Journal of Water Resource and Protection
Vol. 3 No. 6 (2011) , Article ID: 5556 , 8 pages DOI:10.4236/jwarp.2011.36052
Determination of P, Ca, Zn, Cd and Pb Concentrations in Muscle, Gills, Liver, Gonads and Skeletons of Two Natural Populations of Atherina lagunae in North Tunis Lake, Tunisia
1Unit of marine Biology, Faculty of Science, University campus, Tunis, Tunisia
2 Biological Evolution and modeling, University of Provence, Place Victor Hugo, France
3Laboratory of Ichthyology, University Montpellier II, Montpellier, France
Email: nediasakli@yahoo.fr
Received March 16, 2011; revised March 25, 2011; accepted March 28, 2011
Keywords: Heavy Metal, Phosphorus, Calcium, Deformation, Tunis North Lake, Atherina lagunae
ABSTRACT
In this study, zinc (Zn), cadmium (Cd), lead (Pb), phosphorus (P) and calcium (Ca) concentrations in muscles, gills, liver, gonads and skeletons of two natural populations of sand smelt Atherina lagunae (Teleostean, Atherinidae) normal and deformed, as well as bioaccumulation of these elements from the water and the sediment in the North Tunis Lake were investigated. The analysis of Ca was performed with flame atomic absorption spectrometry. The average concentrations of Ca in the different tissues analyzed show higher values in healthy atherines except in gonads where the average concentration of Ca in deformed atherines significantly exceeds that in normal atherines (p < 0.05) and the spine Ca concentrations were similar in the two populations. Zinc, cadmium, lead and phosphorus were determined by inductively coupled plasma atomic emission spectrometry (ICP-AES). The Zn concentrations of Atherina lagunae in North Tunis Lake were very high compared to other studies in other lagoons. The highest concentrations were found in deformed atherines. The differences are significant for all tissues studied (p < 0.05). The average concentration of P in different tissues analyzed shows that the highest values were detected in the normal population of Atherina lagunae. The potential rate of Cd was below the detection limit in the different organs analyzed, in water and sediment.
1. Introduction
The Tunis North Lake, environment receiving domestic and industrial discharge from Tunis City and neighboring towns, has experienced many dystrophies crises. From 1929 to 1980, the Tunis North Lake has been progressively deteriorated by enrichment of its funds by organic matter, the extinction of animal and marine plant and proliferation of Pollution indicator green algae Ulva.
The Tunis North Lake cleansing began in 1985 and lasted three years [1]. The main achievements are:
The deepening of the lake bottom, particularly in the north side, has a dredging volume of about 10,000,000 m3 of solid material.
The dredging of the channel Kheireddine, for a maximum exchange with the sea.
The introduction of hydraulic doors (locks) at the channel Kheireddine (opening and closing triggered by the tide), allowing control of water exchange with the sea water entering sea water from the Nord at high tide and drainage water lagoon to the south at low tide.
The construction of a central dam 8.2 km long in the middle of the lake, connecting the entrance to the Chekli Island and separating the lake into two parts to create a cyclonic circulation in the water.
The filling of nearly 500 ha of land, bringing the surface of the water to 2,500 ha.
Changing the line of banks (as a straight profile) to avoid stagnation of water, leading to 24 km periphery.
Establishing a system of renewal of waters was upon completion of remediation to have a better flow of water into the lake, with a daily input of about 1.6 million m3 seawater and a time to stay relatively small (19 to 27 days).
The drainage works have resulted in improved water quality and hydrodynamic conditions of the environment was responsible for the improvement of biological diversity (over 35 species of fish in 1995 against 24 in 1986 and 38 plant species in 1995 against a dozen in the early 80 s).
The analysis of water carried out in different sites in the North Lake of Tunis showed the detection of many potentially harmful species such as Pseudo-nitzschia spp., Dinophysis spp., Alexandrium spp., Prorocentrum spp., Gyrodinium spp. and Coolia monotis. The potentially harmful phytoplankton group abundance accounted for 42% of all the phytoplankton species detected. The composition of potentially harmful phytoplankton genera and their succession were related to the available nutrients and other environmental factors. Furthermore, there was a highly positive correlation between the occurrence of potentially harmful species and the two following factors: water salinity and temperature [2]. However in 2004, a high rate of spine deformities has been reported affecting fish in the Tunis North Lake such as Atherina lagunae [3,4], Diplodus, Sparus, Dicentrarchus and Syngnathus (unpublished results).
The effects of environmental disturbances studies of physic-chemical parameters on the spine showed that low temperatures and oxygen deficiency can lead to deformities in Salmo gairdneri [5]. High temperatures may also have similar effects in Cyprinus carpio [6]. Toxic substances such as heavy metals considered among the most dangerous contaminants, can cause spine deformities [7-9].
