Paper Menu >>
Journal Menu >>
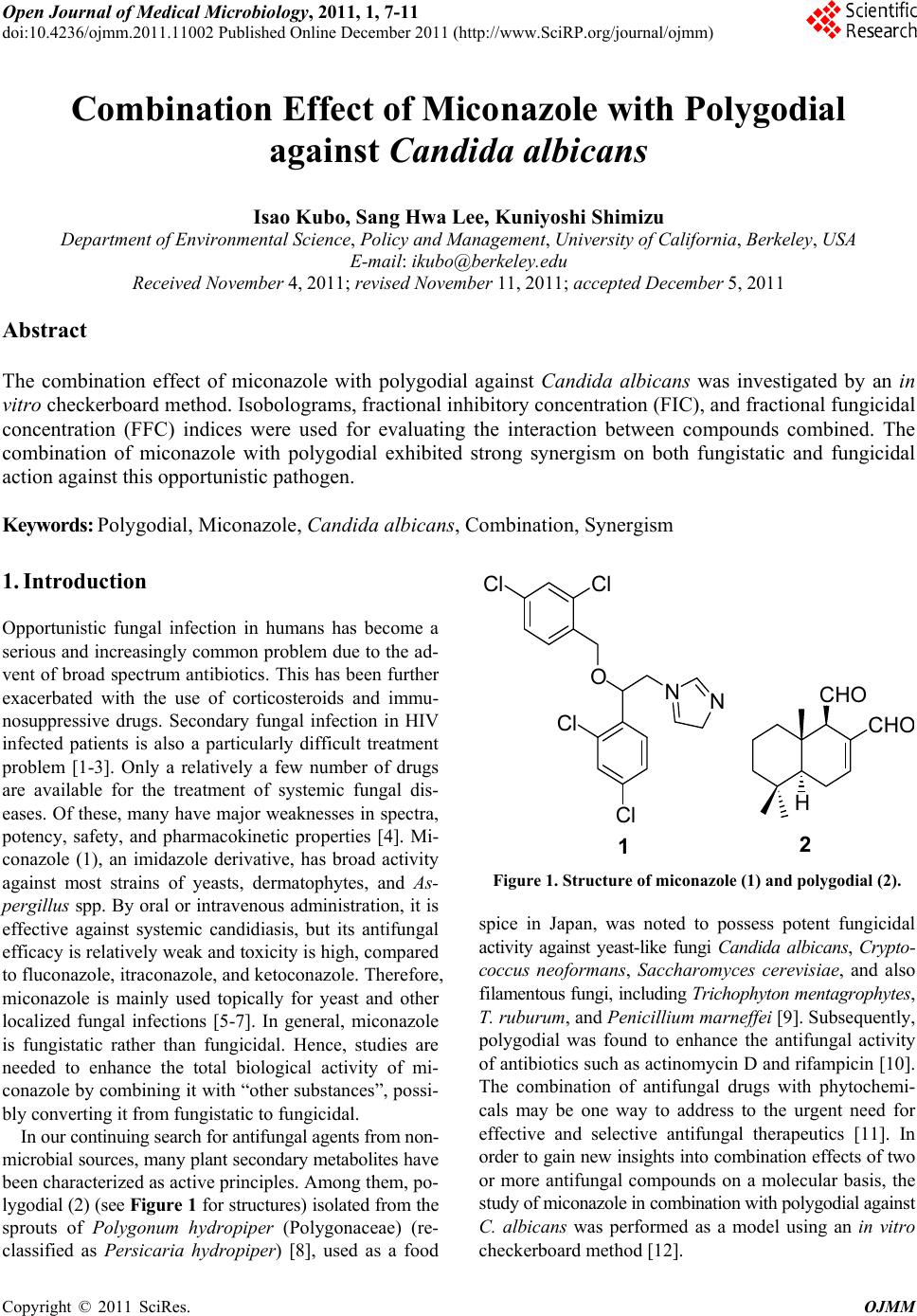 Open Journal of Medical Microbiology, 2011, 1, 7-11 doi:10.4236/ojmm.2011.11002 Published Online December 2011 (http://www.SciRP.org/journal/ojmm) Copyright © 2011 SciRes. OJMM Combination Effect of Miconazole with Polygodial against Candida albicans Isao Kubo, Sang Hwa Lee, Kuniyoshi Shimizu Department of Enviro n mental Science, Policy and Management, University of California, Berkeley, USA E-mail: ikubo@berkeley.edu Received November 4, 2011; revised November 11, 2011; accepted December 5, 2011 Abstract The combination effect of miconazole with polygodial against Candida albicans was investigated by an in vitro checkerboard method. Isobolograms, fractional inhibitory concentration (FIC), and fractional fungicidal concentration (FFC) indices were used for evaluating the interaction between compounds combined. The combination of miconazole with polygodial exhibited strong synergism on both fungistatic and fungicidal action against this opportunistic pathogen. Keywords: Polygodial, Miconazole, Candida albicans, Combination, Synergism 1. Introduction Opportunistic fungal infection in humans has become a serious and increasingly common problem due to the ad- vent of broad spectrum antibiotics. This has been further exacerbated with the use of corticosteroids and immu- nosuppressive drugs. Secondary fungal infection in HIV infected patients is also a particularly difficult treatment problem [1-3]. Only a relatively a few number of drugs are available for the treatment of systemic fungal dis- eases. Of these, many have major weaknesses in spectra, potency, safety, and pharmacokinetic properties [4]. Mi- conazole (1), an imidazole derivative, has broad activity against most strains of yeasts, dermatophytes, and As- pergillus spp. By oral or intravenous administration, it is effective against systemic candidiasis, but its antifungal efficacy is relatively weak and toxicity is high, compared to fluconazole, itraconazole, and ketoconazole. Therefore, miconazole is mainly used topically for yeast and other localized fungal infections [5-7]. In general, miconazole is fungistatic rather than fungicidal. Hence, studies are needed to enhance the total biological activity of mi- conazole by combining it with “other substances”, possi- bly converting it from fungistatic to fungicidal. In our continuing search for antifungal agents from non- microbial sources, many plant secondary metabolites have been characterized as active principles. Among them, po- lygodial (2) (see Figure 1 for structures) isolated from the sprouts of Polygonum