Chinese Medicine
Vol. 3 No. 1 (2012) , Article ID: 18120 , 12 pages DOI:10.4236/cm.2012.31009
Beneficial Effects of Fumaria indica on Chronic Stress-Induced Neurobehavioral and Biochemical Perturbations in Rats*
1Neuropharmacology Research Laboratory, Department of Pharmaceutics, Institute of Technology, Banaras Hindu University, Varanasi, India
2Department of Molecular and Human Genetics, Faculty of Science, Banaras Hindu University, Varanasi, India
3Stettiner Str. 1, D-76138 Karlsruhe, Germany
Email: #vikas.phe@itbhu.ac.in
Received September 30, 2011; revised November 17, 2011; accepted November 28, 2011
Keywords: Fumaria indica; Chronic Stress; Corticosterone; Anti-Oxidant; Cytokines; Adaptogen
ABSTRACT
Objective: To evaluate the anti-stress activity of standardized extract of Fumaria indica (FI) through validated behavioral models of rodents followed by estimation of biochemical changes associated with chronic stress. Methods: Fifty percent ethanolic extract of FI used in this study was standardized on its contents of fumaric acid and its conjugates (0.45% and 0.35% respectively). Stressed Charles Foster rats received unpredictable foot shocks (2 mA, 1 hr, 14 days) through electric grid. FI was given orally as 0.3% carboxymethyl cellulose (CMC) suspension in 100, 200 and 400 mg/kg doses. For comparison, Panax ginseng (PG) extract (100 mg/kg, p.o.) was used as standard adaptogen. Incidence of gastric ulceration, changes in weight of adrenal and spleen, behavioral depression, cognitive dysfunction test and suppression of sexual behavior in male rats were used as validated behavioral models. Plasma corticosterone, brain levels of lipid peroxides (LPO), superoxide dismutase (SOD), catalase (CAT) and reduced glutathione (GSH), and expression of cytokines IL-1β, TNF-α and IL-10 in circulating white blood cells (WBC) were quantified for ascertaining biochemical changes accompanying stress. Results: As compared to vehicle treated stressed rats, the FI and PG treated rats showed fewer incidence of gastric ulceration, reversal of changes in weight of adrenal gland and spleen, reversal of behavioral depression, better performance in passive and active avoidance tests for cognitive function and increased sexual activity. FI and PG significantly decreased chronic unpredictable stress induced elevation of corticosterone, and both extracts normalized also the abnormal oxidative status of the brain observed in stressed rats. FI treatment also suppressed the elevated level of IL-1β, TNF-α and IL-10 in stressed animals. Conclusions: FI could be another adaptogenic herb and fumaric acid and its conjugates are possibly involved in observed bioactivity of its extract.
1. Introduction
Fumaria indica Linn. (Syn: Fumaria parviflora, Fumariaceae) is a wildly growing weed found throughout India and many other parts of the globe. Due to its allelopathic effects on industrial crops and its potential beneficial effects as animal feed, it is now attracting considerable attractions of modern agricultural and veterinary researchers. It is also known since long to be a medicinal plant to the practitioners of Ayurvedic and Unani systems of medicine [1]. It is a common ingredient in several herbal preparations currently commercialized in India for treatments of liver complaints, aches and pains, diarrhea, fever, influenza and diverse other purposes. Numerous alkaloids, organic acids, sterols, and other phytochemicals have been isolated from the plant and diverse therapeutically interesting bioactive of its different types of extracts have also been reported during more recent years. They include their hepato-protective [2], hypoglycemic [3], anti-nociceptive and anti-inflammatory [4], anti-oxidant [5], immune-modulatory activities [6]. Moreover, hypotensive, smooth muscle relaxing, anticonvulsant and diverse CNS function modulating effects of protopine, fumariline and other alkaloids encountered in Fumaria indica has also been described. However, as yet little attention has been paid to evaluate possible involvement of central nervous system functions in diverse known therapeutically interesting pharmacological activities of Fumaria indica extracts.
