International Journal of Clinical Medicine
Vol.2 No.1(2011), Article ID:3924,4 pages DOI:10.4236/ijcm.2011.21001
The Expression of NF-κB in the Glomerulus of Hyperlipidemia Rats
![]()
1Department of Pathophysiology, West China Medical Center of Sichuan University, Chengdu, China; 2Department of Histology and Embryology, West China Medical Center of Sichuan University, Chengdu, China.
Email: {lixuan,pengjin}@scu.edu.cn
Received October 9th, 2010; revised December 14th, 2010; accepted December 21st, 2010.
Keywords: Omponent, Formatting, Style, Styling, Insert
ABSTRACT
Background: The present study was to explore the underlying mechanism of the nuclear factor-kappa B (NF-κB) and the level of monocyte chemoattractant protein-1 (MCP-1) in hyperlipidemia rats. Methods: Rats were given with high fat diet and vitamin D3 by intragastric administration. After four weeks, the level of the plasma cholesterol, low density lipoprotein cholesterol (LDLC), MCP-1 and NF-κB were detected by immunohistochemical method and enzyme linked immunosorbent assay (ELISA). Results: The levels of the plasma cholesterol and LDLC were higher than that of the control group. A significant in-crease for the expressions of MCP-1 and NF-κB was observed. Conclusion: This indicated that the activation of NF-κB could play a crucial role in glomerulus of hyperlipidemia rats.
1. Introduction
Hyperlipidemia is characterized by elevated serum total cholesterol, triglycerides, low HDL and increased apolipoprotein B and/or free fatty acids [1-3]. Hyperlipidemia is one important risk factor of glomeruli sclerosis [4-7]. It is very important to maintain the blood-lipid concentration and early treatment of hyperlipidemia. The report about the expression of NF-κB in the glomerulus of hyperlipidemia is not understood.
Nuclear factor-κB (NF-κB) presents in the cytoplasm of all cell types and comprises a family of dimeric transcription factors that regulate the expression of numerous genes involved in inflammation and cell proliferation [8].
Our study aimed to investigate the expression of NF-κB in the glomerulus of hyperlipidemia rats, so as to explore the underlying mechanism.
2. Material and Methods
2.1. Animal and Grouping
Twelve adult Sprague-Dawley male rats (weighing 200-250 g) were obtained from the Animal Experiment Center of Sichuan University. Animal experiments were carried out according to the NIH (USA) Guide for Care and Use of Laboratory Animal. The animal models of the experimental hyperlipdemia are adopted according to previous report [9]. Rats were randomly divided into the control group and experimental group. The control group was feed by normal food. The experimental group was feed with high fat food for 4 weeks containing 1% cholesterol, 0.35% cholic acid, 5% lard and 0.61% propylthiouracil after three days of VD3 (700,000 IU/Kg weight) gastric perfusion.
2.2. Detection of Blood Lipid
All rats were anesthetized by pentobarbital and the cardiac blood was collected. The plasma was incubated at 4℃ overnight. The serum was collected by centrifuging on 2500 r/min for 10 min. The blood levels of LDL were detected using automatic biochemistry analyzer system.
2.3. Enzyme Linked Immunosorbent Assay (ELISA)
ELISA was performed according to the manufacturer's protocol. A black 96-well Nunc Maxi Sorp plate was coated with strep tavidin in PBS overnight at room temperature, and washed three times with PBS. After washing, 10 μl samples or the standards were added to the appropriate wells. Both standards and samples were done in triplicate. Add 50 μl detection antibodies to each well and incubated for 2 h at room temperature. 50 μl strep tavidin-HRPs (diluted 1:200 in 2%BRA, PBS, 0.1% Tween-20) were added for 20 min at room temperature after washing. After a final wash, 100 μl TMBs (Super Signal ELISA Pico from Pierce) were added. The plate was read immediately on a micro plate reader. The data were calculated.
2.4. Immunohistochemistry
Immunohistochemical method was performed as described previously [10]. Briefly, paraffin sections were dewaxed and added 3% H2O2 to quench the endogenous peroxidase activity. This followed by incubation with NF-κB P65 mouse monoclonal IgG1 antibodies (SC-8008, Santa Cruz, 1:200) at 4℃ overnight. The sections were then washed three times and incubated with biotinylated Goat anti-mouse antibody (1:200, Santa Cruz), incubated in 37℃ 40 min. Positive immunoreactivity was visualized using hematoxylin staining.
The primary antibody was replaced with PBS in negative control group to ascertain the specificity of antibody.
2.5. Statistical Analysis
The data were analyzed statistically with one-way ANOVA by PEMS 3.1 software. The Student-NewmanKeuls (SNK) method was used to estimate the level of significance of differences between means. The statistical significance was defined as P < 0.01.
3. Results
3.1. The Change of Cholesterol and Low Density Lipoprotein Cholesterol (LDLC)
The levels of the plasma cholesterol and LDLC in blood plasma were higher than the control group at high fat diet (HFD) group for 4 weeks (Table 1).
