Journal of Analytical Sciences, Methods and Instrumentation
Vol.09 No.02(2019), Article ID:92888,9 pages
10.4236/jasmi.2019.92002
Triphenylmethanol and Tris(2-(hydroxymethyl)phenol) Derivatives: Synthesis and Application as Indicators for Acid-Base Volumetric Titration
Ryan Beni1,2*, William Boadi1, Jawzah Alnakhli1, Samiyah Alhamed1, Tiffany Robinson1, Melanie Mootry1, Nahom Iyob1, Jamill Jackson1, Natalie Spicer1, Anterrial Harris1, Ibrahim Bamidad1, Renner Antwi1, Shania Richardson1, Tralynn Williams2
1Department of Chemistry, Tennessee State University, Nashville, TN, USA
2Department of Chemistry, Columbus State University, Columbus, GA, USA
Copyright © 2019 by author(s) and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: April 19, 2019; Accepted: June 2, 2019; Published: June 5, 2019
ABSTRACT
Polyphenols are naturally occurring compounds found largely in fruits, vegetables, cereals and beverages. Currently, there is much interest in the potential health benefits of dietary plant polyphenols as antioxidants. The effect of polyphenols on human cancer cells is most often protective and induces a reduction in the number of tumors or rate of growth. During our course of study on anticancer prodrugs, twelve triphenylmethanol and one tris(2-(hydroxymethyl) phenol derivatives were synthesized as a carrier of several drugs with optimized lipophilicity. Besides application of these compounds as a foundation for anticancer drug delivery systems, these compounds were evaluated as indicators for the acid-base volumetric titration of a standard solution of hydrochloric acid with a standard solution of sodium hydroxide. The experiments indicated a moderate-to-sharp color transition of the solutions near the neutralization point for most indicators. These indicators may have potential applications for acid-base titrations in a narrow range.
Keywords:
Polyphenols, Triphenylmethanol, Acid-Base Indicators, Volumetric Titration, Neutralization

1. Background and Introduction
An acid-base indicator is a weak acid or a weak base. Any substance that undergoes a reversible chemical change when pH changes can be used as an acid-base indicator. However, a sharp change in color of the substance is required. The undissociated form of the indicator is a different color from the ionic form of the indicator. An Indicator does not change color from pure acid to pure alkaline at specific hydrogen ion concentration, but rather, a color change occurs over a range of hydrogen ion concentrations. This range is termed as the color change interval. It is expressed as a pH range.
There are several reports about household materials that can be used as acid/base indicators [1] [2] [3] [4] [5] . Most of these materials are natural products derived from plants and so-called phenolic antioxidants. Polyphenols are naturally occurring compounds found largely in fruits, vegetables, cereals and beverages. Fruits like grapes, apples, pears, cherries and berries contain up to 200 - 300 mg of polyphenols per 100 grams fresh weight [6] [7] [8] . In the last decade, there has been much interest in the potential health benefits of dietary plant polyphenols as antioxidants. The effect of polyphenols on human cancer cells is most often protective and induces a reduction in the number of tumors or rate of growth. These effects have been observed at various sites including the mouth, stomach, duodenum, colon, liver, lungs, mammary glands and skin. Many polyphenols, such as quercetin, catechins, isoflavones, lignans, flavanones, ellagic acid, red wine polyphenols, resveratrol and curcumin have been tested; all of them showed protective effects in some models although their mechanisms of action were found to be different. [9] [10] Polyphenols influence the metabolism of pro-carcinogens by modulating the expression of cytochrome P450 enzymes involved in their activation of carcinogens [11] [12] .
To take advantage of the indicator properties of polyphenolic antioxidants, several polyphenolic derivatives were designed and synthesized and their color property at different pH have been studied.
To best of our knowledge, this is the first time these polyphenolic antioxidants are being reported as indicators for acid-base volumetric titration.