Protopine, along with narlumidine, is quantitatively the major alkaloidal constituents of Fumaria indica [7] and this alkaloid is also one of the better pharmacologically studied constituent of the plant. It has been reported to be one of the hepato-protective constituents of hydro-alcoholic extracts of Fumaria indica [8] and its diverse therapeutically interesting bioactivities isolated from other medicinal plants are also known. A report revealing antidepressant like efficacy of protopine [9], triggered our interest to test antidepressant like efficacy of a hydro-alcoholic extract of the plant. Although our initial efforts did not reveal any antidepressants like efficacy of its hydroalcoholic extract [10], dose dependant anxiolytic and other CNS function modulating effects of the extract in a battery of animal models could easily be detected [11,12]. Critical re-analysis of all available preclinical data on Fumaria indica, protopine, and other bioactive secondary metabolite of the plant led to the speculation that therapeutically interesting bioactivities of hydro-alcoholic extracts could as well be due to their stress alleviating properties. Consequently, efforts were made to test its modulating effects on several neurobehavioral and biochemical parameters in chronically stressed animals.
2. Materials and Methods
2.1. Animals
Adult Charles Foster albino rats (150 ± 10 g) of either sex were obtained from the Central Animal House, Institute of Medical Sciences, Banaras Hindu University, Varanasi, and were randomly distributed into different experimental groups. The rats were housed in polypropylene cages at an ambient temperature of 25˚C ± 1˚C and 45% - 55% relative humidity, with a 12:12 h light/dark cycle. Animals were provided with commercial food pellets and water ad libitum unless stated otherwise. They were acclimatized to laboratory conditions for at least one week before using them for the experiments. Principles of laboratory animal care (NIH publication number # 85 - 23, revised in 1985) guidelines were always followed, and prior approval of Institutional Animal Ethics Committee (Dean/2009-10/694) of Banaras Hindu University was obtained.
2.2. Plant Material and Extraction
The plant Fumaria indica was collected locally in Varanasi. It was botanically authenticated by Prof. N. K. Dubey, Department of Botany, Faculty of Science, Banaras Hindu University, and a herbarium specimen (specimen voucher, JAN-2009-01) of the plant was preserved. After shade drying, extraction of the plant was done with soxhlet apparatus using 50% ethanol (v/v) as solvent [4].
2.3. Chemical Characterization of the Extract
Monomethyl fumarate is one of the very first hepatoprotective component of hydro-alcoholic Fumaria indica extracts identified [13], and fumaric acid and its esters are now considered to be potential therapies for multiple sclerosis [14]. Due to feasibility reasons, the tested Fumaria indica extract was provisionally characterized by its conjugated and free fumaric acid contents using High Performance Thin Layer Chromatography (HPTLC) and CAMAG TLC Scanner-III and Camag Linomat applicator IV. Commercially available fumaric acid and dimethyl fumarate (Sigma-Aldrich, USA) were used as authentic markers. For free fumaric acid quantification, the test sample and authentic marker (fumaric acid) were dissolved in methanol, and for estimation of fumaric acid conjugates, test sample and authentic marker (di methyl fumarate) were dissolved in 50 ml of 5N HCL and refluxed for two hours and then dried over water bath and re-dissolved in methanol. The samples and the marker (fumaric acid) were applied to pre-coated silica gel plate (Merck 60F254), and developed in the solvent system Formic Acid: Chloroform: Butanol: Heptane (12:16:32:44) up to 90 mm. Developed plates were dried and scanned under absorbance mode (scanning wavelength λ 260nm), and calculations were based on the area of peaks of sample and corresponding authentic marker [15]. The tested 50% ethanolic extract was found to contain 0.45% w/w of free fumaric acid and 0.35% w/w of fumaric acid conjugates (calculated as di-methyl fumarate).
2.4. Drug Treatment
The Fumaric indica extract (FI) was suspended in 0.3% CMC in distilled water and administered orally (once daily) for 14 days at three dose levels (100, 200 and 400 mg/kg/day). All such treatments were given 60 min before the electroshock procedure. Standard anti-stress agent Panax ginseng (PG; manufactured by Zenith Nutrition, Bangalore, India) was similarly administered as 0.3% CMC suspension at dose of 100 mg/kg for 14 days during the electroshock procedure. Control animals received the vehicle (0.3% CMC suspension) for 14 days without electroshock procedure. In another group, rats received 0.3% CMC suspension 1 h before the electroshock treatments.
2.5. Induction of Chronic Stress
The method of Armando et al. (1993) was used [16]. The stressed rats were subjected daily to 1 h of foot shock through a grid floor in a shock chamber for 14 consecutive days. The duration of each shock (2 mA) and the interval between the shocks was randomly programmed between 3 and 5 sec and 10 and 110 sec, respectively (in order to make the shocks unpredictable). Animals were sacrificed on day 14, 1 h after the last shock procedure, and on completion of other test procedure involved [17]. These other procedures used to quantify stress-induced pathologies are described below.
