 J. Biomedical Science and Engineering, 2011, 4, 788-796 doi:10.4236/jbise.2011.412097 Published Online December 2011 (http://www.SciRP.org/journal/jbise/ JBiSE ). Published Online December 2011 in SciRes. http://www.scirp.org/journal/JBiSE A review of developments of EEG-based automatic medical support systems for epilepsy diagnosis and seizure detection Yuedong Song Computer Laboratory, University of Cambridge, Cambridge, United Kingdom. E-mail: ys340@cam.ac.uk Received 21 October 2011; revised 15 November 2011; accepted 5 December 2011. ABSTRACT Epilepsy is one of the most common neurological disorders-approximately one in every 100 people worldwide are suffering from it. The electroencepha- logram (EEG) is the most common source of infor- mation used to monitor, diagnose and manage neu- rological disorders related to epilepsy. Large amounts of data are produced by EEG monitoring devices, and analysis by visual inspection of long recordings of EEG in order to find traces of epilepsy is not rou- tinely possible. Therefore, automated detection of epilepsy has been a goal of many researchers for a long time. Until now, reviews of epileptic seizure de- tection have been published but none of them has specifically reviewed developments of automatic medical support systems utilized for EEG-based epi- leptic seizure detection. This review aims at filling this lack. The main objective of this review will be to briefly discuss different methods used in this research field and describe their critical properties. Keywords: Electroencephalo gram; Epileptic Seizure; Au- tomatic Diagnostic Systems; Feature Analysis; Recogni- tion 1. INTRODUCTION Epilepsy is a neurological disorder affecting around 1% of the world’s population (about 50 million people) [1]. An epileptic seizure can be characterized by means of paroxysmal occurrence of synchronous oscillations. This kind of seizures can mainly be divided into two classes in terms of the extent of connection of different brain fields: partial seizures and generalized seizures. Partial seizures begin from a circumscribed field of the brain, usually called epileptic foci. Determined by their type, they may or may not impair consciousness. Generalized seizures involve most fields of the brain and may cause loss of consciousness and muscle contractions or stiff- ness. Electroencephalography (EEG) is an important clinical tool, monitoring, diagnosing and managing neu- rological disorders related to epilepsy. In comparison with other approaches such as Magnetoencephalography (MEG) and functional Magnetic Resonance Imaging (fMRI), EEG is a clean, cost effective and safe technique for monitoring brain activity. In spite of available dietary, drug and surgical treat- ment options, currently nearly one out of three epilepsy patients cannot be treated. They are completely subject to the sudden and unforeseen seizures which have a great effect on their daily life, with temporary impair- ments of perception, speech, motor control, memory and/or consciousness. Many new therapies are being investigated and among them the most promising are implantable devices that deliver direct electrical stimula- tion to affected areas of the brain. These treatments will greatly depend on robust algorithms for seizure detection to perform effectively. Because the onset of the seizures cannot be predicted in a short period, a continuous re- cording of the EEG is required to detect epilepsy. How- ever, analysis by visual inspection of long recordings of EEG, in order to find traces of epilepsy, is tedious, time-consuming and high-cost. Therefore, automated detection of epilepsy has been a goal of many research- ers for a long time. Computers have long been suggested for handling this problem and thus, automatic medical support systems for identifying electroencephalographic changes have been under study for many years. The whole procedure can be divided into two modules: fea- ture extraction and classification (shown in Figure 1). The performance of automatic diagnosis systems de- pends on both the feature extraction methods and the classification algorithms applied. Until now, although many methodologies have been developed for automatic epileptic seizure detection, there is no literature specifi- cally contributing to the review of development of automatic medical support systems utilized for EEG- based epileptic seizure detection. In this review, we briefly investigate different approaches used in this re- search field and describe their critical properties. 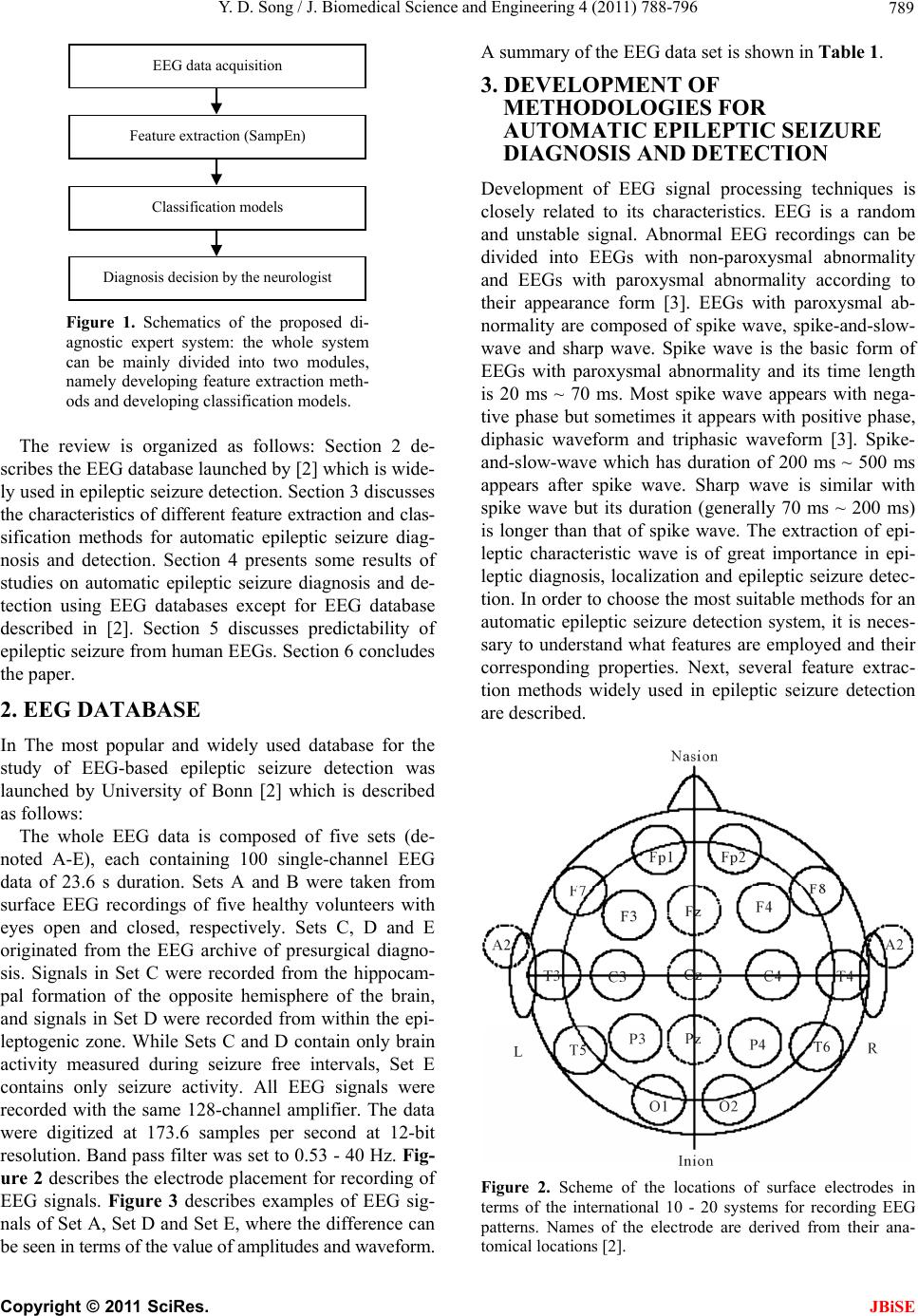 Y. D. Song / J. Biomedical Science and Engineering 4 (2011) 788-796 789 EEG d ata acq uisition Feature extraction (SampEn) Classification models Diagnosis decision by the neurologist Figure 1. Schematics of the proposed di- agnostic expert system: the whole system can be mainly divided into two modules, namely developing feature extraction meth- ods and developing classification models . The review is organized as follows: Section 2 de- scribes the EEG database launched b y [2] which is wide- ly used in epileptic seizure detection . Section 3 discusses the characteristics of differen t feature extraction and clas- sification methods for automatic epileptic seizure diag- nosis and detection. Section 4 presents some results of studies on automatic epileptic seizure diagnosis and de- tection using EEG databases except for EEG database described in [2]. Section 5 discusses predictability of epileptic seizure from human EEGs. Section 6 concludes the paper. 