3.2. The Microanatomy Alternation of Vessel Wall
The light microscope showed aorta with integrated tissue structure, three lays of the vessel wall (tunica intima, tunica media and tunica adventitia) is complete in control group, which is no inflammatory cell infiltration. The experimental group exhibited thicker vessel wall and
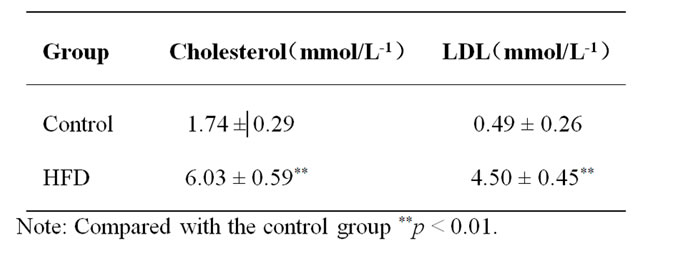
Table 1. The level of the plasma cholesterol and low density lipoprotein cholesterol (LDLC) in blood plasma (mean ± S.E.M).
gibbous tunica intima. Obvious inflammatory cell infiltration and foam cell was found under the tunica intima. Thicker tunica media and disorganized smooth muscle fiber was found in tunica media (Figure 1).
3.3. The Expression of NF-κBp65
No immuno-positive staining was detected in negative control group. NF-κBp65 immuno-positive products were observed mainly in the nucleus as brown-yellow deposits in the glomeruli. Few immune-positive products of NF-κBp65 were showed in the control group. Many immune-positive products for NF-κB were activated in the glomerular after high fat diet for 4 weeks (Figure 1). Positive staining was also observed in the endothelium of glomeruli.
3.4. MCP-1 Expression in Serum
The enzyme-linked immunosorbent assay (ELISA) showed that MCP-1 in serum noticeably increased (P < 0.01) after high fat diet 4 weeks. The level of MCP-1 in control group (62.64 ± 3.1) was lower than that in experimental group (156.37 ± 16.61) (Table 2)(P < 0.01).
4. Discussion
In our study, the plasma cholesterol and OX-LDL increased significantly after high fat diet 4 weeks, which corresponds to the model of hyperlipidemia[1-3]. At the same time, immunohistochemistry showed that NF-κB increased in glomerulus. This indicated NF-κB could play a crucial role in the glomerulus of hyperlipidemia. However, the activation of NF-κB is not only influenced
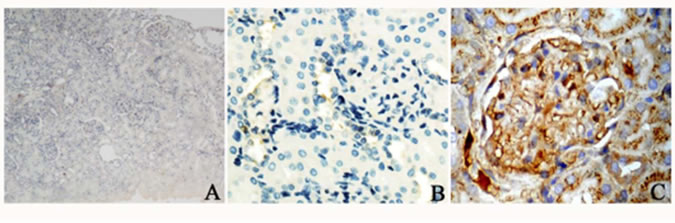
Figure 1. The immune histo-chemical staining of NF-κBp65. No immune-positive staining was detected in negative control group (A). NF-κBp65 immune-positive products were observed mainly in the nucleus in the glomeruli. Few immune-positive products for NF-κBp65 in the control group (B). Many immune positive products of the activated NF-κB in the glomerular in high fat diet group (C).
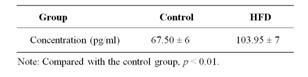
Table 2. The expression of MCP-1 in serum (mean ± S. E. M).
the arterial wall, but also the glomerulus and convoluted tubule [11]. Toma and his colleagues [12] have reported that hyperlipidemia is important in progressive nephron damage, and recent studies also showed the glomerulus has many structural features that resemble arteries commonly involved in atherosclerosis [13].
ELISA exhibited MCP-1 in serum noticeably increased after high fat diet 4 weeks. MCP-1 is crucial in recruiting and activating monocytes, mast cells and basophils during inflammation [14]. MCP-1 is produced by fibroblasts, endothelial cells, and so on [15]. The biological role of MCP-1 in vivo is still not entirely understood. Hyperlipidemia could increase monocyte adherence to vascular endothelium. This process is intermediated by MCP-112. Monocyte recruitment into sub endothelial space is primarily mediated by NF-κB-dependent gene expression. Once the NF-κB was active by the inflammatory response, the MCP-1 in plasma will increased. This increase would lead to multiple organs injury with the structural features with aorta endothelium [2]. The kidney parenchyma showed chronic inflammation responses in some degree after the hyperlipidemia induced by high fat diet.
This is the first time to report that NF-κB is expressed not only on the glomerulus but also in convoluted tubule. Since inflammatory cytokines is expressed in some vessel wall cell such as endothelium and smooth muscle cell, this suggests two possible explanations why the NF-κB is observed in the convoluted tubule. First, the inflammatory markers such as MCP-1 increasing in blood plasma could lead to generalized NF-κB’s activation. Second, the high blood lipids could lead to the activation of oxidative modification of low-Density lipoprotein (OX-LDL), and OX-LDL could directly affect the activation of NF-κB in convoluted tubule.