2.5.1. Gastric Ulceration
The stomachs of rats were removed and split open along the greater curvature. The numbers of discrete ulcers was noted by the help of a magnifying glass. The severity of the ulcers was scored after histological confirmations, 0 = no ulcers, 1 = changes limited to superficial layers of the mucosa with no congestion, 2 = half the mucosal thickness showing necrotic changes and congestion, 3 = more than two-third of mucosal thickness showing necrotic changes and congestion, and 4 = complete destruction of the mucosa with marked hemorrhage.
2.5.2. Adrenal Gland and Spleen Weights
The adrenal gland and spleen were removed and weighed. Organ weights were normalized for the body weights of the animal by calculating the ratio: mg organ weight/100 g body weight [17].
2.5.3. Behavioral Perturbation
2.5.3.1. Depression
The following two tests were used to assess behavioral depression:
“Behavioral Despair” Test
Rats were forced to swim individually in a polypropylene vessel (45 × 40 × 30 cm) with a water level of 20 cm, which ensured that the rat’s feet does not touch the floor of the vessel and that it could not climb out of it. The rat was allowed to swim for 10 min. Thereafter, during the next 5 min, the total period of immobility, characterized by complete cessation of swimming with the head floating above water level, was noted. This immobility period, after initial frenzied attempts to escape, was postulated to represent “behavioral despair” [18].
Learned Helplessness Test
On day 12 of the investigation, rats were subjected to additional foot shocks (60 scrambled shocks, 15 sec duration, 0.8 mA, and every min) in a two compartment jumping box with the escape door to the adjoining unelectrified compartment closed. Duration of this training was 1 h. On day 14, i.e. 48 h later, they were subjected again to avoidance training, using the same apparatus but keeping the escape route to the unelectrified chamber open. During this avoidance training the rats were placed in the electrified chamber and allowed to acclimatize for 5 min before being subjected to 30 avoidance trials, with an inter-trial interval of 30 sec. During the first 3 sec of the trial, a buzzer stimulus (conditioned stimulus, CS) was presented followed by electroshock (unconditioned stimulus, UCS) (0.8 mA) delivered via the grid floor for the next 3 sec. The avoidance response was characterized by escape to the adjoining “safe” chamber during CS. Failure to escape during UCS within 15 sec assessed as “escape” failure which is postulated to indicate despair or depression [19].
2.5.3.2. Cognitive Dysfunction
The following two tests were used to assess the effect of stress on retention of a learned task as memory:
Active Avoidance Test
Rats were trained for an active avoidance task before subjecting them to stress. During training, the rats were placed in the right electrified compartment of a shuttle box and allowed to acclimatize for 5 min. Thereafter, the rats were subjected to 15 sec of a buzzer stimulus (conditioned stimulus) which was followed by electric shock (1 mA, 50 Hz) given through the grid floor (unconditioned stimulus). On day 1of the experiment, the rats were given at least 10 trials, with an inter-trial interval of 60 min, until they reached the criterion of 100% avoidance response of jumping to the unelectrified left chamber of the shuttle box during conditioned stimulus. The test was repeated on day 14 in order to assess the retention of the active avoidance learning [20].
Passive Avoidance Test
The test apparatus used was a rectangular box (45 × 30 × 40) with an electrified grid floor. An 8 cm high platform (17 × 12 cm) was fixed to the centre of the floor. A rat was placed on the platform and allowed to step down. On day 1 of the experiment, i.e. 24 h after the first exposure to the chamber, the rat was again placed on the platform and on stepping down, received foot shock (0.75 mA, 2 sec) through the grid floor. Every rat was given such consecutive trials until the latency of step down had stabilized. The test was repeated on day 14 of the experiment, which enabled quantification of the retention capacity of the memory of the learned task [21].
2.5.3.3. Sexual Behavior
Male rats used in this study were individually placed together with 2 oestrinised (sequentially treated with oestradiol valerate 5 µg/rat, followed by hydroxyprogesterone 1.5 mg/rat, s.c., 48 h later) female rats in cage situated in a dimly lighted room for 10 min. The total numbers of mounts were counted [22].
2.5.4. Biochemical Parameters Estimation
2.5.4.1. Plasma Corticosterone
Plasma corticosterone was estimated using ELISA kit (Assay Design, USA). Immediately after the last stress regimen, animals were sacrificed by decapitation and blood was collected in EDTA coated tubes kept in ice and centrifuged at 1000 × g for 20 min at 4˚C. Plasma was separated and aliquots were stored at −70˚C for corticosterone estimation. Manufacturer’s instructions were followed for quantification of plasma corticosterone using ELISA kit.
