(100) Single Crystal Surfaces
Takaomi Suzuki*, Yuya Yamada
Department of Environmental Science and Technology, Faculty of Engineering, Shinshu University, Wakasato, Nagano, Japan
Email: *takaomi@shinshu-u.ac.jp
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 14 May 2015; accepted 29 June 2015; published 3 July 2015
ABSTRACT
Contact angle of ethylene glycol and formamide on (100) faces of NaCl, KCl, and KBr single crystal was measured, and the specific surface free energy (SSFE) was calculated. Dispersion component of the SSFE was 90.57, 93.78, and 99.52 mN∙m−1 for NaCl, KCl, and KBr, respectively. Polar component of the SSFE was 1.05, 0.65, and 0.45 mN∙m−1 for NaCl, KCl, and KBr. Such a large ratio of dispersion component of SSFE results from the neutrality of the crystal surface of alkali halide. Lattice component of alkali halide is 780, 717 and 689 kJ∙mol−1 for NaCl, KCl, and KBr. The larger lattice enthalpy decreases dispersion component, and increases polar component of the SSFE. The larger lattice enthalpy is considered to enhance the rumpling of the crystal surface more strongly, and such rumpling is considered to decrease the neutrality of the crystal surface.
Keywords:
Component, Specific Surface Free Energy, Crystal Growth, Mineral Salt, Morphology

1. Introduction
The relationship between the specific surface free energy (SSFE) and the contact angle of a liquid is shown by Young’s equation [1] :
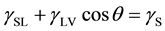 (1)
(1)
where 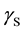 is the SSFE of the solid,
is the SSFE of the solid, 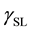 is the interfacial tension between the solid and the liquid, and
is the interfacial tension between the solid and the liquid, and 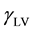 is the surface tension of the liquid. Because there are two unknown parameters
is the surface tension of the liquid. Because there are two unknown parameters 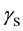 and
and 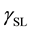 in Equation (1), we
in Equation (1), we
cannot introduce 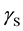 from a single contact angle,
from a single contact angle, 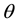 , only. In order to evaluate the SSFE from the contact angle of liquid, several models are proposed [2] - [6] . For example, Fowkes [5] proposed that the surface tension could be described as a sum of the dispersion component and the polar component as,
, only. In order to evaluate the SSFE from the contact angle of liquid, several models are proposed [2] - [6] . For example, Fowkes [5] proposed that the surface tension could be described as a sum of the dispersion component and the polar component as,
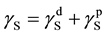 (2)
(2)
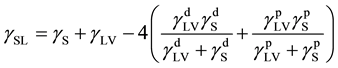 (3)
(3)
where 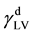 and
and 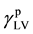 are dispersion and polar component of the surface tension of the liquid, respectively. The values of
are dispersion and polar component of the surface tension of the liquid, respectively. The values of 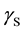 can be obtained from
can be obtained from 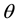 as
as
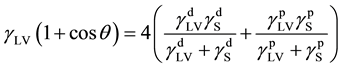 (4)
(4)
2. Experimental
Three kinds of synthetic alkali halide single crystals, NaCl, KCl, and KBr from Furu-Uchi Chemicals were used as sample crystals. Each alkali halide crystal was cleaved using sharp edge of a knife and (100) surface was prepared. Droplet of formamide or ethylene glycol was dropped on the cleaved face of each crystal using micropipette. The droplets sized ~0.1 mm3 were observed using digital camera with a magnifying lens. We took more than 40 photographs for each crystal face and used the photographs in which the boundary between the liquid and solid was clearly recognized the contact angles of the droplets were measured manually using printed photographs.
3. Results and Discussion
Average and standard deviation of contact angles of ethylene glycol and formamide on NaCl, KCl, and KBr are summarized in Table 1.
The contact angles of liquids on alkali halide crystal are much smaller than those on inorganic oxide crystals [10] . The values of dispersion and polar component of the SSFE can be calculated from the contact angle of liquids using Equation (4). The dispersion and polar components of ethylene glycol are 30.1 and 17.6 mN∙m−1, and those of formamide are 39.5 and 18.7 mN∙m−1 [2] .
Calculated SSFE of NaCl, KCl, and KBr and dispersion and polar components of them are summarized in Table 2. In our previous research, we did not discuss the dispersion and polar component separately, but we discussed summed SSFE only [7] - [9] .
Table 1. Average and standard deviation of the contact angles of ethylene glycol 

Here we re-calculated 





Polar component of SSFE is caused by the potential energy of interaction between two permanent dipoles. On the other hand, the dispersion component results from interaction between two induced dipoles. On (100) face of these alkali halide crystals, same number of anion and cation arrange alternately, which compose neutral surface as shown in Figure 1(a). Therefore, interaction between induced-dipoles is dominant and the dipole-dipole interaction should be subordinate.
The relationship between dispersion component of SSFE and lattice enthalpy was compared as shown in Figure 2, and the polar components of SSFE are also compared with lattice enthalpy as shown in Figure 3. The lattice enthalpy for NaCl, KCl, and KBr is 787, 717, and 689 kJ∙mol−1, respectively [11] .