2. EEG DATABASE In The most popular and widely used database for the study of EEG-based epileptic seizure detection was launched by University of Bonn [2] which is described as follows: The whole EEG data is composed of five sets (de- noted A-E), each containing 100 single-channel EEG data of 23.6 s duration. Sets A and B were taken from surface EEG recordings of five healthy volunteers with eyes open and closed, respectively. Sets C, D and E originated from the EEG archive of presurgical diagno- sis. Signals in Set C were recorded from the hippocam- pal formation of the opposite hemisphere of the brain, and signals in Set D were recorded from within the epi- leptogenic zone. While Sets C and D contain only brain activity measured during seizure free intervals, Set E contains only seizure activity. All EEG signals were recorded with the same 128-channel amplifier. The data were digitized at 173.6 samples per second at 12-bit resolution. Band pass filter was set to 0.53 - 40 Hz. Fig- ure 2 describes the electrode placement for recording of EEG signals. Figure 3 describes examples of EEG sig- nals of Set A, Set D and Set E, where the difference can be seen in terms of the value of amplitudes and waveform. A summary of the EEG data set is shown in Table 1. 3. DEVELOPMENT OF METHODOLOGIES FOR AUTOMATIC EPILEPTIC SEIZURE DIAGNOSIS AND DETECTION Development of EEG signal processing techniques is closely related to its characteristics. EEG is a random and unstable signal. Abnormal EEG recordings can be divided into EEGs with non-paroxysmal abnormality and EEGs with paroxysmal abnormality according to their appearance form [3]. EEGs with paroxysmal ab- normality are composed of spike wave, spike-and-slow- wave and sharp wave. Spike wave is the basic form of EEGs with paroxysmal abnormality and its time length is 20 ms ~ 70 ms. Most spike wave appears with nega- tive phase but sometimes it appears with positive phase, diphasic waveform and triphasic waveform [3]. Spike- and-slow-wave which has duration of 200 ms ~ 500 ms appears after spike wave. Sharp wave is similar with spike wave but its duration (generally 70 ms ~ 200 ms) is longer than that of spike wave. The extraction of epi- leptic characteristic wave is of great importance in epi- leptic diagnosis, localization and epileptic seizure detec- tion. In order to choose the most suitable methods for an automatic epileptic seizure detection system, it is neces- sary to understand what features are employed and their corresponding properties. Next, several feature extrac- tion methods widely used in epileptic seizure detection are described. Figure 2. Scheme of the locations of surface electrodes in terms of the international 10 - 20 systems for recording EEG patterns. Names of the electrode are derived from their ana- omical locations [2]. t C opyright © 2011 SciRes. JBiSE  Y. D. Song / J. Biomedical Science and Engineering 4 (2011) 788-796 Copyright © 2011 SciRes. 790 (a) (b) (c) Figure 3. Sample EEG recordings. (a) Normal EEG; (b) Interictal EEG; (c) Ictal EEG. JBiSE 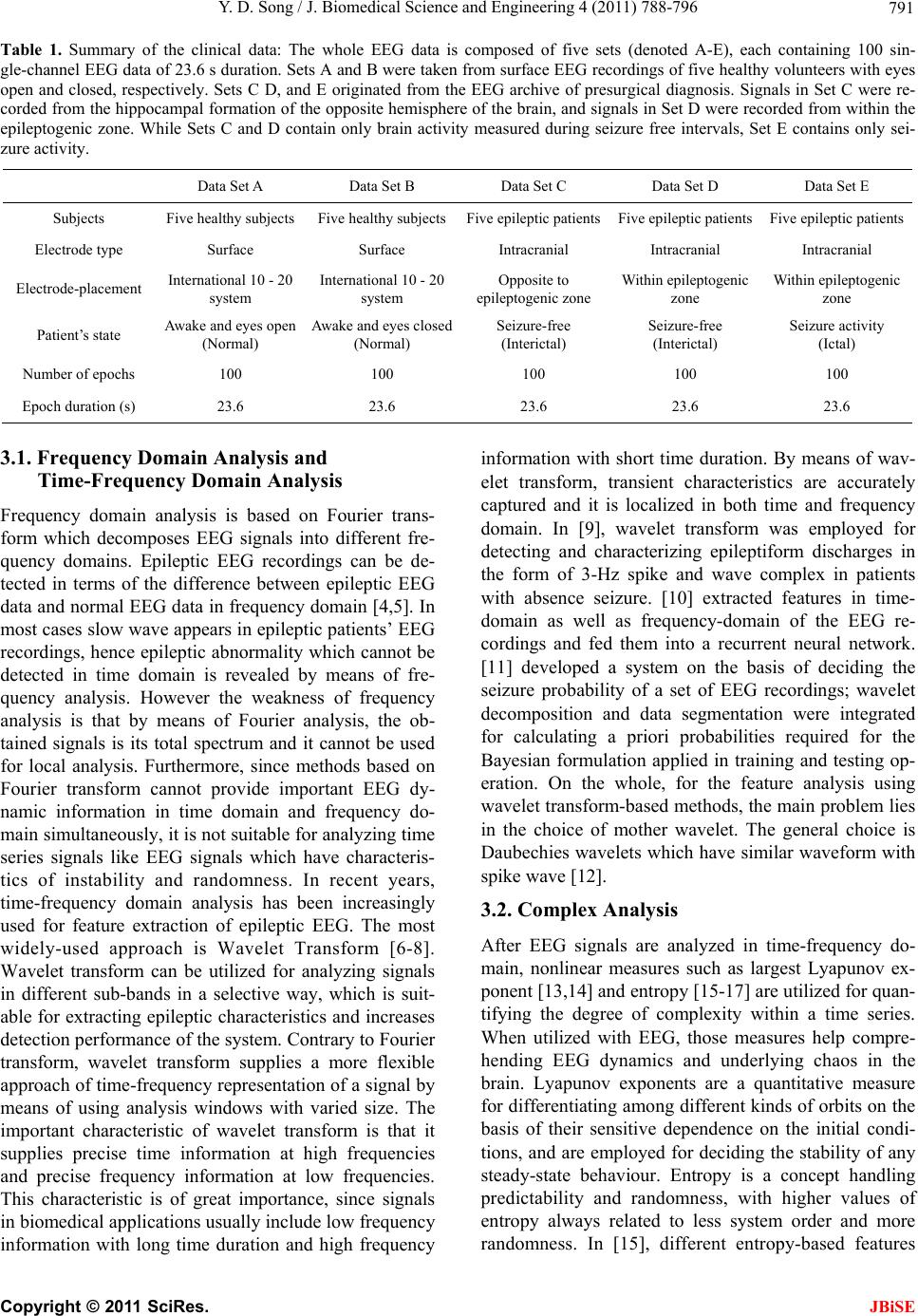 Y. D. Song / J. Biomedical Science and Engineering 4 (2011) 788-796 791 Table 1. Summary of the clinical data: The whole EEG data is composed of five sets (denoted A-E), each containing 100 sin- gle-channel EEG data of 23.6 s duration. Sets A and B were taken from surface EEG recordings of five healthy volunteers with eyes open and closed, respectively. Sets C D, and E originated from the EEG archive of presurgical diagnosis. Signals in Set C were re- corded from the hippocampal formation of the opposite hemisphere of the brain, and signals in Set D were recorded from within the epileptogenic zone. While Sets C and D contain only brain activity measured during seizure free intervals, Set E contains only sei- zure activity. Data Set A Data Set B Data Set C Data Set D Data Set E Subjects Five healthy subjects Five healthy subjectsFive epileptic patientsFive epileptic patients Five epileptic patients Electrode type Surface Surface Intracranial Intracranial Intracranial Electrode-placement International 10 - 20 system International 10 - 20 system Opposite to epileptogenic zone Within epileptogenic zone Within epileptogenic zone Patient’s state Awake and eyes open (Normal) Awake and eyes closed (Normal) Seizure-free (Interictal) Seizure-free (Interictal) Seizure activity (Ictal) Number of epochs 100 100 100 100 100 Epoch duration (s) 23.6 23.6 23.6 23.6 23.6 3.1. Frequency Domain Analysis and Time-Frequency Domain Analysis Frequency domain analysis is based on Fourier trans- form which decomposes EEG signals into different fre- quency domains. Epileptic EEG recordings can be de- tected in terms of the difference between epileptic EEG data and normal EEG d ata in frequency domain [4,5]. In most cases