The present study provided a new insight to understand the role of NF-κB and MCP-1 in the glomerulus of hyperlipidemia rats. The activation of NF-κB and the upregulation of MCP-1 were observed after glomerulus injury. These findings indicated that chronic inflammation induced by hyperlipidemia could relate with renal injury.
5. Acknowledgements
We would like thank Dr Luo ZT for statistic counseling and Miss Zou Haoli for reviewing the English Language.
REFERENCES
- J. L. Beaumont, L. A. Carlson, G. R. Cooper, Z. Fejfar, D. S. Fredrickson and T. Strasser, “Classification of Hyperlipidaemias and Hyperlipoproteinaemias,” Bull World Health Organ, Vol. 43, No. 6, 1970, pp. 891-915.
- D. S. Fredrickson, R. I. Levy and R. S. Lees, “Fat Transport in Lipoproteins—An Integrated Approach to Mechanisms and Disorders,” New England Journal of Medicine, Vol. 276, No. 5, 1967, pp. 273-281. doi:10.1056/NEJM196702022760507
- R. J. Havel, “Pathogenesis, Differentiation and Management of Hypertriglyceridemia,” Advances in Internal Medicine, Vol. 15, 1969, pp. 117-154.
- A. D. Schachter, J. Strehlau, D. Zurakowski, et al., “Increased Nuclear Factor-KappaB and Angiotensinogen Gene Expression in Posttransplant Recurrent Focal Segmental Glomerulosclerosis,” Transplantation, Vol. 70, No. 7, 2000, pp. 1107-1110. doi:10.1097/00007890-200010150-00021
- C. C. Yu, C. W. Yang, M. S. Wu, et al., “Mycophenolatemofetil Reduces Renal Cortical Inducible Nitric Oxide Synthase mRNA Expression and Diminishes Glomerulosclerosis in MRL//pr Mice,” Journal of Laboratory and Clinical Medecine, Vol. 138, No. 1, 2001, pp. 69-77. doi:10.1067/mlc.2001.115647
- F. Zheng, Q. L. Cheng, A. R. Plati, et al., “The Glomerulosclerosis of Aging in Females: Contribution of the Proinflammatorymesangial Cell Phenotype to Macrophage Infiltration,” American Journal of Pathology, Vol. 165, No. 5, 2004, pp. 1789-1798. doi:10.1016/S0002-9440(10)63434-7
- A. Yamamoto, A. Kawaguchi, M. Harada-Shiba, M. Tsushima and S. Kojima, “Apheresis Technology for Prevention and Regression of Atherosclerosis: An Overview,” Therapeutic Apheresis, Vol. 1, No. 3, 1997, pp. 233-241. doi:10.1111/j.1744-9987.1997.tb00144.x
- A. Hoffmann and D. Baltimore, “Circuitry of Nuclear Factor Kappa B Signaling,” Immunological Reviews, Vol. 210, No. 4, 2006, pp. 171-186. doi:10.1111/j.0105-2896.2006.00375.x
- K. W. Walton, “Fat Transport by Lipoproteins in Health and Disease,” Journal of Atherosclerosis Research, Vol. 7, No. 5, 1967, pp. 533-536. doi:10.1016/S0368-1319(67)80031-0
- D. X. Qin, X. Zou, W. Luo, et al., “Expression of Some Neurotrophins in the Spinal Motoneurons after Cord Hemisection in Adult Rats,” Neuroscience letters, Vol. 410, No. 3, 2006, pp. 222-227. doi:10.1016/j.neulet.2006.10.006
- J. Morrissey and S. Klahr, “Transcription Factor NF-KappaB Regulation of Renal Fibrosis during Ureteral Obstruction,” Seminars in Nephrology, Vol. 18, No. 6, 1998, pp. 603-611.
- L. Toma, C. S. Stancu, G. M. Botez, A. V. Sima and M. Simionescu, “Irreversibly Glycated LDL Induce Oxidative and Inflammatory State in Human Endothelial Cells; Added Effect of High Glucose,” Biochemical and Biophysical Research Communications, Vol. 390, No. 3, 2009, pp. 877-882. doi:10.1016/j.bbrc.2009.10.066
- S. Wahyudi and D. Sargowo, “Green Tea Polyphenols Inhibit Oxidized LDL-Induced NF-KB Activation in Human Umbilical Vein Endothelial Cells,” Acta Medica Indonesiana, Vol. 39, No. 2, 2007, pp. 66-70.
- R. K. Tangirala, K. Murao and O. Quehenberger, “Regulation of Expression of the Human Monocyte Chemotactic Protein-1 Receptor (hCCR2) by Cytokines,” Journal of Biological Chemistry, Vol. 272, No. 12, 1997, pp. 8050-8056. doi:10.1074/jbc.272.12.8050
- K. S. Weber, P. J. Nelson, H. J. Grone and C. Weber, “Expression of CCR2 by Endothelial Cells: Implications for MCP-1 Mediated Wound Injury Repair and in Vivo Inflammatory Activation of Endothelium,” Arteriosclerosis, Thrombosis, and Vascular Biology, Vol. 19, No. 9, 1999, pp. 2085-2093.