2. Discussion
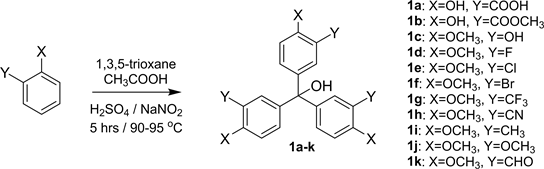
The synthesis of triphenylmethanol derivatives 1a-k was accomplished by using a modified method based on literature [13] [14] (Scheme 1). In this regard, 1,3,5-trioxane was added to a solution of phenol or anisol derivatives followed
Scheme 1. Synthesis of triphenylmethanol derivatives 1a-k.
by heating of the mixtures. Then, a mixture of sulfuric acid in glacial acetic acid was added to the mixtures and the stirring of the solutions was continued. Finally, the reaction mixtures were cooled down and a solution of sodium nitrite and phenol or anisol derivatives in concentrated sulfuric acid were added to the reaction mixtures. The mixtures were then stirred at room temperature to give the triphenylmethanol 1a-k derivatives which were further purified on CombiFlash Rf-200 chromatography to yield pure products (63% - 87%).
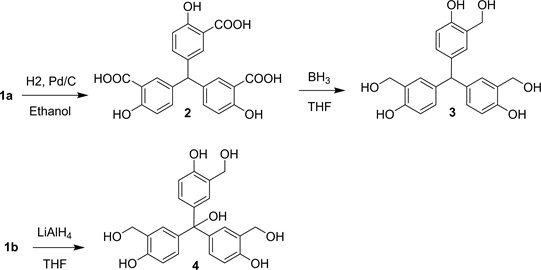
For the synthesis of tris(2-(hydroxymethyl)phenol derivatives, the reduction of 5,5',5"-(hydroxymethanetriyl)tris(2-hydroxybenzoic acid) (1a) in the presence of hydrogen (H2) and palladium/charcoal afforded 5,5',5"-methanetriyltris (2-hydroxybenzoic acid) (2). Further reduction of 2 with a borane solution in THF gave 4,4',4"-methanetriyltris(2-(hydroxymethyl)phenol) (3). The reduction of (1b) with a suspension of LiAlH4 in THF produced 4,4',4"-(hydroxymethanetriyl)tris(2-(hydroxymethyl)phenol) (4). Scheme 2 illustrates the synthesis of tris(2-(hydroxymethyl)phenol) derivatives 3, 4.
The synthesized compounds 1a-k, 3 and 4 were then evaluated as indicators for the acid-base volumetric titration of a standard solution of 0.001 M HCl with a standard solution of 0.001 M NaOH. The experiments showed a sharp color change of the solution from light yellow to light green at pH = 6.44 - 7.08 for 1a, from light yellow to dark green at pH = 6.59 - 8.18 for 1b, from light violet to dark red at pH = 6.56 - 7.82 for 1c, from light yellow to dark violet at pH = 6.45 - 7.53 for 1d, from yellow to dark green at pH = 6.87 - 8.24 for 1e, from orange to dark violet at pH = 6.55 - 7.78 for 1f, from colorless to light red at pH = 6.79 - 8.34 for 1g, from colorless to red at pH = 7.02 - 7.53 for 1h, from yellow to dark violet at pH = 6.85 - 7.59 for 1i, from light violet to red at pH = 7.24 - 7.78 for 1j, from colorless to blue violet at pH = 6.70 - 7.86 for 1k, from dark orange to light yellow at pH = 7.43 - 8.12 for 3 and from dark red to light yellow at pH = 6.45 - 7.23 for 4. These indicators may have potential applications for acid-base titrations in a narrow range.
Scheme 2. Synthesis of triphenylmethanoltris(2-(hydroxymethyl)phenol derivatives 3, 4.
3. Experimental
General Information: The chemical structures of products were characterized by a PE Biosystems Mariner API time-of-flight electrospray mass spectrometer. The volumetric titration with standard solutions of 0.001 M NaOH and 0.001 M HCl were done at 25˚C and pKa values were calculated by Henderson–Hasselbalch equation for the neutralization equilibrium point. The product yields for triphenylmethanol derivatives (1a-k) were calculated based on 1,3,5-trioxane starting material as a limiting reagent, i.e. the weight of pure isolated product was divided to the theoretical weight of the product assuming complete reaction and consumption of limiting reagent (trioxane) in course of the reaction.