hydropiper (Polygonaceae) (re- classified as Persicaria hydropiper) [8], used as a food ClCl ONN Cl Cl H CHO CH O 12 Figure 1. Structure of miconazole (1) and polygodial (2). spice in Japan, was noted to possess potent fungicidal activity against yeast-like fungi Candida albicans, Crypto- coccus neoformans, Saccharomyces cerevisiae, and also filamentous fungi, including Trichophyton mentagrophytes , T. rubu rum , and Penicillium marneffei [9]. Subsequently, polygodial was found to enhance the antifungal activity of antibiotics such as actinomycin D and rifampicin [10]. The combination of antifungal drugs with phytochemi- cals may be one way to address to the urgent need for effective and selective antifungal therapeutics [11]. In order to gain new insights into combination effects of two or more antifungal compounds on a molecular basis, the study of miconazole in combination with polygodial against C. albicans was performed as a model using an in vitro checkerboard method [12]. 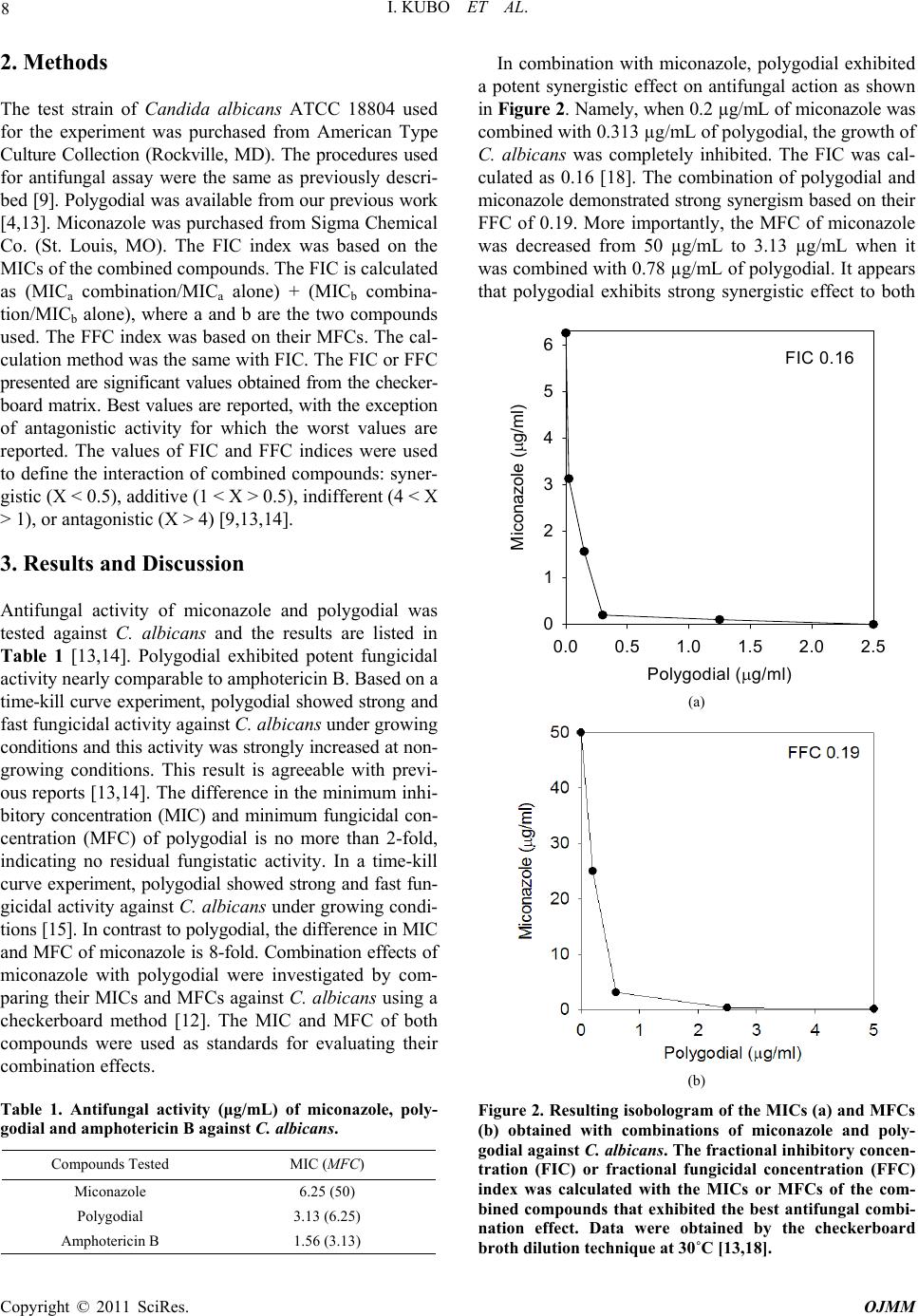 I. KUBO ET AL. Copyright © 2011 SciRes. OJMM 8 2. Methods The test strain of Candida albicans ATCC 18804 used for the experiment was purchased from American Type Culture Collection (Rockville, MD). The procedures used for antifungal assay were the same as previously descri- bed [9]. Polygodial was available from our previous work [4,13]. Miconazole was purchased from Sigma Chemical Co. (St. Louis, MO). The FIC index was based on the MICs of the combined compounds. The FIC is calculated as (MICa combination/MICa alone) + (MICb combina- tion/MICb alone), where a and b are the two compounds used. The FFC index was based on their MFCs. The cal- culation method was the same with FIC. The FIC or FFC presented are significant values obtained from the checker- board matrix. Best values are reported, with the exception of antagonistic activity for which the worst values are reported. The values of FIC and FFC indices were used to define the interaction of combined compounds: syner- gistic (X < 0.5), additive (1 < X > 0.5), indifferent (4 < X > 1), or antagonistic (X > 4) [9,13,14]. 3. Results and Discussion Antifungal activity of miconazole and polygodial was tested against C. albicans and the results are listed in Table 1 [13,14]. Polygodial exhibited potent fungicidal activity nearly comparable to amphotericin B. Based on a time-kill curve experiment, polygodial showed strong and fast fungicidal activity against C. albicans under growing conditions and this activity was strongly increased at non- growing conditions. This result is agreeable with previ- ous reports [13,14]. The