slow wave appears in epileptic patients’ EEG recordings, hence epileptic abnormality which cannot be detected in time domain is revealed by means of fre- quency analysis. However the weakness of frequency analysis is that by means of Fourier analysis, the ob- tained signals is its total spectrum and it cannot be used for local analysis. Furthermore, since methods based on Fourier transform cannot provide important EEG dy- namic information in time domain and frequency do- main simultaneously, it is not suitab le for analyzing time series signals like EEG signals which have characteris- tics of instability and randomness. In recent years, time-frequency domain analysis has been increasingly used for feature extraction of epileptic EEG. The most widely-used approach is Wavelet Transform [6-8]. Wavelet transform can be utilized for analyzing signals in different sub-bands in a selective way, which is suit- able for extracting epileptic characteristics and increases detection performance of the system. Contrary to Fourier transform, wavelet transform supplies a more flexible approach of time-frequency representation of a signal by means of using analysis windows with varied size. The important characteristic of wavelet transform is that it supplies precise time information at high frequencies and precise frequency information at low frequencies. This characteristic is of great importance, since signals in biomedical applications usually include low frequency information with long time duration and high frequency information with short time duration. By means of wav- elet transform, transient characteristics are accurately captured and it is localized in both time and frequency domain. In [9], wavelet transform was employed for detecting and characterizing epileptiform discharges in the form of 3-Hz spike and wave complex in patients with absence seizure. [10] extracted features in time- domain as well as frequency-domain of the EEG re- cordings and fed them into a recurrent neural network. [11] developed a system on the basis of deciding the seizure probability of a set of EEG recordings; wavelet decomposition and data segmentation were integrated for calculating a priori probabilities required for the Bayesian formulation applied in training and testing op- eration. On the whole, for the feature analysis using wavelet transform-based methods, the main problem lies in the choice of mother wavelet. The general choice is Daubechies wavelets which have similar waveform with spike wave [12]. 3.2. Complex Analysis After EEG signals are analyzed in time-frequency do- main, nonlinear measures such as largest Lyapunov ex- ponent [13,14] and entro py [15-17] are utilized for quan- tifying the degree of complexity within a time series. When utilized with EEG, those measures help compre- hending EEG dynamics and underlying chaos in the brain. Lyapunov exponents are a quantitative measure for differentiating among different kinds of orbits on the basis of their sensitive dependence on the initial condi- tions, and are employed for deciding the stability of any steady-state behaviour. Entropy is a concept handling predictability and randomness, with higher values of entropy always related to less system order and more randomness. In [15], different entropy-based features C opyright © 2011 SciRes. JBiSE  Y. D. Song / J. Biomedical Science and Engineering 4 (2011) 788-796 792 that are utilized to normal and epileptic electroencepha- logram recordings were compared and then were tested by applying the adaptive neuro-fuzzy inference systems. The above-mentioned feature extraction methods used for EEG signal analysis include an assumption that the underlying signal dynamic mechanism is composed of a linear superposition of complex exponentials. But the intrinsic basis functions are usually presumed a priori rather than extracted from the EEG recordings in an adaptive way. The obtained power spectrum derived from the analysis involves spu rious power readings if the EEG time series signals we are interested in include more than pure low frequency functions, and contain energy that always stands for nonlinearities in the ana- lyzed EEG recordings. Th e reason is that nonlinearity in the EEG data will be stand for within the power spec- trum as higher-order harmonics because the transform itself employs an accumulation of trigonometric func- tions. As long as the signal transform is formed, it is hard to discriminate true power-frequency EEG signals from spurious energy representation because of nonlin- earities. Hence every time-varying frequen cy representa- tion will be averaged out within the power spectrum. Therefore some novel signal decomposition approaches are required to obtain underlying oscillators originated from a seizure signal without any assumptions of the underlying waveform or specific time-scales of the os- cillatiors, which is capable of presenting the dynamic of EEG signals in an adaptive way. 3.3. Classification Models After features in EEG signals are extracted utilizing the above-mentioned signal processing methods, different techniques based on pattern recognition are then devel- oped for classifying