Lead can be introduced into the aquatic environment through soil erosion, as atmospheric dust, by oil combustion, by both domestic and industrial landfills and by precipitation [10]. According to Moriarty (1990), Lead is ubiquitous in the aquatic ecosystem. It is bioaccumulative. It is present as inorganic or organic element. Seymore et al. (1995) showed that Lead can be metabolized with calcium and subsequently accumulates in bone tissue. It also accumulates in muscle, bone, kidney, gills and liver [11]. The toxicity of Lead depends on the fish age, pH and water hardness [11].
Zinc is a trace element essential for fish metabolism. Several studies have examined both deficiencies and excesses of Zn. The toxic concentrations of Zn vary among aquatic organisms, the exposure time and environmental conditions [12]. Zinc accumulates mainly in the gills, intestines and liver.
Cadmium is known for its toxic effects in fish. It causes lesions in the testes and ovaries [13], reducing the consumption of oxygen by the gills [14] Pathological changes of kidney and intestinal tissues [15] and lesions and skeletal deformities [15-21].
• The axial skeletal deformities have been linked to dysfunction of bone metabolism in larvae of Lates calcarifer [22] and also the muscle degeneration in Danio rerio [23].
• Phosphorus, constituting cell membrane and essential in the phosphorylation reactions plays a major role in the development of bone tissue. The phosphorus absorption by gills cannot meet the phosphorus requirements. Deficiency of this element causes a growth decrease, bone demineralization and skeletal deformities [24].
• Calcium, such as the phosphorus, plays a vital role in bone mineralization.
• In this work, the presence of P, Ca, Zn, Cd and Pb were measured in various organs of A. lagunae in both normal and deformed individuals, in water and sediment from North Tunis Lake using several analytical techniques.
2. Materials and Methods
2.1. Study Area and Sampling
The atherines used in this study were captured from October 2004 to August 2005 in the Northwest of the Tunis North Lake (36˚50N - 10˚12E). The fish were first wrapped into polyethylene plastic, put into an isolated container, and brought to the Biology Laboratory of Tunis Sciences Faculty. After biometric measurements, the fishes were immediately frozen and stored at −80˚C until dissection. Caught fish were counted. Since spinal deformities were visible on the fish body immediately upon catching, the percentage of deformed fish was calculated. A fish showing a representative deformity were radiographed using a medical X-ray system and the radiographs were used for an examination of the skeleton. A sample of normal fish, based on external morphology, was also radiographed in order to validate the results of the macroscopic evaluation. A total of 300 fish were analyzed, including 150 with spine abnormalities (kyphosis, lordosis and scoliosis) and 150 normal.
2.2. Sample Preparation
To minimize contamination, all the materials used in the experiments were previously washed in ultra pure water, and a stainless steel knife was used to cut the tissues. Before analysis the fish were thawed and a 0.5 g sample was taken from each tissue (muscle, gill, gonads, skeletal and liver). All tissue samples were transferred into 100 ml Teflon beakers. Thereafter, 10 ml ultrapure concentrated nitric acid was added slowly to the sample. The Teflon beaker was covered with a watch glass, and heated at 200˚C on a hot plate for 3 h, until the solution evaporate slowly to near dryness. Two milliliters of 1 N HNO3 was added to the residue and the solution was evaporated again on the hot plate. By repeating the additional digestion twice, all organic materials in each sample were completely digested. After cooling, 2.5 ml of 1 N HNO3 was added to digested residue and was transferred to 25 ml volumetric flasks, then diluted to level with deionized water. Before analysis, the samples were filtered through a 0.45 ml nitrocellulose membrane filter. Sample blanks were prepared in the laboratory in a similar manner to the field samples [25]. Metal contents were expressed as µg/g wet weight for tissues.
2.3. Chemical Analysis
All samples analyses were repeated three times for Cd, P, Pb and Zn by ICP-AES Horiba Jobin Yvon. Standard solutions were prepared from stock solutions (Merck, multi element standard). The accuracy and precision of our results were checked by analyzed certified reference material (CRM, Dorm-2, Dorm-3). The results showed good agreement between the certified and the analytical values (Table 1), the recovery of elements being partially complete for most of them. The absorption wavelengths for Cd, Zn, Pb, and P were 226.502, 213.858, 220.353 and 214.914 nm, respectively.
Ca was determined by atomic absorption spectrometry with flame (Analytik Jena Nava 400).