2.5.4.2. Antioxidant Activity in Brain Tissue Homogenate
For these studies, the rats were sacrificed by decapitation after last foot shock treatment on day 14. Brain tissue were removed, weighed and homogenized as 1:10 in icecold 50 mM potassium phosphate buffer (pH 7.4) containing 1 mM EDTA. Protein was estimated using the method of Lowry et al., 1951 [23]. Other parameters quantified are described as follows:
Lipid Peroxides (LPO)
Lipid peroxidation was quantified by measuring the level of malonaldehyde (MDA) according to the method of Ohkawa et al., 1979 [24]. 100 μl tissue homogenate was added to 50 µl of 8.1% sodium dodecyl sulfate, vortexed and incubated for 10 min at room temperature. 375 µl of 20% acetic acid and 375 µl of thiobarbituric acid (0.6%) was added and placed in boiling water bath in sealed tubes for 60 min. The samples were allowed to cool at room temperature. Thereafter 1.25 ml of butanol: pyridine (15:1) was added, vortexed and centrifuged at 2000 × g for 5 min. Optical density of five hundred micro liters of the pink colored organic layer was measured at 532 nm on a spectrophotometer using. 1,1,3,3-Tetra-ethoxypropane was used as standard for MDA, and its concentration was expressed as n mol MDA/mg protein.
Superoxide Dismutase (SOD)
SOD activity was estimated by following the method of Kakkar et al., 1984 [25]. The inhibition of reduction of nitro blue tetrazolium (NBT) to blue colored formozan in presence of phenazine metha sulphate (PMS) and NADH was measured at 560 nm using n-butanol as blank. One unit of enzyme activity was defined as the amount of enzyme that inhibits rate of reaction by 50% in one min under the defined assay conditions and the results were expressed as units (U) of SOD activity/mg protein.
Catalase (CAT)
Catalase activity was assayed by the method of Sinha (1972) [26]. The reaction mixture contained 1.0 ml of 0.01 M phosphate buffer. 0.1 ml of brain homogenate and 0.4 ml of 2M H2O2. The reaction was stopped by the addition of 2.0 ml of dichromate acetic acid reagent (5% potassium dichromate and glacial acetic acid mixed in 1:3 ratio). Absorbance of reaction mixture was read at 620 nm. CAT activity was expressed as U/min/mg protein.
Glutathione (GSH)
One ml of brain homogenate was precipitated with 1.0 ml sulfosalicylic acid (4%). The samples were kept at 4˚C for 1 h and then subjected to centrifugation at 1200 × g for 15 min at 4˚C. The assay mixture contained 0.1 ml filtered aliquot, 2.7 ml phosphate buffer (0.1 M, pH 7.4) and 0.2 ml DTNB (0.4% in phosphate buffer 0.4 M, pH 7.4) in a total volume of 3.0 ml. The yellow colour developed was read immediately at 412 nm. The results were expressed as µM GSH/mg protein [27].
2.5.4.3. Cytokines in Blood Cells
RNA Isolation: Rat whole blood (1 ml) was mixed with 4 ml of RBC lysis buffer (155 mM NH4Cl, 12 mM NaHCO3, 0.1 mM EDTA) and incubated at room temperature for 10 min to lyse the red blood cells (RBCs). The remaining white blood cells (WBCs) were washed with phosphate buffer saline (PBS) and lysed in TRI Reagent™ (Sigma- Aldrich, USA). The total RNA was isolated from this lysate according to manufacturer’s instructions. The quality of RNA was assessed by running the RNA samples on 1% denaturating formaldehyde-agarose gel. The RNA was quantified using Nano Drop Spectrophotometer (Thermo Scientific, USA) and equal amount of total RNA from each rat was pooled in each study group.
2.5.4.4. Reverse Transcription-Polymerase Chain Reaction
(a) cDNA Preparation: In order to avoid amplification of contaminating genomic DNA, total RNA was treated with RNase-free DNase (New England Biolabs, USA). DNase treated RNA (2 µg) was reverse transcribed by using a high capacity cDNA reverse transcription kit (Applied biosystems, USA). Briefly, 10 μl of nuclease free water containing 2 µg of RNA was added to 10 µl of RT mix containing 2 μl of RT random primer (10X), 0.8 μl of dNTPs mix (100 mM), 2 μl of RT buffer (10X), 1 μl of MultiScribe™ Reverse Transcriptase (50 U/µl) and 1 µl of human placental ribonuclease inhibitor (10 U/µl). The samples were then incubated at 25˚C for 10 min. followed by incubation at 37˚C for 2 h. The reverse transcriptase was then inactivated by heating the reaction mixture at 85˚C for 5 min.