difference in the minimum inhi- bitory concentration (MIC) and minimum fungicidal con- centration (MFC) of polygodial is no more than 2-fold, indicating no residual fungistatic activity. In a time-kill curve experiment, polygodial showed strong and fast fun- gicidal activity against C. albicans under growing condi- tions [15]. In contrast to polygodial, the difference in MIC and MFC of miconazole is 8-fold. Combination effects of miconazole with polygodial were investigated by com- paring their MICs and MFCs against C. albicans using a checkerboard method [12]. The MIC and MFC of both compounds were used as standards for evaluating their combination effects. Table 1. Antifungal activity (μg/mL) of miconazole, poly- godial and amphotericin B against C. albicans. Compounds Tested MIC (MFC) Miconazole 6.25 (50) Polygodial 3.13 (6.25) Amphotericin B 1.56 (3.13) In combination with miconazole, polygodial exhibited a potent synergistic effect on antifungal action as shown in Figure 2. Namely, when 0.2 µg/mL of miconazole was combined with 0.313 µg/mL of polygodial, the growth of C. albicans was completely inhibited. The FIC was cal- culated as 0.16 [18]. The combination of polygodial and miconazole demonstrated strong synergism based on their FFC of 0.19. More importantly, the MFC of miconazole was decreased from 50 µg/mL to 3.13 µg/mL when it was combined with 0.78 µg/mL of polygodial. It appears that polygodial exhibits strong synergistic effect to both FIC 0.16 Polygodial (g/ml) 0.0 0.5 1.0 1.5 2.0 2.5 Miconazole (g/ml) 0 1 2 3 4 5 6 (a) (b) Figure 2. Resulting isobologram of the MICs (a) and MFCs (b) obtained with combinations of miconazole and poly- godial against C. albicans. The fractional inhibitory concen- tration (FIC) or fractional fungicidal concentration (FFC) index was calculated with the MICs or MFCs of the com- bined compounds that exhibited the best antifungal combi- nation effect. Data were obtained by the checkerboard broth dilution technique at 30˚C [13,18]. 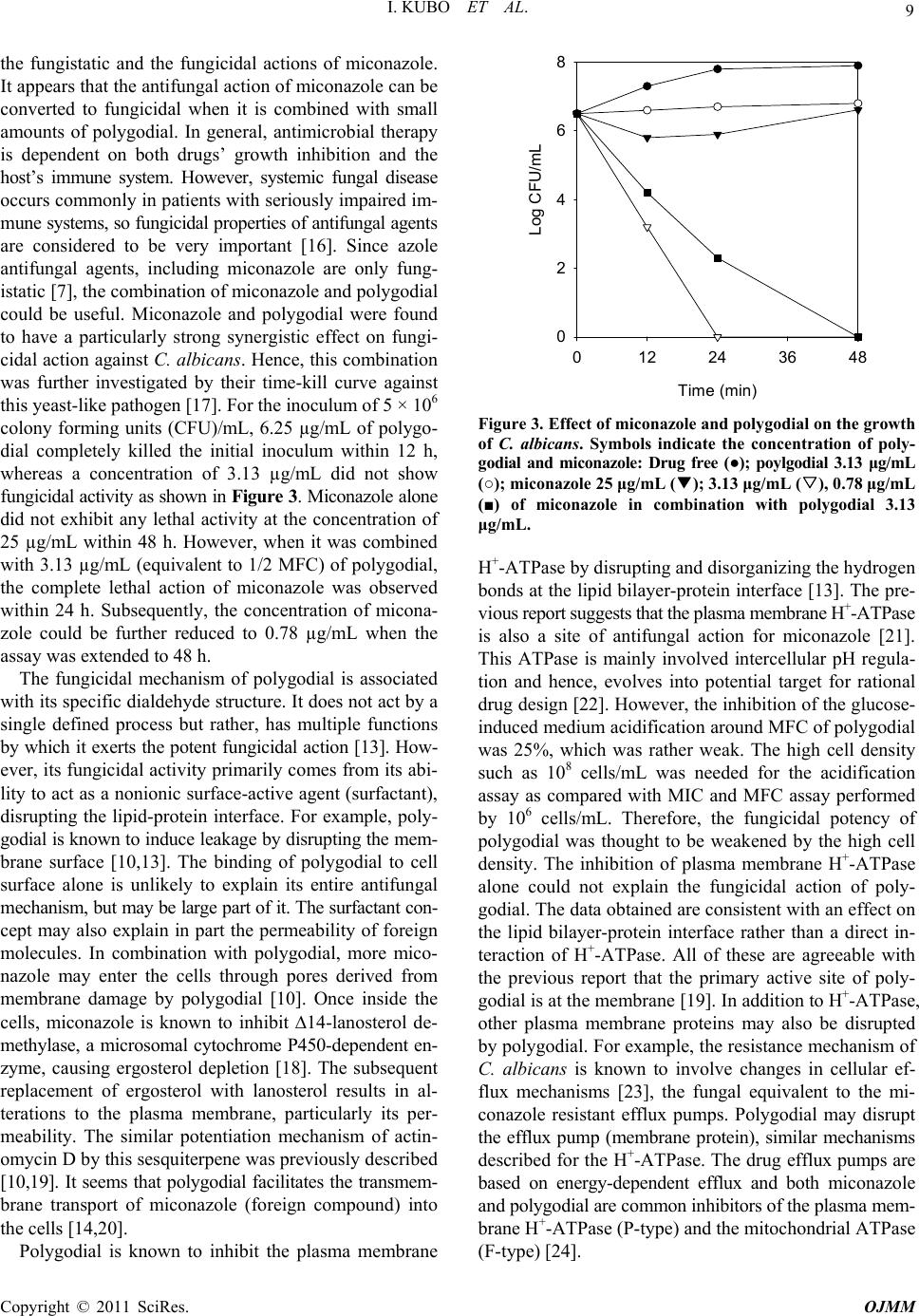 I. KUBO ET AL. Copyright © 2011 SciRes. OJMM 9 the fungistatic and the fungicidal actions of miconazole. It appears that the antifungal action of miconazole can be converted to fungicidal when it is combined with small amounts of polygodial. In general, antimicrobial therapy is dependent on both drugs’ growth inhibition and the host’s immune system. However, systemic fungal disease occurs commonly in patients with seriously impaired im- mune systems, so fungicidal properties of antifungal agents are considered to be very important [16]. Since azole antifungal agents, including miconazole