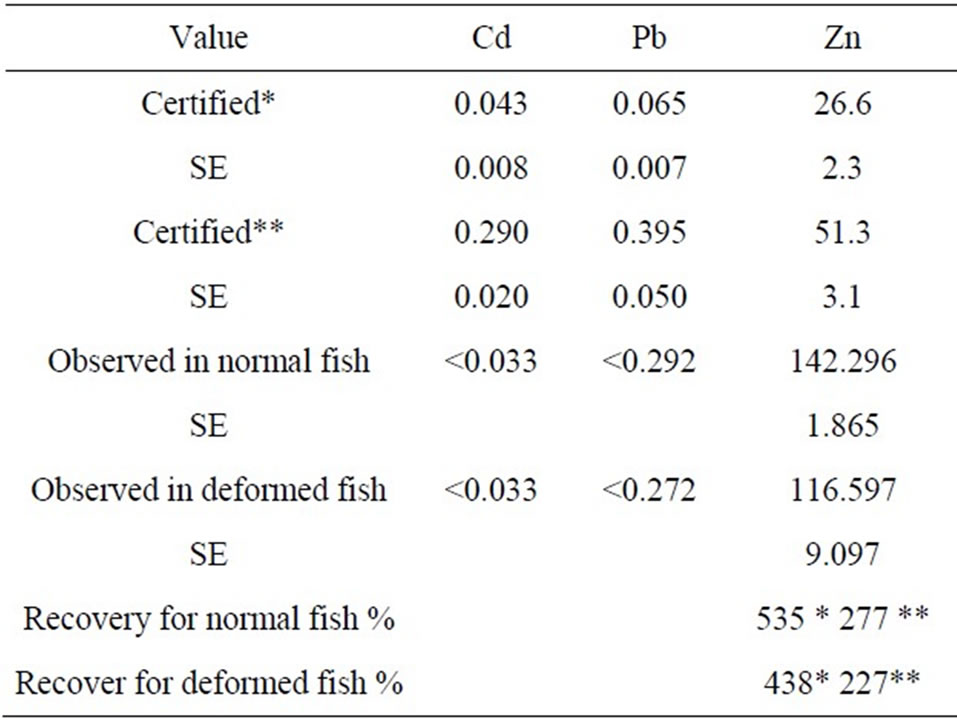
Table 1. Concentration of metals found in muscle from normal and deformed atherines and in Certified Reference Dorm-2 (Dogfish muscle)* and Dorm-3 (fish) **from the National Research Council, Canada (all data as mean ± standard errors, in µg/g dry weight).
2.4. Statistical Analyses
Each reported result was the average value of the three analyses. The results were offered as means ± SEM. A logarithmic transformation was done on the data to improve normality. To test the differences between the concentrations in tissues of fish, one way ANOVA was performed. Post hoc test (Duncan) was applied to determine statistically significant differences following ANOVA. Possibilities less than 0.05 were considered statistically significant (p < 0.05). All statistical calculations were performed with SPSS 13.0 for Windows.
3. Results
3.1. Incidence of Deformities
In the fish population studied, the global incidence of malformations was high. The proportion of kyphosis (Ki), lordosis (Li) and scoliosis (Si) were respectively 9.75% and 8.78%, and 2% of total captured fish. The rate of deformities decreased with age (e.g. 64.94% for size class 25 - 30 mm and <5% for size classe 50 - 55 mm). Kyphosis being the main deformity scored in this way (70% of the observed deformities) [3]. External scoliosis had the low incidence level. Among captured population a sample of 300 fish were analyzed (150 normal and 150 deformed fish).
3.2. Fish
3.2.1. Calcium
Calcium was determined by flame atomic absorption with a detection limit of 0.1713 mg/L. Ca was detected in all muscle samples (deformed fish and healthy). The average concentration is (11.51 ± 0.84) mg/g dry weight in the deformed atherines and (16.55 ± 0.19) mg/g dry weight in healthy atherines. Both samples were analyzed three times. The standard variation respectively in specimens deformed and healthy is of 7.53% and 1.2%. The difference between concentrations was significant (p < 0.05). Ca was also detected in the 300 livers tested. The average concentration respectively in the deformed and healthy fish is respectively 5.61 ± 0.86 mg/g dry weight and 7.08 ± 0.4 mg/g dry weight. The standard variation in the deformed is 15.35% and among healthy is 5.72%. Statistical analysis revealed an insignificant difference. The analysis of the skeleton showed a slight non-significant difference between the healthy and deformed samples (56.59 ± 4.73 mg/g dry weight vs. 55.64 ± 1.62 mg/g dry weight). Ca was detected in all samples of gills with average concentrations of 50.401 ± 6.738 mg/g dry weight in deformed atherines and 62.085 ± 1.939 mg/g dry weight in normal atherines. The standard variations are respectively in the deformed and healthy 13.37% and 3.14%. The highest concentration of Ca measured in the gonads was found in the deformed unlike other organs analyzed (3.59 ± 0.17 mg/g dry weight vs. 2.40 ± 0.19 mg/g dry weight). The difference is statistically signifycant (p < 0.005).