3.1. Preparation of Triphenylmethanol Derivatives (1a-k)
1,3,5-trioxane (15 mmole) was added to salicylic acid, methyl salicylate, 2-methoxyphenol, 2-fluoroanisole, 2-chloroanisole, 2-bromoanisole, 2-(trifluoromethyl) anisole, 2-methoxybenzonitrile, 2-methylanisole, 1,2-dimethoxybenzene or 2-methoxybenzaldehyde solutions (100 mmole) in 10 mL glacial acetic acid. The mixtures were heated to reach to 90˚C - 95˚C, then 1 mL of a mixture of sulfuric acid: glacial acetic acid (1:5, v/v) was added dropwise and the solution and the stirring were continued for 5 h at 90˚C - 96˚C. The reaction mixture was then cooled down to 0˚C using an ice bath and a homogenous solution of sodium nitrite (1.0 g, 15 mmole) and salicylic acid, methyl salicylate, 2-methoxyphenol, 2-fluoroanisole, 2-chloroanisole, 2-bromoanisole, 2-(trifluoromethyl) anisole, 2-methoxybenzonitrile, 2-methylanisole, 1,2-dimethoxybenzene or 2-methoxybenzaldehyde (15 mmole) in 10 ml concentrated sulfuric acid was added to the reaction mixture. The ice bath was removed and the stirring was continued at room temperature for an additional 24 hr. The mixture was then poured into crushed ice (100 g) with stirring. The precipitate was filtered off, dried under vacuum and was further purified on C18 column and hexanes/ethyl acetate as solvent using a TeledyneCombiFlash® Rf-200 chromatography machine with the gradient system set at a constant flow rate of 25 ml/min to yield pure products in 63% - 87% yield. 5,5',5''-methanetriyltris (2-hydroxybenzoic acid) (1a) (4.43 g, 67%), MS (ESI-TOF) (m/z) calcd. 441.1, found 441.2 [M + H]+; trimethyl 5,5',5''-(hydroxymethanetriyl) tris(2-hydroxybenzoate) (1b), (5.57 g, 77%), MS (ESI-TOF) (m/z) calcd. 483.1, found 483.1 [M + H]+; tris(3-hydroxy-4-methoxyphenyl)methanol (1c), (4.72 g, 79%), MS (ESI-TOF) (m/z) calcd. 421.1, found 421.2 [M + Na]+; tris(3-fluoro-4-methoxyphenyl)methanol (1d), (3.83 g, 63%), MS (ESI-TOF) (m/z) calcd. 405.1, found 405.0 [M + H]+; tris(3-chloro-4-methoxyphenyl)methanol (1e), (4.51 g, 66%), MS (ESI-TOF) (m/z) calcd. 453.0, found 452.9 [M + H]+; tris(3-bromo-4-methoxyphenyl)methanol (1f), (6.43 g, 73%), MS (ESI-TOF) (m/z) calcd. 584.9, found 584.9 [M + H]+; tris(3-trifluoromethyl-4-methoxyphenyl)methanol (1g), (5.91 g, 71%), MS (ESI-TOF) (m/z) calcd. 555.1, found 555.1 [M + H]+; tris(3-cyano-4-methoxyphenyl)methanol (1h) (4.59 g, 72%), MS (ESI-TOF) (m/z) calcd. 448.1, found 448.0 [M + Na]+; tris(3-methyl-4-methoxyphenyl)methanol (1i), (4.77 g, 81%), MS (ESI-TOF) (m/z) calcd. 415.2, found 415.3 [M + Na]+; tris(3,4-dimethoxyphenyl)methanol (1j), (5.75 g, 87%), MS (ESI-TOF) (m/z) calcd. 441.2, found 441.1 [M + H]+; 5,5',5''-(hydroxymethanetriyl)tris(2-methoxybenzaldehyde) (1k), (4.37 g, 67%), MS (ESI-TOF) (m/z) calcd. 457.1, found 457.1 [M + Na]+.