are only fung- istatic [7], the combination of miconazole and polygodial could be useful. Miconazole and polygodial were found to have a particularly strong synergistic effect on fungi- cidal action against C. albicans. Hence, this combination was further investigated by their time-kill curve against this yeast-like pathogen [17]. For the inoculum of 5 × 106 colony forming units (CFU)/mL, 6.25 µg/mL of polygo- dial completely killed the initial inoculum within 12 h, whereas a concentration of 3.13 µg/mL did not show fungicidal activity as shown in Figure 3. Miconazole alone did not exhibit any lethal activity at the concentration of 25 µg/mL within 48 h. However, when it was combined with 3.13 µg/mL (equivalent to 1/2 MFC) of polygodial, the complete lethal action of miconazole was observed within 24 h. Subsequently, the concentration of micona- zole could be further reduced to 0.78 µg/mL when the assay was extended to 48 h. The fungicidal mechanism of polygodial is associated with its specific dialdehyde structure. It does not act by a single defined process but rather, has multiple functions by which it exerts the potent fungicidal action [13]. How- ever, its fungicidal activity primarily comes from its abi- lity to act as a nonionic surface-active agent (surfactant), disrupting the lipid-protein interface. For example, poly- godial is known to induce leakage by disrupting the mem- brane surface [10,13]. The binding of polygodial to cell surface alone is unlikely to explain its entire antifungal mechanism, but may be large part of it. The surfactant con- cept may also explain in part the permeability of foreign molecules. In combination with polygodial, more mico- nazole may enter the cells through pores derived from membrane damage by polygodial [10]. Once inside the cells, miconazole is known to inhibit 14-lanosterol de- methylase, a microsomal cytochrome P450-dependent en- zyme, causing ergosterol depletion [18]. The subsequent replacement of ergosterol with lanosterol results in al- terations to the plasma membrane, particularly its per- meability. The similar potentiation mechanism of actin- omycin D by this sesquiterpene was previously described [10,19]. It seems that polygodial facilitates the transmem- brane transport of miconazole (foreign compound) into the cells [14,20]. Polygodial is known to inhibit the plasma membrane Time (min) 0 12243648 Log CFU/mL 0 2 4 6 8 Figure 3. Effect of miconazole and polygodial on the growth of C. albicans. Symbols indicate the concentration of poly- godial and miconazole: Drug free (●); poylgodial 3.13 μg/mL (○); miconazole 25 μg/mL (▼); 