3.2.2. Zinc
Zn was detected in all tissues analyzed both in healthy and deformed fish. The highest concentrations were found in deformed atherines. The differences are signifycant for all tissues studied p < 0.05). The maximum value was found in the vertebral column of deformed specimens (309.7 ± 0.76 µg/g dry weight). In muscle, the average concentration was 142.30 ± 1.87 µg/g in deformed and 116.59 ± 9.09 µg/g dry weight in healthy. In the gills, the concentrations are similar in both samples (267.35 ± 0.6 for normal and 295.98 ± 13.06 µg/g dry weights in deformed) and the differences are not signifycant. Zn was also detected in all livers analyzed. The average concentrations are respectively in deformed and normal atherines 209.28 ± 10.26 µg/g and 37.73 ± 3.67 µg/g dry weights. The difference is highly significant (p < 0.01). The highest concentration was 216.53 µg/g in deformed atherines and 40.33 µg/g in the normal fish. In the gonads, the concentrations of Zn in deformed specimens were found to be higher than in healthy ones (180.93 ± 10.72 vs. 35.97 ± 0.32 µg/g dry weight) (p < 0.01).
3.2.3. Phosphorus
Phosphorus was detected in all organs analyzed in both healthy and deformed atherines. The differences were not significant in muscles, gills and skeletons. In liver, concentrations in normal atherines were twice time of the deformed fish (14.83 mg/g dry weight vs. 7.25 mg/g). The highest values were detected in the skeleton (53.25 mg/g dry weight in normal vs. 50.09 mg/g dry weight in deformed). The lowest values are noted in the gonads (8.07 mg/g dry weight vs. 5.59 mg/g dry weight). Statistical analysis showed significant differences (p < 0.05).
3.2.4. Cadmium and Lead
Cadmium and lead were below the limit detection in the different organs analyzed (liver, gills, muscles, gonads and skeleton), the limit of detection (LOD) is respecttively 33.1 ng/l and 272 ng/l. All samples are analyzed three times, but the concentrations of these two elements are always below the detection limits.
3.3. Water
The potential rate of Pb, Cd and Zn were below the limit of detection. LOD (Pb) = 11 µg/l; LOD (Cd) = 10.6 µg/l; LOD (Zn) = 18 µg/l. The average concentration of Ca and P were respectively 531.3 ± 63.76 mg/l and 3.39 mg/l.
3.4. Sediment
The concentration of Pb, Zn, P and Ca were respectively 38.01 ppm; 640.60 ppm; 452.60 ppm and 28.4%. The Cd concentration in sediment was below the limit of detection (LOD = 1.0 ppm).
4. Discussion
In this study, three types of spinal deformities consisting of a kyphosis, lordosis and scoliosis which frequently co-occur in varying degrees of severity are described, to our knowledge for the first time, in A. lagunae from the Tunis North Lake (Tunisia) [3,4,26,27]. These deformities were similar to those described in Z. ophiocephalus from the Karin Sea Eastern Middle Adriatic [28], in Atherina boyeri from the estuary of the Neretva [29] and reared Sparus aurata [30]. Spinal deformities in A. lagunae were found to be significantly associated with fish length. In both sexes, the highest occurrence of deformities was observed in the 20 - 25 mm class decreasing thereafter with fish length. The same result was obtained by Antunes and Lopes Da Cunha [31], who showed, in Gobius niger from Sado estuary in Portugal, that vertebral deformities were more frequent in the 60 - 69 mm class decreasing thereafter with fish length. These results also agree with those reported by Tutman et al., who noticed a decrease in spinal deformities with age in A. boyeri. The absence of older deformed fish could be explained by higher mortality of deformed fish as compared to normal fish. In fact, different possible effects on the individual due to spinal deformities are described in the literature: impaired swimming performance [32], decreased ability to escape and hindrance to catching food [33].
Many environmental factors can cause spinal deformities in wild fish populations, making detection of causal relationships difficult. The skeletal deformities can be environmentally induced by alteration of biological processes necessary for maintaining the biochemical integrity of bone, or neuromuscular effects, which lead to deformities without a chemical change in vertebral composition [34]. Our assessment of factors that may be causing the high incidence of deformities in sand smelt populations was based on comparisons of calcium and phosphorus, and some contaminants (Cd, Pb and Zn), with published information on relationships between environmental variables and fish deformities.


Table 2. Comparison of heavy metal concentration in fish with value taken from open literature, in µg/g dry weight).