3.2. Preparation of 5,5',5''-Methanetriyltris(2-Hydroxybenzoic Acid) (2)
5,5',5''-(hydroxymethanetriyl)tris(2-hydroxybenzoic acid); (1) (2.20 gm, 5 mmol) was added into absolute ethanol (50 mL). Palladium/charcoal (10%, 100 mg) was added to the solution and the mixture was shaken under hydrogen gas at 60 psi using a hydrogenation apparatus for 96 h. Then the Palladium/charcoal catalyst was filtered off using celite and washed with ethanol. The solvent of filtrate was removed under reduced pressure to yield 5,5',5''-methanetriyltris(2-hydroxybenzoic acid) (2) (1.89 g, 89%). MS (ESI-TOF) (m/z) calcd. 425.0873, found 425.0894 [M + H]+, Melting point 280˚C - 282˚C. This dark red product was used for the next step of reaction without further purification.
3.3. Preparation of 4,4',4''-Methanetriyltris(2-(Hydroxymethyl)Phenol) (3)
5,5',5''-methanetriyltris(2-hydroxybenzoic acid) (2), (1.50 gm, 3.5 mmol) was dissolved in 50 mL THF under anhydrous condition [15] [16] . The solution which was equipped with a magnetic stirrer and ice bath, was cooled down to 0˚C and then a 1M borane solution in THF (45 mL, 45 mmol) was added dropwise over a period of 90 min. After the completion of addition of 1M borane solution, the ice bath was removed, and the reaction mixture was allowed to warm up to room temperature. The resulting mixture was stirred for 1 hr at room temperature and then was refluxed at 65˚C for 3 hrs. At the end of which analysis of a small aliquot of the reaction mixture indicated the completion of the reaction. The reaction mixture was carefully quenched with 15 mL of water, concentrated by rotary evaporation, and then was made acidic with a pH of 6.5 by addition of 1N HCl and subsequently extracted with Et2O. The organic layer was dried over MgSO4, filtered off and the solvent was removed by rotary evaporation. The product was isolated by flash chromatography on silica gel using EtOAc: MeOH (1:1 v/v) as eluent to yield pure 4,4',4''-methanetriyltris(2-(hy- droxymethyl)phenol) (3) (1.02 g, 76%). MS (ESI-TOF) (m/z) calcd. 383.1495, found 383.1612 [M + H]+. The compound’s color was pinkish red.
3.4. Preparation of 4,4',4''-(Hydroxymethanetriyl)tris(2-(hydroxymethyl) phenol)4
A suspension of LiAlH4 (3.0 g, 80 mmol) in 50 mL anhydrous THF was prepared. The suspension was cooled down to 0˚C using an ice bath. Then a solution of trimethyl 5,5',5''-(hydroxymethanetriyl)tris(2-hydroxybenzoate) (1b), (2 g, 4.15 mmol) in 50 mL anhydrous THF was added dropwise to the LiAlH4 solution during 1 h at 0˚C. Then the ice bath was removed and after reaching room temperature, the mixture was refluxed for 4 h. The reaction mixture was then cooled to room temperature and quenched by the sequential addition of 3 mL of water, 15 mL of 10% sulfuric acid. Then the organic solvent was removed under vacuum and the residue was extracted using of diethyl ether (3 × 50 mL). The combined filtrate was dried over anhydrous sodium sulphate and the solvent was removed under vacuum. The product was isolated by flash chromatography on silica gel using Et2O: MeOH (1:1 v/v) as eluent to yield 4,4',4''-(hydroxymethanetriyl)tris(2-(hydroxymethyl)phenol) (4), (1.16 g, 70%). The color of the product was dark red. MS (ESI-TOF) (m/z) calcd. 399.1444, found 399.1456 [M + H]+.