these obtained feature vectors. In order to select the most suitable classifier for a set of features, the properties of the available classifiers have to be understood. In recent years, several classification models have been developed for handling EEG signals classification for epileptic seizure detection, and among these methods, Neural Network-based methods and Support Vector Machine-based methods (SVM) are two widely-used classification paradigms. Artificial Neural Networks (ANN) has been widely used in pattern recog- nition, signal prediction and feature extraction due to its excellent self-learning capability, self-adaptive capabil- ity and strong parallel processing mechanism. A variety of algorithms on the basis of ANN have been employed in EEG signal classification and epileptic seizure detec- tion [18-22]. The learning mechanism of neural net- works can be mainly divided into two categories, namely supervised learning and unsupervised learning. Super- vised learning needs prior knowledge of the analysed data and the back-propagation methods are implemented for the training of weights in neural networks. The un- supervised learning paradigm, on the contrary, has fewer requirements for the prior knowledge of data, and pat- terns with similar characteristics are clustered together by systems. Initial EEG data points and some extracted features using other methods such as waveform charac- teristic parameters detected by utilizing time domain analysis, results of wavelet decomposition, etc., can be- come inputs of neural networks. However the use of neural network refers to many parameters and options such as training parameters, network structures and ini- tial weights and so on, which may have great impact on the training procedure of neural networks. A large num- ber of experiments are thus required to choose optimal parameter sets and a large amount of data is also needed for testing performance of neural networks. The conflict between performance and computation complexity in artificial neural networks is usually figured out by means of trial and the problem regarding how to select optimal number of hidden nodes in neural networks still remains unsolved. In [23], a method based on iteration was de- veloped to handle EEG signals piecewise, which reduces the computation time and cost of neural networks. The Support Vector Machine (SVM) is a supervised machine learning paradigm capable of solving linear and non-linear classification and regression problems [23]. SVM paradigm was first proposed in [24] based on the ideas of statistical learning theory and structural risk minimization. Due to its accuracy and capabilit y of han- dling a great number of predictors, it has been widely used in EEG signal classification and epileptic seizure detection [25-29]. Most of classification models divide categories utilizing hyperplanes which separate the categories by means of a flat plane in the predictor space. Support vector machines expand the concept of hyper- plane separation to data which cannot be divided linearly, through mapping the predictors into a higher-dimen- sional space where data can be divided linearly. SVM classification models have many advantages. A special global optimum for its parameters, such as the degree d of the kernel function and misclassification trade-off factor c controling the trade-off between the maximum margin and the minimum training error, can be found by means of quadratic programming optimization. Nonlin- ear boundaries are able to be utilized without much extra computational effort. Furthermore the performance of SVM is very competitive with other classification mod- els. A weakness SVM has is that the problem complexity is related to the order of the number of patterns rather than the order of the dimension of the patterns. The gen- eral quadratic programming algorithm will usually fail and unique-purpose optimizers employing problem- C opyright © 2011 SciRes. JBiSE  Y. D. Song / J. Biomedical Science and Engineering 4 (2011) 788-796 793 specific speedups need to be utilized for resolving the optim i z ation problems. The above-mentioned methods for automatic epileptic seizure detection have their own characteristic; the per- formance of detecting epileptic seizure using these de- veloped systems will be increased if we can integrate these methods for enhancing their self- adaptive capabil- ity. In order to obtain power spectra in patients with sei- zures, multiple signal classification methods were de- veloped in [30]. Methodologies on the basis of the com- bination of statistical time series analysis, k-nearest neighbour clustering and chaos theory were proposed in [31]. Although many methods for EEG-based epileptic seizure detection have been developed recently and have shown good experimental results, there are still some problems which need to be solved when applied in clinical settings. In the study of EEG-based epileptic seizure detection, due to the lack of publically available EEG databases and the limitation of clinical data sam- ples, most proposed methods were developed using only EEG databases with small number of data samples and it is very likely that they are not applicable in real situa- tions, which makes it difficult to conduct an in-depth investigation of adaptive methodologies for clinical ap- plication. In addition, the EEG data compression is also a problem in this research field. In clinical epileptic sei- zure detection from human Electroencephalograms, the systems used usually have 8, 16, 32 or more electrode channels and the duration of EEG recordings are very long. Huge number of data processing tasks will have direct impact on the applicability of the developed algo- rithms, making it difficult to detect epileptic seizures in a real-time situation efficiently. 