Several physiological, environmental, genetic, xenobiotic and nutritional factors have been linked to this problem during larval and juvenile development stages of cultured freshwater and marine fish. These factors include phosphorus and calcium deficiencies and toxicities [35]. Skeletal abnormalities can also be caused by genetic factors such as mutations, hybridization or inbreeding [36]. Generally deformities are a complex mixture of different bone disorders including vertebral and spinal malformations such as kyphosis, lordosis and scoliosis. The frequency and severity of skeletal abnormalities can be directly related to age, as noted in halibut [37], while an indirect relationship has been observed in sharpsnout seabream [38]. Calcium, zinc and phosphorus either promote bone formation or mineralization [37]. Calcium and phosphorus are closely related to the development and maintenance of the skeletal system and the stability of the vertebrae are maintained by a solid phase of calcium phosphate. Fish and other aquatic organisms absorb Ca and P from water and their Ca requirement is met by their ability to absorb this element directly from water.
Unlike terrestrial animals, bone is not the major site of Ca regulation in fish. Fish gills provide them with continuous access to an unlimited Ca reservoir and Ca regulation occurs at the gills, fins, and oral epithelia tissues. The absorbed calcium is deposited in bone, scale and skin [37]. In this study, the concentrations of Ca in gills were significantly lower in deformed fish than in healthy ones (50.401 ± 6.738 mg/g dry weight in athérines deformed and 62.085 ± 1.939 mg/g dry weight in normal atherines). Calcium deficiency is not common in fish. Phosphorus deficiency signs include reduced growth, decreased feed efficiency, reduced bone mineralization and skeletal abnormalities. The concentrations of phosphorus in deformed atherines were lower in all samples analyzed. The phosphorus liver concentrations in healthy specimen were twice that those deformed. This differences was highly significant (p < 0.01).
The concentrations of metals in the liver represent the storage of metals from the water where the fish species live [39]. Thus, the liver is more often recommended as environmental indicator organ of water pollution than any other fish organs. We have found that Zn concentrations in muscle, gonads, gill and in liver of deformed A. lagunae from the Tunis North Lake were significantly higher than normal atherina. Zn in muscle of Atherina lagunae exceeds the values found in Sardina pilchardus (34.58 µg/g) and Atherina hepsetus (24.34 µg/g) [40], Sarda sarda (48.7 µg/g) [41]; Liza ramada (12.28 µg/g); Shelon labrax (7.24 µg/g) Mugil cephalus (40.2 µg/g); Lithognathus mormyrus (5.83 µg/g) [42] (Table 2). The liver concentrations were higher than those found in Carasobarbus luteus (1.36 µg/g) and Clarias gariepinus (4.39 µg/g) [43]. High values of Zn were reported in L. aurata (200.90 µg/g), C. labrax (190.20 µg/g), M. cephalus (161.30 µg/g), S. aurata (265.20µg/g) and L. ramada (339.79 µg/g) [42]. However, other studies show concentrations of Zn in the gills very low in Carasobarbus luteus (0.685 µg/g) and Clarias gariepinus (0.924 µg/g) [43]. Considering the heavy metal concentrations in the liver, the deformed fish exhibited higher accumulated liver concentrations of Zn indicative of a differenttial ability to handle the metal.
In accordance with the present study, Bengtsson et al. [16] have found high frequency of vertebral deformities in Myoxocephalus quadricornis exposed to heavy metal pollution in the Gulf of Bothnia (Baltic Sea). Bengtsson [16] suggested that vertebral damage caused by Zn might be attributed to its effect on the muscle action potential and thus to an effect on the neuromuscular system. In fact, field surveys [16,44] provide evidence of environmental influence on induction of skeletal abnormalities, including spinal and vertebral deformities.
A causal relationship between high environmental levels of chlorinated hydrocarbons or heavy metals and skeletal anomalies in fish has been suggested [18]. Induction of spinal deformities in fish can be confounded by a number of biotic factors (e.g. hereditary defects, parasite infections) and abiotic factors (e.g. vitamin deficiencies, electrical shock...) which are not related to pollution. Cadmium (Cd) is considered to be one of most toxic heavy metals. It enters the environment from natural and, essentially, anthropogenic sources [45]. Cd dissolved in water or deposit in sediment constitutes a contamination source for the various aquatic food chain links [46]. On the basis of the obtained results and the above mentioned requirements we propose to use the incidence of spinal deformities in A. lagunae as a biomarker for future monitoring programs, to evaluate the evolution of heavy metal pollution in the Tunis North Lake. However, this study must be regarded as a preliminary approach to this problem, due to the low number of sites examined.
REFERENCES
- G. Pergnent and N. Ben Maiz, “Le lac de Tunis, Unexemple de Restauration d’une lagune Méditerranéenne,” Actes de colloque, Brest, 7-8 November 2000, pp. 168-178.