3.5. Volumetric Titration of a Standard Solution of 0.001 M HCl with a Standard Solution of 0.001 M NaOH Using (1a-k), (3) and (4) as Acid-Base Indicator
The solutions of synthesized indicator compounds (1a-k), (3) and (4) were prepared by dissolving 0.25 g of each compound in ethanol to the final volume of 25 mL at room temperature followed by stirring for 10 min. Then the indicator was poured into a 25 mL bottle fitted with an eye dropper. 25 mL of a standard solution of 0.001 M HCl was placed in a 100 mL Erlenmeyer flask and after addition of 4 - 6 drops of indicator (1a-k), (3) and (4) solution, the titration was started with a standard solution of 0.001 M NaOH. The pH of HCl solution in the Erlenmeyer flask was carefully monitored with a FisherbrandTM accumetTM AB15 Basic pH Meter equipped with a conventional glass pH electrode during the titration. The color of the solution around the neutralization point was suddenly changed. Further increasing of the pH did not significantly change the color of the solution. The experiments were repeated 3 times for each compound.
4. Results
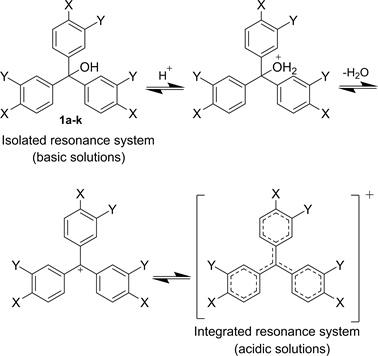
The triphenylmethanol derivatives are aromatic compounds, however in basic solutions, the aromaticity of phenolic rings is isolated. By decreasing the pH in acidic solutions, the hydroxy group of triphenylmethanol can absorb a proton and be eliminated from the molecule. The driving force for such a phenomenon is the chance of forming an integrated resonance system that is more stable. This phenomenon is responsible for changing the color in acidic and basic solutions. i.e. the isolated and integrated resonance systems absorb different wavelengths of light, therefore show different colors. Scheme 3 illustrates a mechanism of resonance structure conversion of triphenylmethanol derivatives 1a-k in basic and acidic solutions. Similar mechanisms may apply to indicator 3 and 4.
Table 1 indicates the color change, pH range for the color transition and pKa values of indicator compounds (1a-k), (3) and (4). The pKa values were calculated
Scheme 3. Resonance structure of triphenylmethanol derivatives 1a-k in basic and acidic solutions.
Table 1. Color change, pH range for the color transition and pKa values of indicator compounds 1a-k, 3 and 4.
according to Henderson-Hasselbalch equation for the neutralization equilibrium point at 25˚C. Table 1 shows the titration results and pKa values of indicators.
5. Conclusion
In summary, twelve triphenylmethanol and one tris(2-(hydroxymethyl)phenol derivatives were synthesized and tested as acid-base indicators. The experiments showed a sharp color change of the solutions near neutralization point for most indicators. These indicators may have potential applications for acid-base titrations in a narrow range.
Acknowledgements
We acknowledge the financial support from the National Cancer Institute, MMC-Vanderbilt-TSU Partners in Eliminating Cancer Disparities (MVTCP), Grant Number 5U54CA163066-03. We also acknowledge the financial support from the USDA National Institute of Food and Agriculture, Grant# TENX-1608-FS. We thank US Department of Education, Title III Part B, grant number P031B090214 for partial financial support. Jawzah Alnakhli and Samiyah Alhamed acknowledge the scholarship provided by the Saudi Arabian Cultural Mission to the US (SACM).
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Cite this paper
Beni, R., Boadi, W., Alnakhli, J., Alhamed, S., Robinson, T., Mootry, M., Iyob, N., Jackson, J., Spicer, N., Harris, A., Bamidad, I., Antwi, R., Richardson, S. and Williams, T. (2019) Triphenylmethanol and Tris(2-(hydroxymethyl)phenol) Derivatives: Synthesis and Application as Indicators for Acid-Base Volumetric Titration. Journal of Analytical Sciences, Methods and Instrumentation, 9, 13-21. https://doi.org/10.4236/jasmi.2019.92002
References
- 1. Atkins, P.W. (1987) Molecules. W. H. Freeman, Oxford, England.