4. STUDIES ON EPILEPTIC SEIZURE DIAGNOSIS AND DETECTION USING OTHER EEG Most studies about developing epileptic seizure diagno- sis and detection systems that were mentioned above are mainly based on the EEG database described in [2]. In addition to this EEG database, some studies are also conducted using other EEG resources. [32] developed a fuzzy rule-based seizure detection system on the basis of knowledge from experts’ reasoning. A total of 302.7 hours of intracranial EEG data recordings obtained from 21 patients with 78 seizures was employed for assessing the system. Spectral, temporal and complexity features were extracted from IEEG recordings and joined by utilizing the fuzzy rule-based system in a spatio-tempo- ral way for detecting epileptic seizures. The system showed an excellent performance with a sensitivity of 98.7%, an average detection latency of 11 seconds and a false detection rate of 0.27/h. [33] defined a generalized nonlinear method for identifying seizure EEG segments from non-seizure segments using nonlinear decision functions with the flexibility in selecting any degree of complexity and with any number of dimensions. A per- formance assessment of the correlation sum according to sensitivity, specificity and accuracy in its capability of discriminating seizure signals from non-seizure signals was supplied. A total of 126 EEG signals from 11 se- quential patients were handled and the correlation sum was calculated from non-overlapping scrolling windows with 1 second duration. The experimental observations showed a significant decrease in the amplitude of the correlation sum prior to the onset of seizures. The ap- proach with k-fold cross validation conducted with a sensitivity of 92.31%, a specificity of 91.67% and an accuracy of 91.84%, which shows its suitability for off- line seizure detection. [34] tried to identify the seizure onset patterns by using an evolutionary scheme which searches for optimal kernel types and parameters for support vector machine. They considered the fractal di- mension, Lyapunov exponent and wavelet entropy for feature extraction and the classification accuracy of this method was evaluated using the CHB-MIT dataset. A comparison of experimental results revealed that the proposed approach outperformed that of general support vector machine, and the accuracy rate achieved 96.29% for sensitivity and 100% for specificity. In [35], a novel algorithm based on wavelet analysis was proposed for detecting epileptic seizures from scalp EEG signals. They used wavelet packet transform to decompose the EEG data from each channel. In terms of the obtained wavelet coefficients, a patient-specific measure was de- veloped for quantifying the separation between non- seizure and seizure sign als within the frequency ra nge of 1 - 30 Hz. The measure was utilized for determining a normalized index called combined seizure index which is obtained for each EEG channel. Significant increase during seizure on set is observed using combined seizure index and channel alarms were then generated by one- sided cumulative sum test on the basis of this normalized index. The approach was evaluated on EEG recordings originated from fourteen patients with sixty-three sei- zures during 75.8 hours. The results showed a low false detection rate of 0.51/h, a high sensitivity of 90.5% and a median detection delay of seven seconds. 5. PREDICTABILITY OF EPILEPTIC SEIZURES FROM HUMAN EEGS The human brain is considered as a dynamic system, because epileptic networks in human beings are compli- cated nonlinear architectures and the interactions are supposed to reveal nonlinear behaviour. These ap- proaches support the point that quantification of changes C opyright © 2011 SciRes. JBiSE  Y. D. Song / J. Biomedical Science and Engineering 4 (2011) 788-796 794 in the human brain originating from EEG may predict epileptic seizures, but conventional approaches are not able to identify particular change before seizure happens. [36] utilized nonlinear dynamics methods into clinical epilepsy analysis and their point is that seizure can be thought of as a change of the brain with epilepsy from chaotic to a more regular circumstances. Hence the spa- tial-temporal characteristics of the brain with epilepsy are not the same for various clinical circumstances. They conduct more investigations on the basis of temporary evolution of a nonlinear dynamic analysis method called the largest Lyapunov exponent for patients having tem- porary lobe epilepsy [37] and concluded that