- S. Turki, Z. Armi, E. Trabelsi and B. Maiz, “Nutrient Loading and Occurrence of Potentially Harmful Phytoplankton Species in the North Lake of Tunis Tunisia,” Cahier de Biologie Marine, Vol. 49, 2008, pp. 311-321.
- N. Ayed, E. Faure, J. P. Quignard, F. Maamouri and M. Trabelsi, “Incidence of Kyphosis Deformities in Natural Population of Atherina lagunae (Trabelsi et al. 2002) from the Tunis North Lake, Tunisia,” Marine Biology, 2008, Vol. 153, No. 3, pp. 319-325. doi:10.1007/s00227-007-0813-y
- N. Ayed, R. M. Barthélémy, J. L. Da Prato, J. P. Quignard and M. Trabelsi, “Accumulation de Métaux (aluminium et cuivre) Chez Atherina lagunae du lac nord de Tunis,” Revue Social Science Nata De Tunisie, Vol. 3, 2009, pp. 1-9.
- L. Tomasik, W. Wawrzyniak and A. Wnnicki, “Oxygen Deficiency and Negative Temperature as Teratogenic Factors in Rainbow Trout Salmo gairdneri,” Acta Icthyologique Piscat, Vol. 12, 1982, pp. 93-99.
- J. Mis, K. Bieniarz, P. Epler, M. Sokolowska-Mikolajczyk and J. Chyb, “Incubation of Fertilized Common Carp (Cyprinus carpio) Eggs in Different Concentrations of Copper,” Polymer Arch Hydrobiology, Vol. 42, 1995, pp. 269-276
- J. H. Beattie and D. Pascoe, “Cadmium Uptake by Rainbow Trout, Salmo gairdneri Eggs and Alevins,” Journal of Fish Biology, Vol. 13, No. 5, 1978, pp. 631-637. doi:10.1111/j.1095-8649.1978.tb03477.x
- G. W. Holcombe, D. A. Benoit, E. N. Leonard and J. M. McKim, “Long-Term Affects of Lead Exposure on Three Generations of Brook Trout (Salvelinus fontinalis),” Journal of the Fisheries Research Board of Canada, Vol. 35, 1976, pp. 1084-1088.
- J. Mis and J. Bigaj, “Hatching Glands of Carp (Cyprinus Carpio) Embryos from the Eggs Incubated at Various Oncentrations of Zinc or Copper,” Polymer Arch Hydrobiology, Vol. 44, 1997, pp. 153-155.
- J. Harte, C. Holdren, R. Schneider and C. Sfdrley, “Toxic A to Z: A Guide to Everyday Pollution Hazards,” University of California Press, Berkeley, 1991.
- G. Nussey, J. H. Van Vuren and H. H. Du Preez, “Bioaccumulation of Chromium, Manganese, Nickel and Lead in the Tissues of the Moggel, Labeo Umbratus (Cyprinidae), from Witbank Dam, Mpumalanga,” Water SA, Vol. 26, No. 2, 2000.
- R. N. Jackson, D. Baird and S. Els, “The Effect of the Heavy Metals Lead (Pb2+) and Zinc (Zn2+) on the Brood and Larval Development of the Burrowing Crustacean Callianassa kraussi,” Water SA, Vol. 31, 2005.
- G. B. Sangalang and M. J. O’Halloran “Adverse Effects of Cadmium on Brook Trout Testis and on in Vitro Testicular Androgen Synthesis,” Biology of Reproduction, Vol. 9, 1973, pp. 394-403.
- A. Calabrese, F. P. Thurbermg, M. A. Dawsona and D. R. Wenzloff, “Sublethal Physiological Stress Induced by Cadmium and Mercury in the Winter Flounder,” Pseudopleuronectes cmmericanus, 1975, pp. 15-21.
- J. Koyama and Y. Itazawa, “Effect of Oral Administration of Cadmium on Fish I. Analyticl Results of The Blood and Bones,” Bulletin of the Japanese Society of Scientific Fisheries, Vol. 43, 1977, pp. 523-526.
- B. E. Bengtsson, “Vertebral Damage in Wsh Induced by Pollutants,” In: J. E. Brown, Ed., Sublethal Effects of Toxic Chemicals on Aquatic Animals, Elsevier, Amsterdam, 1975, pp. 22-30.
- T. R. Henry, J. M.Spitsbergen, M. W. Hornung, C. C. Abnet and R. E. Peterson, “Early Life Stage Toxicity of 2,3,7,8-Tetrachlorodibenzo-P-Dioxin in Zebrafish (Danio Rerio),” Toxicology Applied Pharmacology, Vol. 142, No. 1, 1997, pp. 56-68.
- P. M. Mehrle, T. A Haines, S. Hamilton, J. L. Ludke, T. L. Maye and M. A. Ribick, “Relation between Body Contaminants and Bone Development in East-Coast Striped Bass,” Transactions of the American Fisheries Society, Vol. 111, 1982, pp. 231-241.