(b) PCR: The gene specific primers were either synthesized at Integrated DNA Technologies (USA) or were purchased from New England Biolabs (USA). Primer sequences and corresponding product size are mentioned in Table 1. The PCR reaction was carried out in a thermal cycler (Applied biosystems, USA) in a 15 μl final volume containing 0.5 μl of cDNA, 1.5 μ1of PCR buffer (10X), 0.15 μl of Taq polymerase (5 U/μl), 0.9 μl of MgCl2 (25 mM), 0.3 μl of each forward and reverse primer (10 pM). After an initial denaturation step at 94˚C for 5 min, temperature cycling was initiated. Each cycle consisted of denaturation step at 94˚C for 30 sec., annealing at primer specific temperatures for 30 sec. and extension at 72˚C for 30 sec. After 35 cycles, a final extension at 72˚C for 7 min. was done. PCR products were separated on 2% agarose gel and visualized using ethidium bromide staining. The density of each band was measured using densitometric software provided with Alpha Imager gel documentation system (Alpha Imager, India). The cytokine mRNA expressions were expressed as a ratio of cytokine band intensity to GAPDH housekeeping control.
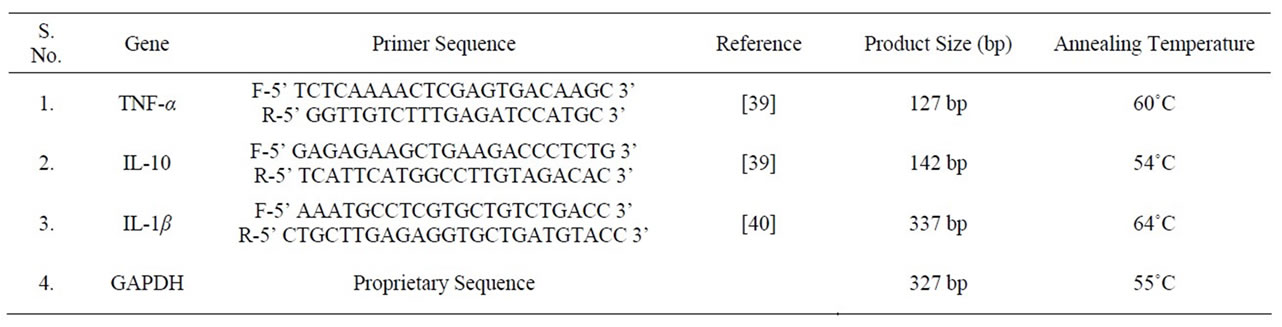
Table 1. Primers used for RT-PCR analysis.
2.6. Dose Finding Experiments in Unstressed Animals
These experiments were carried out using 20, 60 and 180 mg/kg (p.o.) doses of FI. Antidepressants like activities were tested in both rats and mice after its acute doses in forced swim test model [18], whereas anxiolytic activity was assessed only in rats, using suppressed feeding latency test and social interaction test [28] and after its single as well as seven daily oral doses.
2.7. Statistical Analysis
The data are expressed as means ± SD for each treatment group. The data obtained from each response measures were subjected to Kruskal-Wallis one way analysis of variance (ANOVA) and inter group comparisons was made by Mann-Whitney-U-test (two-tailed) for only those responses which yielded significant treatment effects in the ANOVA test.
3. Results
3.1. Body Weights and Gastric Ulceration
During the 14 days of chronic stress the vehicle treated rats lost their body weights, whereas weight gains were observed in the unstressed vehicle treated ones. As summarized in Table 2, stress induced body weight losses in the FI and PG treated groups were significantly lower than that of the vehicle treated stressed one. However, this effect of FI was not dose dependant, and both the plant extracts tested did not completely reverse the stressed-induced effects on body weight losses. Moreover, although the effects of FI on stress-induced gastric ulceration were dose dependant, its observed dose effect relationship for this effect was not very steep, and even after the highest dose of FI tested it did not completely protected all animals against stress induced ulcers. Observed effects of PG (100 mg/kg) on gastric ulcers were more pronounced than that of the maximally observed one after the highest FI (400 mg/kg/day) dose as mentioned in Table 2.