the EEG action is growingly less chaotic when the seizure moves towards. Because of these pioneering researches, non- linear approaches originated from dynamical system the- ory have been used for quantifying the transitions of human brain dynamics prior to the beginning of seizures. [38] performed investigation on the increase of non linear complexity from human neuronal networks before sei- zure happens on the basis of the information from changes in the neuron al complexity lo ss, wh ich ou tlin ing the complicated content of the correlation dimension. [39] noticed that the alterations in the correlation integral can be utilized for pursuing precisely the beginning of seizure for a patient with temporal lobe epilepsy, where- as [40] showed that by means of changes of the fre- quency and amplitude, those alterations in the correla- tion integral can be fully explained. In [41], a sudden decrease in the dynamical similarity during the period before seizur e happens w as observed and that action was getting more and more noticeable when the beg inning of seizure moved forwards. [42] found that the energy in human EEG signals raises before seizure happens, and in their following studies the pro of of epileptic seizure pre- dictability on the basis of the choice of diverse of nonlin- ear and linear characteristics of the EEG was supplied [43,44] made use of 4 different nonlinear quantification approaches under the framework of the Lyapunov theory and observed important preictal changes. Most of the above-mentioned researches in epilepsy prediction are conducted on the basis of intracranial EEG recordings. However two problems need to be considered and solved when it comes to the study of scalp EEG recordings. 1) scalp EEG data are more subject to eye and muscle arte- facts as well as environmental noise than the intracranial EEG data; 2) the significant information in EEG signals are weakened and mixed in the propagation by means of soft bone and tissue. Conventional nonlinear analysis approaches like sample entropy or the Lyapunov expo- nents are influenced by the above-mentioned two prob- lems and hence they cannot be used to discriminate be- tween slightly different chaotic rules in the scalp EEG [45]. One method for handling those problems is to de- fine various nonlinear measures generating better results in comparison with the conventional nonlinear analysis methods for the scalp EEG recordings. [46] followed this method for analyzing scalp EEGs and developed an approach on the basis of the phase-space dissimilarity measures to predict epileptic events from human scalp EEG recordings. The method developed on the basis of dynamical entrainment has revealed good results as well on human scalp EEG recordings for epileptic seizure predictability [47,48]. 6. CONCLUSIONS Diagnosing epilepsy needs acquisition of patients’ EEG recording and collecting additional clinical information. Large amounts of data are produced by EEG monitoring devices and analysis by visual inspection of long re- cordings of EEG in order to find traces of epilepsy is not routinely possible. Research into automatic detection systems for epilepsy has been increasingly popular dur- ing these years. The problem of signal classification for epileptic seizure detection is considered as a typical pat- tern-recognition problem which includes feature extrac- tion and classification. In this paper, we briefly reviewed different methods developed for automatic epileptic sei- zure detection and describe their critical properties. Various feature extraction techniques on the basis of frequency domain analysis, time-frequency domain ana- lysis and complex analysis were discussed; respectively and classification models employed for designing medi- cal support systems of auto matic epileptic seizur e detec- tion were also discussed. On the other hand, although predictability of epileptic seizure originating from hu- man intracranial and scalp EEGs has been approved, more studies need to be conducted for increasing the accuracy of prediction. REFERENCES [1] Iasemidis, L.D., Shiau, D.S., Chaovalitwongse, W., Sac- kellares, J.C., Parda los, P.M., Principe, J.C., Ca rney, P. R., Prasad, A., Veeramani, B. and Tsakalis, K. (2003) Adap- tive epileptic seizure prediction system. IEEE Transac- tion on Biomedical Engineering, 50, 616-627. doi:10.1109/TBME.2003.810689 [2] Andrzejak, R.G., Lehnertz, K., Mormann, F., Rieke, C., David, P. and Elger, C.E. (2001) Indications of nonlinear deterministic and finite-dimensional structures in time se- ries of brain elect rical activity: Dependence on recording region and brain state. Physical Reviews E, 64, 061907. doi:10.1103/PhysRevE.64.061907 [3] IFSECN (1974) A glossary of terms most commonly used by clinical electroencephalographers. Electroencephalogra- phy and Clinical Neurophysiology, 37, 538-548. doi:10.1016/0013-4694(74)90099-6 [4] Gotman, J., Flanagan, D., Zhang, J. and Rosenblatt, B. C opyright © 2011 SciRes. JBiSE  Y. D. Song / J. Biomedical Science and Engineering 4 (2011) 788-796 795 (1997) Automatic seizure detection in the newborn: Me- thods and initial evaluation. Electroencephalography and