- P. E. Olsson, L. Westerlund, S. J. Teh, K. Billsson, A. H. Berg, M. Tysklind, J. Nilsson, L. O. Eriksson and D. E. Hinton, “Effects of Maternal Exposure to Estrogen and PCB on Different Life Stages of Zebrafish (Danio Rerio),” Royal Swedish Academy of Sciences, 1999, Vol. 28, pp. 100-106.
- H. Teraoka, W. Dong, S. Ogawa, S. Tsukiyama, Y. Okuhara, M. Niiyama, N. Ueno, R. Peterson and T. Hiraga, “2,3,7,8-Tetrachlorodibenzo-P-Dioxin Toxicity in the Zebrafish Embryo: Altered Regional Blood Flow and Impaired Lower Jaw Development,” Toxicological Sciences, Vol. 65, 2002, pp. 192-199. doi:10.1093/toxsci/65.2.192
- S. H. Cheng, A. W. K. Wai, C. H. So and R. S. S. Wu, “Cellular and Molecular Basis of Cadmium Induced Deformities in Zebrafish Embryos,” Environmental Toxicology and Chemistry, Vol. 19, No. 12, 2000, pp. 3024- 3031. doi:10.1002/etc.5620191223
- M. R. Fraser, T. A. Anderson and R. De Nys, “Ontogenetic Development of the Spine and Spinal Deformities in Larval Barramundi (Lates Calcarifer) Culture,” Aquacult, Vol. 242, No. 1-4, 2004, pp. 697-711.
- G. Gerhard, E. Kauffman, X. Wang, R. Stewart, J. Moore, C. Kasales, E. Demidenko and K. Cheng, “Life Spans and Senescent Phenotypes in Two Strains of Zebrafish (Danio Rerio),” Experimental Gerontology, Vol. 37, 2002, p. 1055. doi:10.1016/S0531-5565(02)00088-8
- S.-J. Kaushik, “Besoins et Apport en Phosphore Chez Les Poissons,” INRA Production Animales, Vol. 18, No. 3, 2005, pp. 203-208
- M. G. M. Alam, A. Tanaka, G. Allinson, L. J. B. Laurenson, F. Stagnitti and E. Snow, “A Comparison of Trace Element Concentrations on Cultured and Wild Carp (Cyprinus Carpio) of Lake Kasumigaura, Japan,” Ecotoxicology and Environmental Safety, Vol. 53, 2002, pp. 348-354. doi:10.1016/S0147-6513(02)00012-X
- N. Ayed, E. Faure, J.-P. Quignard, F. Maamouri and M. Trabelsi, “Données Preliminaries Sur les Déformations du Squelettes Axial de Atherina lagunae (Trabelsi et al. 2002) Du Lac Nord De Tunis,” Congrès Franco-Canadien de Zoologie, 21-23 Septembre 2005, Université de Montréal, Québec, Canada.
- N. Ayed, E. Faure, J. P. Quignard and M. Trabelsi, “Etude De La Cyphose Chez Atherina Lagunae Du Lac Nord De Tunis,” Troisième Rencontres Icthyologique de France, 28-31 Mars 2006, Paris, France.
- J. Dulcic, “Incidence of Spinal Deformities in Natural Populations of Grass Goby, Zosterisessor Ophiocephalus from the Karin Sea, Eastern Middle Adriatic,” Cybium, Vol. 28, 2004, pp. 7-11.
- P. Tutman, B. Glamuzina, B. Skaramuka, V. Koqul, N. Glavi and D. Dului, “Incidence of Spinal Deformities in Natural Populations of Sandsmelt, Atherina boyeri (Risso, 1810) in the Nerevta River Estuary, Middle Adreatic,” Fish Research, Vol. 45, 2000, pp. 61-64. doi:10.1016/S0165-7836(99)00098-3
- J. M. Afonso, D. Monter, L. Robaina, N. Astorga, M. S. Izquierdo and R. Gines, “Association of a LordosisScoliosis-Kyphosis Deformity in Gilthead Sea Bream (Sparus aurata) with Family Structure,” Fish Physiology and Biochemistry, Vol. 24, 2000, pp. 159-163. doi:10.1023/A:1007811702624
- M. Antunes and L. Da P. Cunha, “Skeletal Anomalies in Gobius Niger (Gobiidae) from Sado Estuary, Portugal,” Cybium, Vol. 26, 2002, pp. 1791-1784.