Figure 1. Neutral surface of NaCl (a) and rumpled surface (b).
Figure 2. Dispersion component of the SSFE of NaCl, KCl, and KBr, as a function of lattice enphalpy.
Table 2. Specific surface free energy (SSFE) and dispersion and polar components of that on (100) face of NaCl, KCl, and KBr. The SSFE of some inorganic oxide re-calculated from our former experimental results are show in the bottom. The values have 5% - 10% fluctuation depending on individual samples of inorganic oxide crystals.
Figure 3. Polar component of SSFE of NaCl, KCl, and KBr, as a function of lattice enthalpy.
The larger lattice enthalpy causes the smaller dispersion component of SSFE. The larger enthalpy causes the larger value of polar component of the SSFE of each alkali halide crystal. Though the potential energy for each ion in the alkali halide crystal is symmetric, the potential for the surface ions is asymmetric. The ions are attracted inside of the crystal, which caused the rumpling of the crystal surface. The structure of crystal surface of NaCl, KCl, and KBr is studied by Vogt and Weiss using LEED, and reported that the rumpling of the first layer (Δ) are 0.007, 0.003, and 0.002 nm for NaCl, KCl, and KBr, respectively [12] [13] . The crystal with the larger lattice enthalpy has the larger rumpling. As shown in Figure 1(b), the surface of alkali halide is neutral, but the rumpling of the crystal face decreases the neutrality, and the surface became a little polar. NaCl crystal has the largest lattice enthalpy and the largest rumpling. Therefore, NaCl has the largest ratio of polar component and the smallest dispersion component. On the other hand, KBr has the smallest lattice enthalpy and the smallest rumpling, and KBr has the smallest rate of polar component and largest dispersion component.
4. Conclusions
Although contact angle of liquid on crystal surface is macroscopic value as we can see by our naked eye, the contact angle of liquid includes microscopic information such as atomic scale roughness of the crystal surface.
References
- Good, R.J. (1992) Contact Angle, Wetting, and Adhesion: A Critical Review. Journal of Adhesion Science and Technology, 6, 1269-1302. http://dx.doi.org/10.1163/156856192X00629
- Shimizu, R.N. and Demarquette, N.R. (2000) Evaluation of Surface Energy of Solid Polymers Using Different Models. Journal of Applied Polymer Science, 76, 1831-1845. http://dx.doi.org/10.1002/(SICI)1097-4628(20000620)76:12<1831::AID-APP14>3.0.CO;2-Q
- Spelt, J.K and Li, D. (1996) In: Neumann, W.A. and Spelt, J.K., Eds., Applied Surface Thermodynamics, Marcel Dekker, New York, Chap. 5.
- Fox, H.W. and Zisman, W.A. (1950) The Spreading of Liquids on Low Energy Surfaces. I. Polytetrafluoroethylene. Journal of Colloid Science, 5, 514-531. http://dx.doi.org/10.1016/0095-8522(50)90044-4
- Fowkes, F.M. (1964) Attractive Forces at Interfaces. Industrial & Engineering Chemistry Research, 56, 40-52. http://dx.doi.org/10.1021/ie50660a008
- Wu, S. (1972) Calculation of Interfacial Tnsion in Polymer Systems. Journal of Polymer Science Part C, 34, 19-30. http://dx.doi.org/10.1002/polc.5070340105
- Suzuki, T. and Oda, M. (2011) Specific Surface Free Energy and the Morphology of Synthesized Ruby Single Crystals. Journal of Crystal Growth, 318, 76-78. http://dx.doi.org/10.1016/j.jcrysgro.2010.11.058
- Suzuki, T., Takahashi, K., Kawasaki, M. and Kagami, T. (2014) Specific Surface Free Energy of As-Grown and Polished Faces of Synthetic Quartz. Journal of Crystallization Process and Technology, 4, 177-184. http://dx.doi.org/10.4236/jcpt.2014.44022
- Suzuki, T., Takemae, H. and Yoshida, M. (2013) Thermodynamic Interpretation of the Morphology Individuality of Natural and Synthesized Apatite Single Crystals. Journal of Crystallization Process and Technology, 3, 119-122. http://dx.doi.org/10.4236/jcpt.2013.34019
- Suzuki, T., Nakayama, K. and Oishi, S. (2004) Surface Tension of Barium Chlorapatite Crystals Grown from Flux. Bulletin of Chemical Society of Japan, 77, 109-113. http://dx.doi.org/10.1246/bcsj.77.109
- Atkins, P. and de Paula, J. (2014) Atkins’ Physical Chemistry. 10th Edition, Oxford University Press, Oxford.
- Vogt, J. and Wess, H. (2001) The Structure of NaCl(100) and KCl(100) Single Crystal Surfaces: A Tensor Low Energy Electron Diffraction Analysis. Surface Science, 491, 155-168. http://dx.doi.org/10.1016/S0039-6028(01)01391-7
- Vogt, J. and Wess, H. (2002) The Structure of KBr(100) and LiF(100) Single Crystal Surfaces: A Tensor Low Energy Electron Diffraction Analysis. Surface Science, 501, 203-213. http://dx.doi.org/10.1016/S0039-6028(01)01963-X
NOTES
*Corresponding author.