Clinical Neurophysiology, 103, 356-36 2. doi:10.1016/S0013-4694(97)00003-9 [5] Polat, K. and Günes, S. (2007) Classification of epilepti- form EEG using a hybrid system based on decision tree classifier and fast fourier transform. Applied Mathemat- ics and Computation, 187, 1017-1026. doi:10.1016/j.amc.2006.09.022 [6] Übeyli, E.D. (2009) Combined neural network model employing wavelet coefficients for EEG signals classifi- cation. Digital Signal Processing, 19, 297-308. [7] Subasi, A. (2007) EEG signal classification using wave- let feature extraction and a mixture of expert model. Ex- pert Systems with Applications, 32, 1084-1093. doi:10.1016/j.eswa.2006.02.005 [8] Zandi, A.S., Javidan, M., Dumont, G.A. and Tafreshi, R. (2010) Automated real-time epileptic seizure detection in scalp eeg recordings using an algorithm based on wavelet packet transform. IEEE Transactions on Biomedical En- gineering, 57, 1639-1651. doi:10.1109/TBME.2010.2046417 [9] Adeli, H., Zhou, Z. and Dadmehr, N. (2003) Analysis of EEG records in an epileptic patient using wavelet trans- form. Journal of Neuroscience Methods, 123, 69-87. doi:10.1016/S0165-0270(02)00340-0 [10] Srinivasan, V., Eswaran, C. and Sriraam, N. (2005) Arti- ficial neural network based epileptic detection using time- domain and frequency-domain features. Journal of Medi- cal Systems, 29, 647-660. doi:10.1007/s10916-005-6133-1 [11] Saab, M.E. and Gotman, J. (2005) A system to detect the onset of epileptic seizures in scalp EEG. Clinical Neuro- physiology, 116, 427-442. doi:10.1016/j.clinph.2004.08.004 [12] Indiradevi, K.P., Elias, E., Sathidevi, P.S., Dinesh, S. and Radhakrishnan, K. (2008) A multi-level wavelet approach for automatic detection of epileptic spikes in the electro- encephalogram. Computers in Biology and Medicine, 38, 805-816. doi:10.1016/j.compbiomed.2008.04.010 [13] Übeyli, E.D. (2009) Automatic detection of electroence- phalographic changes using adaptive neuro-fuzzy inference system employing Lyapunov exponents. Expert Systems with Applic atio ns , 36, 9031-9038. doi:10.1016/j.eswa.2008.12.019 [14] Ghosh-Dastidar, S., Adeli, H. and Dadmehr, N. (2007) Mixed-band wavelet-chaos-neural network methodology for epilepsy and epileptic seizure detection. IEEE Trans- actions on Biomedical Engineering, 54, 1545-1551. doi:10.1109/TBME.2007.891945 [15] Kannathal, N., Choo, M.L., Rajendra, A.U. and Sada- sivan, P.K. (2005) Entropies for detection of epilepsy in EEG. Computer Method and Programs in Biomedicine, 80, 187-194. doi:10.1016/j.cmpb.2005.06.012 [16] Ocak, H. (2008) Optimal classification of epileptic sei- zures in EEG using wavelet analysis and genetic algo- rithm. Signal Processing, 88, 1858-1867. doi:10.1016/j.sigpro.2008.01.026 [17] Srinivasan, V., Eswaran, C. and Sriraam, N. (2007) Appro- ximate entropy-based epileptic EEG detection using arti- ficial neural networks. IEEE Transactions on Information Technology in Biomedicine, 11, 288-295. doi:10.1109/TITB.2006.884369 [18] Orhan, U., Hekim, M., Ozer, M. (2011) EEG signals clas- sification using the K-means clustering and a multilayer perceptron neural network model. Expert Systems with Applications, 38, 13475-13481. doi:10.1016/j.eswa.2011.04.149 [19] Kumar, S.P., Sriraam, N., Benakop, P.G. and Jinaga, B.C. (2009) Entropies based detection of epileptic seizures with artificial neural network classifiers. Expert Systems with Applic atio ns , 37, 3284-3294. doi:10.1016/j.eswa.2009.09.051 [20] Naghsh-Nilchi, A.R. and Aghashahi, M. (2010) Epilepsy seizure detection using eigen-system spectral estimation and multiple layer perceptron neural network. Biomedi- cal Signal Processing and Control, 5, 147-157. doi:10.1016/j.bspc.2010.01.004 [21] Kocyigit, Y., Alkan, A. and Erol, H. (2008) Classification of EEG recordings by using fast independent component analysis and artificial neural network. Journal of Medical Systems, 32, 17-20. doi:10.1007/s10916-007-9102-z [22] Guo, L., Rivero, D., Dorado, J., Rabunal, J.R. and Pazos, A. (2010) Automatic epileptic seizure detection in EEGs based on line length feature and artificial neural networks. Journal of Neuroscience Methods, 191, 101-109. doi:10.1016/j.jneumeth.2010.05.020 [23] Boser, B.E. (1992) A training algorithm for optimal mar- gin classifiers. Proceedings of 5th Annual Workshop of Computational Learning Theory, Pennsylvania, 144-152. [24] Cortes, C. and Vapnik, V. (1995) Support vector n etworks. Machine Learning, 20, 273-297. doi:10.1007/BF00994018 [25] Guler, I. and Übeyli, E.D. (2007) Multiclass Support Vec- tor Machines for EEG-Signals Classification. IEEE Tran- sactions on Information Technology in Biomedicine, 11, 117-126. doi:10.1109/TITB.2006.879600 [26] Gardner, A.B., Krieger, A.M., Vachtsevanos, G. and Litt, B. (2006) One-class novelty detection for seizure analy- sis from intracranial EEG. Journal of Machine Learning Research, 7, 1025-1044. [27] Hsu, K.C. abd Yu, S.N. (2010) Detection of seizures in EEG using subband nonlinear parameters and genetic algorithm. Computers in Biology and Medicine, 40, 823- 830. doi:10.1016/j.compbiomed.2010.08.005 [28] Chandaka, S., Chatterjee, A. and Munshi, S. (20 09) Cross- correlation aided support vector