- J. S. Weiss and P. Weiss, “Abnormal Locomotion Associated with Skeletal Malformations in the Sheep Head Minnow, Cyprinidon variegatus, Exposed to Malathion,” Environmental Research, Vol. 12, 1976, pp. 196-200
- R. I. Kroger and J. F. Guthrie, “Incidence of Crooked Vertebral Columns in Juvenile Atlantic Menhaden, Brevoortia tyrannus,” Chesapeake Science, Vol. 2, 1971, pp. 276-278.
- S. P. Lall, L. M. Lewis-McCrea, “Role of Nutrients in Skeletal Metabolism and Pathology in Fish–An Overview,” Aquaculture, Vol. 267, 2007, pp. 3-19. doi:10.2307/1350917
- P. Divanach, C. Boglione, B. Menu, G. Koumoundouros, M. Kentouri and S. Cataudella, “Abnormalities in Finfish Mariculture: An Overview of the Problem, Causes and Solutions,” In: M. C. Saroglia, J. Sweetman and P. Lavens, Eds., Seabass and Seabream Culture: Problems and Prospects, Oostende, 1996, pp. 45-66. doi:10.1016/j.aquaculture.2007.02.053
- L. Madsen, J. Arnbjerg and I. Dalsgaard, “Radiological Examination of the Spinal Column in Farmed Rainbow Trout Oncorhynchus mykiss (Walbaum): Experiments with Flavobacterium psychrophilum and Oxytetracycline,” Aquaculture Research, Vol. 32, 2001, pp. 235- 241.
- L. M. Lewis, S. P. Lall and P. E. Witten, “Morphological Descriptions of The Early Stages of Spine and Vertebral Development in Hatchery-Reared Larval and Juvenile Atlantic Halibut (Hippoglossus Hippoglossus),” Aquaculture, Vol. 241, No. 1-4, 2004, pp. 47-59. doi:10.1046/j.1365-2109.2001.00552.x
- E. Favaloro and A. Mazzola, “Meristic Character Analysis and Skeletal Anomalies during Growth in Reared Sharpsnout Seabream,” Aquaculture, Vol. 8, 2000, pp. 417-430. doi:10.1016/j.aquaculture.2004.08.018
- H. Karadede, S. A. Oymak and E. U¨nlu¨, “Heavy Metals in Mullet, Liza abu, and Catfish, Siluris triostegus, from the Atat¨urk Dam Lake (Euphrates), Turkey,” Environment International, Vol. 30, 2004, pp. 183-188. doi:10.1023/A:1009284421354
- M. Canli and G. Atli, “The Relationships between Heavy Metal (Cd, Cr, Cu, Fe, Pb, Zn) Levels and Size of Six Mediterranean Fish Species,” Environmental Pollution, Vol. 121, 2003, pp. 129-136. doi:10.1016/S0160-4120(03)00169-7
- O. D. Uluozlu, M. Tuzen, D. Mendil and M. Soylak, “Trace Metal Content in Nine Species of Fish from the Black and Aegean Seas, Turkey,” Food Chemistry, Vol. 104, No. 2, 2007, pp. 835-840. doi:10.1016/S0269-7491(02)00194-X
- K. Uysal, Y. Emre and E. Köse ,“The Determination of Heavy Metal Accumulation Ratios in Muscle, Skin and Gills of Some Migratory Fish Species by Inductively Coupled Plasma-Optical Emission Spectrometry (ICPOES) in Beymelek Lagoon (Antalya/Turkey),” Microchemical Journal, Vol. 90, No. 1, 2008, pp. 67-70. doi:10.1016/j.foodchem.2007.01.003
- M. Türkmen and C. Ciminli, “Determination of Metals in Fish and Mussel Species by Inductively Coupled PlasmaAtomic Emission Spectrometry,” Food Chemistry, Vol. 103, 2007, pp. 670-675. doi:10.1016/j.microc.2008.03.005
- C. Boglione, C. Costa, M. Giganti, M. Cecchetti, P. Didato and M. Scardi, “Biological Monitoring of Wild Thicklip Grey Mullet (Chelon Labrosus), Goldengrey Mullet (Liza Aurata), Thinlip Mullet (Liza Ramada) and Flathead Mullet (Mugil Cephalus) (Pisces: Mugilidae) from Different Adriatic Sites: Meristic Counts and Skeletal Anomalies,” Ecological Indicators, Vol. 6, 2006, pp. 712-732.
- J. Burger, “Assessment and Management of Risk to Wildlife from Cadmium,” Science of the Total Environment, Vol. 389, 2008, pp. 37-45. doi:10.1016/j.ecolind.2005.08.032
- M. Romeo and M. Gnassia-Barelli, “Metal Distribution in Different Tissues and in Subcellular Fractions of the Mediterranean Clam Ruditapes Decussatus Treated With Cadmium, Copper, or Zinc,” Comparative Biochemistry and Physiology, Vol. 111, 1995, pp. 457-463.