3.13 μg/mL (▽), 0.78 μg/mL (■) of miconazole in combination with polygodial 3.13 μg/mL. H+-ATPase by disrupting and disorganizing the hydrogen bonds at the lipid bilayer-protein interface [13]. The pre- vious report suggests that the plasma membrane H+-ATPase is also a site of antifungal action for miconazole [21]. This ATPase is mainly involved intercellular pH regula- tion and hence, evolves into potential target for rational drug design [22]. However, the inhibition of the glucose- induced medium acidification around MFC of polygodial was 25%, which was rather weak. The high cell density such as 108 cells/mL was needed for the acidification assay as compared with MIC and MFC assay performed by 106 cells/mL. Therefore, the fungicidal potency of polygodial was thought to be weakened by the high cell density. The inhibition of plasma membrane H+-ATPase alone could not explain the fungicidal action of poly- godial. The data obtained are consistent with an effect on the lipid bilayer-protein interface rather than a direct in- teraction of H+-ATPase. All of these are agreeable with the previous report that the primary active site of poly- godial is at the membrane [19]. In addition to H+-ATPase, other plasma membrane proteins may also be disrupted by polygodial. For example, the resistance mechanism of C. albicans is known to involve changes in cellular ef- flux mechanisms [23], the fungal equivalent to the mi- conazole resistant efflux pumps. Polygodial may disrupt the efflux pump (membrane protein), similar mechanisms described for the H+-ATPase. The drug efflux pumps are based on energy-dependent efflux and both miconazole and polygodial are common inhibitors of the plasma mem- brane H+-ATPase (P-type) and the mitochondrial ATPase (F-type) [24].  I. KUBO ET AL. Copyright © 2011 SciRes. OJMM 10 In addition to polygodial, anethole also acts synergis- tically with several antifungal agents. For example, ane- thole was reported to enhance the fungicidal activity of polygodial 128-fold against C. albicans in combination with a sublethal concentration of anethole [17]. On the other hand, anethole exhibited a strong synergistic effect on fungistatic action of an azole antifungal agent, mico- nazole, against this opportunistic fungus but a marginal synergistic effect on fungicidal action. Thus, C. albicans cells appeared to adapt to this combination stress, even- tually recovering and growing normally. Interestingly, a structurally similar phenyl propanoid, eugenol character- ized from the same source, did not show this synergistic activity at all, although it was found to exhibit fungicidal activity