machine classifier for classification of EEG signals. Expert Systems with Ap- plications, 36, 1329-1336. doi:10.1016/j.eswa.2007.11.017 [29] Übeyli, E.D. (2008) Analysis of EEG signals by com- bining eigenvector methods and multiclass support vec- tor machines. Computers in Biology and Medicine, 38, 14-22. doi:10.1016/j.compbiomed.2007.06.002 [30] Alkan, A., Koklukaya, E. and Subasi, A. (2005) Automa- tic seizure detection in EEG using logistic regression and artificial neural network. Journal of Neuroscience Meth- ods, 148, 167-176. doi:10.1016/j.jneumeth.2005.04.009 [31] Chaovalitwongse, W.A., Fan, Y. and Sachdeo, E.C. (2007) On the time series k-nearest neighbour classification of abnormal brain activity. IEEE Transactions on Systems, Man and Cybernetics-Part A: Systems and Humans, 37, 1005-1016. doi:10.1109/TSMCA.2007.897589 [32] Aarabi, A., Fazel-Rezai, R. and Aghakhani, Y. (2009) A C opyright © 2011 SciRes. JBiSE  Y. D. Song / J. Biomedical Science and Engineering 4 (2011) 788-796 Copyright © 2011 SciRes. 796 JBiSE fuzzy rule-based system for epileptic seizure detection in intracranial EEG. Clinical Neurophysiology, 120, 1648- 1657. doi:10.1016/j.clinph.2009.07.002 [33] Tito, M., Cabrerizo, M., Ayala, M., Barreto, A., Miller, I, Jayakar, P. and Adjouadi, M. (2009) Classification of elec- troencephalographic seizure recordings into ictal and in- terictal files using correlation sum. Computers in Biology and Medicine, 39, 604-614. doi:10.1016/j.compbiomed.2009.04.005 [34] Zavar, M., Rahati, S., Akbarzadeh-T, M.-R. and Ghase- mifard, H. (2011) Evolutionary model selection in a wave- let-based support vector machine for automated seizure detection. Expert Systems with Applications, 38, 10751- 10758. doi:10.1016/j.eswa.2011.01.087 [35] Zandi, A.S., Javidan, M., Dumont, G.A. and Tafreshi, R. (2010) Automated real-time epileptic seizure detection in scalp EEG recordings using an algorithm based on wave- let packet transform. IEEE Transactions on Biomedical Engineering, 57, 1639-1651. doi:10.1109/TBME.2010.2046417 [36] Iasemidis, L.D., Shiau, D.S., Sackellares, J.C., Pardalos, P.M. and Prasad A. (2004) A dynamical resetting of the human brain at epileptic seizures: Application of nonlin- ear dynamics and global optimization techniques. IEEE Transactions on Biomedical Engineering, 51, 493-506. doi:10.1109/TBME.2003.821013 [37] Iasemidis, L.D., Sackellares, J.C., Zaveri, H.P. and Wil- lians, W.J. (1990) Phase space topography and the Lya- punov exponent of electrocorticograms in partial seizures. Brain Topography, 2, 187-201. doi:10.1007/BF01140588 [38] Lehnertz, K. and Elger, C.E. (1995) Spatio-temporal dy- namics of the primary epileptogenic area in temporal lobe epilepsy characterized by neuronal complexity loss. Elec- troencephalogram Clinical Neurophysiology, 95, 108-117. doi:10.1016/0013-4694(95)00071-6 [39] Lerner, D.E. (1996) Monitoring changing dynamics with correlation integrals: Case study of an epileptic seizure. Physica D, 97, 563-576. doi:10.1016/0167-2789(96)00085-1 [40] Osorio, I., Harrison, M.A.F., Lai, Y.C. and Frei, M.G. (2001) Observations on the application of the correlation dimension and correlation integral to the prediction of seizures. Journal of Clinical Neurophysiology, 18, 269- 274. doi:10.1097/00004691-200105000-00006 [41] Van Quyen, M.L., Martinerie, J., Baulac, M. and Varela, F.J. (1999) Anticipating epileptic seizures in real time by a non-linear analysis of similarity between EEG record- ings. NeuroReport, 10, 2149-2155. doi:10.1097/00001756-199907130-00028 [42] Litt, B., Estellera, R., Echauz, J., D’Alessandro, M., Shor, R., Henry, T., Pennell, P., Epstein, C., Ba kay, R., Dichter, M. and Vachtsevanos, G. (2001) Epileptic seizures may begin hours in advance of clinical onset: A report of five patients. Neuron, 30, 51-64. doi:10.1016/S0896-6273(01)00262-8 [43] D’Alessandro, M., Esteller, R., Vachtsevanos, G., Hinson, A., Echauz J. and Litt B. (2003) Epileptic seizure predic- tion using hybrid feature selection over multiple intrac- ranial eeg electrode contacts: A report of four patients. IEEE Transactions on Biomedical Engineering, 50, 603- 615. doi:10.1109/TBME.2003.810706 [44] M oser, H.R., We ber, B ., Wieser, H.G. and Meier, P.F. (1 999) Electroencephalogram in epilepsy: Analysis and seizure prediction within the fra mework of Lyapunov theory. Phy- sica D, 130, 291-305. doi:10.1016/S0167-2789(99)00043-3 [45] Hively, L.M., Protopopescu, V.A. and Gailey, P.C. (2000) Timely detection of dynamical change in scalp EEG sig- nals. Chaos, 10, 864-875. doi:10.1063/1.1312369 [46] Hively, L.M. and Protopopescu, V.A. (200 3) Channel-con- sistent forewarning of epileptic events from scalp EEG. IEEE Transactions on Biomedical Engineering, 50, 584- 593. doi:10.1109/TBME.2003.810693 [47] Sackellares, J., Iasemidis, L., Shiau, D., Gilmore, R. and Ro- per, S. (1999) Detection of the preictal transition from scalp EEG recordings. Epilepsia, 40, 176. [48] Shiau, D., Iasemidis, L., Suharitdamrong, W., Dance, L., Chaovalitwongse, W., Pardalos, P., Carney, P. and Sac- kellares, J. (2003) Detection of the preict al period by dy- namical analysis of scalp EEG. Epilepsia, 44, 233-234.
|