- 2. Li, K.C. and Wagenknecht, A.C. (1956) Anthocyanin Pigments of Sour Cherries. Journal of the American Chemical Society, 78, 979-980. https://doi.org/10.1021/ja01586a029
- 3. Francis, F.J., Harborne, J.B. and Barker, W. (1966) Anthocyanins in the Lowbush Blueberry, Vaccinium Angusifolium. Journal of Food Science, 31, 583-587. https://doi.org/10.1111/j.1365-2621.1966.tb01908.x
- 4. Fuleki, T. (1969) The Anthocyanins of Strawberry, Rhubarb, Radish and Onion. Journal of Food Science, 34, 365-369. https://doi.org/10.1111/j.1365-2621.1969.tb10367.x
- 5. Lukton, A., Chichester, C.O. and Mackinney, G. (1955) Characterization of a Second Pigment in Strawberries. Nature, 176, 790-792. https://doi.org/10.1038/176790a0
- 6. Scalbert, A., Manach, C., Morand, C. and Remesy, C. (2005) Dietary Polyphenols and the Prevention of Diseases. Critical Reviews in Food Science and Nutrition, 45, 287-306. https://doi.org/10.1080/1040869059096
- 7. Spencer, J.P., El Mohsen, M.M.A., Minihane, A.M. and Mathers, J.C. (2008) Biomarkers of the Intake of Dietary Polyphenols: Strengths, Limitations and Application in Nutrition Research. British Journal of Nutrition, 99, 12-22. https://doi.org/10.1017/S0007114507798938
- 8. Bhooshan, P.K. and Rizv, S.I. (2009) Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Medicine and Cellular Longevity, 2, 270-278. https://doi.org/10.4161/oxim.2.5.9498
- 9. Yang, C.S., Landau, J.M., Huang, M.T. and Newmark, H.L. (2001) Inhibition of Carcinogenesis by Dietary Polyphenolic Compounds. Annual Review of Nutrition, 21, 381-406. https://doi.org/10.1146/annurev.nutr.21.1.381
- 10. Johnson, I.T., Williamson, G. and Musk, S.R.R. (1994) Anticarcinogenic Factors in Plant Foods: A New Class of Nutrients? Nutrition Research Reviews, 7, 175-204. https://doi.org/10.1079/NRR19940011
- 11. Talalay, P., De Long, M.J. and Prochaska, H.J. (1988) Identification of a Common Chemical Signal Regulating the Induction of Enzymes That Protect against Chemical Carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America, 85, 8261-8265. https://doi.org/10.1073/pnas.85.21.8261
- 12. Khan, N. and Mukhtar, H. (2008) Multitargeted Therapy of Cancer by Green Tea Polyphenols. Cancer Letters, 269, 269-280. https://doi.org/10.1016/j.canlet.2008.04.014
- 13. Shrestha, S., Bhattarai, B.R., Chang, K.J., Lee, K.-H. and Cho, H. (2007) Methylenedisalicylic Acid Derivatives: New PTP1B Inhibitors that Confer Resistance to Diet-Induced Obesity. Bioorganic & Medicinal Chemistry Letters, 17, 2760-2764. https://doi.org/10.1016/j.bmcl.2007.02.069
- 14. Mark, C., Suseela, K., Erik, D.C., Dominique, S., Mark, E.G. and Julie, A.B. (1991) Synthesis and anti-HIV activities of low molecular weight aurintricarboxylic acid fragments and related compounds. Journal of Medicinal Chemistry, 34, 337-342. https://doi.org/10.1021/jm00105a053
- 15. Brown, H.C., Heim, P. and Yoon, N.M. (1970) Selective Reductions. XV. Reaction of Diborane in Tetrahydrofuran with Selected Organic Compounds Containing Representative Functional Groups. Journal of the American Chemical Society, 92, 1637-1646. https://doi.org/10.1021/ja00709a037
- 16. Yoon, N.M., Pak, C.S., Brown, H.C., Krishnamurthy, S. and Stocky, T.P. (1973) Selective Reductions. XIX. Rapid Reaction of Carboxylic Acids with Borane-Tetrahydrofuran. Remarkably Convenient Procedure for the Selective Conversion of Carboxylic Acids to the Corresponding Alcohols in the Presence of Other Functional Groups. The Journal of Organic Chemistry, 38, 2786-2792. https://doi.org/10.1021/jo00956a011