against S. cerevisiae and C. albicans with each MFC of 800 μg/mL. Anethole was previously described to exhibit in vivo synergistic effect on the fungicidal activity of the anethole/- polygodial-containing compound against C. albicans, supporting its present clinical application [25]. On the basis of the result obtained with polygodial, the study was further extended to see if the enhancing activity to mi- conazole is specific to only polygodial’s dialdehyde struc- tural feature. In order to facilitate it, a structurally simple primary aliphatic alcohol, decanol was selected since amphipathic primary aliphatic alcohols exhibit antifungal activity against C. albicans. Among these alcohols, de- canol was noted to exhibit the most potent fungicidal activity with an MFC of 50 µg/mL against C. albicans. Hence, miconazole was combined with decanol to see if the same combination effect can also be observed against C. albicans. The combination of miconazole and decanol synergistically retarded the growth of C. albicans, but had only marginal synergism on their fungicidal action. Thus, C. albicans cells appeared to adapt to this combi- nation stress, eventually recovering and growing normally. To reveal the different combination effect between po- lygodial and decanol, the leakage of 260 nm absorbing materials and K+ ion from C. albicans cells was checked. Polygodial induced leakage of 260 nm absorbing materi- als, similar to those described for combination against S. cerevisiae [10]. In contrast to polygodial, decanol did not induce a leak of 260 nm absorbing materials although it did induce a leak of K+ ion. The leakage of K+ ion by decanol was observed within 30 min treatment accompa- nied by significant loss of cell viability, suggesting that decanol quickly affects the plasma membrane of C. al- bicans cells forming rather smaller size pores than poly- godial. The size formed may not be large enough to fa- cilitate the transmembrane transport of miconazole into the cells. In conclusion, the combination effect of miconazole with polygodial is truly synergistic, as shown by the va- rious concentrations less than half-MIC or MFC of the combined counterpart. It seems that the combination of miconazole and polygodial targets the extracytoplasmic region, and thus does not need to enter the cell, thereby avoiding most cellular pump-based resistance mecha- nisms. The combination strategy of antifungal drugs and phytochemicals for the purpose of effectively controlling systemic fungal pathogens is promising. 4. Acknowledgements The authors are grateful to Dr. M. Lewin for his critical reading of the manuscript, and Dr. M. Himejima and Dr. K. Fujita for performing antifungal assay at earlier stage of the work. 5. References [1] American Thoracic Society, “Fungal Infection in HIV- Infected Persons,” American Journal of Respiratory and Critical Care Medicine, Vol. 152, No. 2, 1995, pp. 816- 822. [2] D. M. Dixon, M. M. McNeil, M. L. Cohen, B. G. Gellin and J. R. La Montagne, “Fungal Infections—A Growing Threat,” Public Health Reports, Vol. 111, No. 3, 1996, pp. 226-235. [3] J. R. Graybill, “Treatment of Systemic Mycoses in Pa- tients with AIDS,” Archives of Medical Research, Vol. 24, No. 4, 1993, pp. 403-412. [4] N. H. Georgopapadakou and T. J. Walsh, “Human My- coses—Drugs and Targets for Emerging Pathogens,” Sci- ence, Vol. 264, No. 5157, 1994, pp. 371-373. doi:10.1126/science.8153622 [5] R. Y. J. Cartwright, “Anti Fungal Drugs,” Journal of An- timicrobial Chemotherapy, Vol. 1, No. 2, 1975, pp. 141- 162. doi:10.1093/jac/1.2.141 [6] J. A. Como and W. E. Dismukes, “Oral Azole Drugs as Systemic Antifungal Therapy,” New England Journal of Medicine, Vol. 330, No. 4, 1994, pp. 263-272. doi:10.1056/NEJM199401273300407 [7] R. A. Fromtling, “Overview of Medically Important An- tifungal Azole Derivatives,” Clinical Microbiology Re- view, Vol. 1, No. 2, 1988, pp. 187-217. [8] C. Barnes and J. Loder, “Structure of Polygodial—A New Sesquiterpene Dialdehyde from Polygonum hydro- poper L,” Australian Journal of Chemistry, Vol. 15, No. 2, 1962, pp. 322-327. doi:10.1071/CH9620322 [9] S. H. Lee, J. R. Lee, C. S. Lunde and I. Kubo, “In Vitro Anti-Fungal Susceptibilities of Candida albicans and Other Fungal Pathogens to Polygodial, a Sesquiterpene Dialdehyde,” Planta Medica, Vol. 65, No. 3, 1999, pp. 204-208. doi:10.1055/s-1999-13981 [10] I. Kubo and M. Taniguchi, “Polygodial, an Antifungal Potentiator,” Jourmal of Natural Products, Vol. 51, No. 1, 1988, pp. 22-29. doi:10.1021/np50055a002  I. KUBO ET AL. Copyright © 2011 SciRes. OJMM 11 [11] S. Sternberg, “The Emerging Fungal Threat,” Science, Vol. 266, No. 5191, 1994, pp. 1632-1634. doi:10.1126/science.7702654 [12] C. W. Norden, H. Wenzel and E. J. Keleti, “Comparison of Techniques for Measurement of in Vitro Antibiotic Synergism,” Journal of Infectious Diseases, Vol. 140, No. 4, 1979, pp. 629-633. doi:10.1093/infdis/140.4.629 [13] I. Kubo, K. Fujita and S. H. Lee, “Antifungal Mechanism of Polygodial,” Journal of Agricultural and Food Chem- istry, Vol. 49, No. 3, 2001, pp. 1607-1611. doi:10.1021/jf000136g [14] I. Kubo and S. H. Lee, “Potentiation of Antifungal Activ- ity of Sorbic Acid,” Journal of Agricultural and Food Chemistry, Vol. 46, No. 10, 1998, pp. 4052-4055. doi:10.1021/jf980174o [15] M. Himejima and I. Kubo, “Fungicidal Activity of Poly- godial in Combination with Anethole and Indole against Candida albicans,” Journal of Agricultural and Food Chemistry, Vol. 41, No. 10, 1993, pp. 1776-1779. doi:10.1021/jf00034a048 [16] J. R. Graybill, “The Future of Antifungal Therapy,” Cli- nical Infectious Diseases, Vol. 22, Supplement 2, 1996, pp. S166-S178. doi:10.1093/clinids/22.Supplement_2.S166 [17] I. Kubo and M. Himejima, “Anethole, a Synergist of Polygodial against Filamentous Microorganisms,” Jour- nal of Agricultural and Food Chemistry, Vol. 39, No. 12, 1991, pp. 2290-2292. doi:10.1021/jf00012a040 [18] V. H. Boscche, “Biochemical Targets for Antifungal Azole Derivatives: Hypothesis on the Mode of Action,” In: M. R. McGinnis, Ed., Current Topics in Medical My- cology, Springer-Verlag, New York, 1985, pp. 313-351. [19] M. Taniguchi, Y. Yano, K. Motoba, S. Oi, H. Haraguchi, K. Hashimoto and I. Kubo, “Polygodial-Induced Sensi- tivity to Rifampicin and Actinomycin D of Saccharomy- ces cerevisiae,” Agricultural and Biological Chemistry, Vol. 52, No. 7, 1988, pp. 1881-1883. doi:10.1271/bbb1961.52.1881 [20] M. Taniguchi, Y. Yano, E. Tada, K. Ikenishi, S. Oi, H. Haraguchi, K. Hashimoto and I. Kubo, “Mode of Action of Polygodial, an Antifungal Sesquiterpene Dialdehyde,” Agricultural and Biological Chemistry, Vol. 52, No. 6, 1988, pp. 1409-1414. doi:10.1271/bbb1961.52.1409 [21] R. Surarit and M. G. Shepherd, “The Effects of Azole and Polyene Antifungals on the Plasma Membrane Enzymes of Candida albicans,” Journal of Medical and Veterinary Mycology, Vol. 25, No. 6, 1987, pp. 403-413. doi:10.1080/02681218780000491 [22] N. H. Georgopapadakou and T. J. Walsh, “Antifungal Agents: Chemotherapeutic Targets and Immunologic Stra- tegies,” Antimicrobial Agents and Chemotherapy, Vol. 40, No. 2, 1996, pp. 279-291. [23] S. Kanazawa, M. Driscoll and K. Struhl, “ATR1, a Sac- charomyces cerevisiae Gene Encoding a Transmembrane Protein Required for Aminotriazole Resistance,” Mole- cular and Cellular Biology, Vol. 8, No. 2, 1988, pp. 664- 673. [24] C. S. Lunde and I. Kubo, “Effect of Polygodial on the Mitochondrial ATPase of Saccharomyces cerevisiae,” Antimicrobial Agents and Chemotherapy, Vol. 44, No. 7, 2000, pp. 1943-1953. doi:10.1128/AAC.44.7.1943-1953.2000 [25] Y. Naito, C. C. Wu, M. G. Seal, F. Gelosa, M. Yoshioka, P. Safran and F. Marotta, “Protective Effect of a Poly- godial/Anethole-Containing Natural Product against Can- dida albicans Gastrointestinal Colonization and Dis- semination,” International Medical Journal, Vol. 8, No. 1, 2001, pp. 3-9. |