3.2. Adrenal Gland and Spleen Weight
Table 3 summarizes the observed variation of adrenal and spleen weights in different experimental groups. Chronic stress induced adrenal hypertrophy and hypotrophy of the spleen. The two higher FI doses (200 and 400 mg/kg/day) and PG (100 mg/kg/day) completely reversed these stress induced effects, and no statistically significant differences between the mean values of these two groups and that of the vehicle treated unstressed control group were observed. Statistically significant beneficial effects of the lowest FI dose tested (100 mg/kg/day) on both the stress induced pathologies was partial only. These observations reveal again that the standard herbal adaptogen PG used in this study is somewhat more potent than FI. They suggest though, that minimal effective doses of FI for affording protection against stress induced pathologies will be lower than 100 mg/kg/day.

Table 2. Effect of Fumaria indica on body weights and chronic stress induced gastric ulceration in rats.
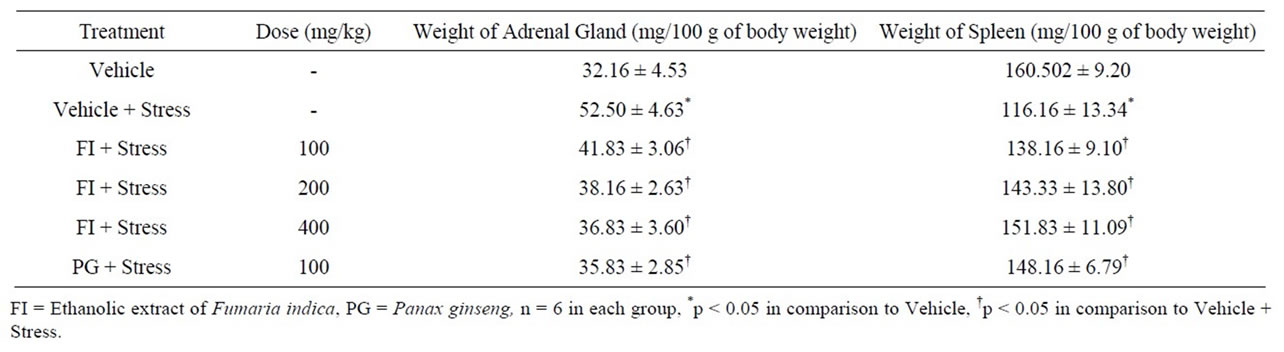
Table 3. Effect of Fumaria indica on chronic stress induced change in weight of adrenal gland and spleen.
3.3. Stress Induced Behavioral Perturbations
Earlier behavioral observation in our laboratories revealed dose dependant anxiolytic and memory function improving effects of FI. But even after its highest doses tested (400 mg/kg/day) it had no effects in several animal models for antidepressants [10-12]. However, depression is one of the major psychopathologies resulting from chronic stress, and antidepressant like effects of PG and other herbal adaptogens in stressed animals are also known. Consequently, the effects of FI on stress-induced depression and cognitive abnormalities were tested. In addition, effects of the extract on chronic stress induced sexual behavior were also tested. Observations made during these efforts are summarized as follows:
3.3.1. Behavioral Despair Test
Immobility period, i.e. an indicator of depression, was significantly higher in the vehicle treated and chronically stressed group than in all others. No statistically significant differences between the mean immobility periods of the two lower dose (100 or 200 mg/kg/day) of FI treated and the non-stressed group were observed. However, these values for the 400 mg/kg/day FI, or 100 mg/kg/day PG, treated groups were significantly lower than that of the vehicle treated non-stressed group (p < 0.001 for both the treated groups). These observations clearly demonstrate dose dependant antidepressant like efficacy of FI in chronically stressed animals, and that even its lowest dose regimen tested (100 mg/kg/day) completely protected the animals against stress induced behavioral despair. The results are depicted in Figure 1.
3.3.2. Learned Helplessness Test
Results and statistical analysis of the observations made in this test are summarized in Table 4. Escape failures and avoidance responses in the vehicle treated chronically stressed rats were the highest and the lowest respectively than those of all other groups. Daily treatments with FI, or with PG, completely blocked both the quantified stress-induced behavioral abnormalities induced in the vehicle treated stressed animals. In this test, the performances of the PG and FI (400 mg/kg/day) treated groups were significantly better than those of the vehicle treated control animals not subject to chronic stress. In view of the fact that FI is ineffective (even after 400 mg/kg/day doses) as an antidepressant in unstressed animals [10], the observed effects seems to be due to its stress-alleviating properties.
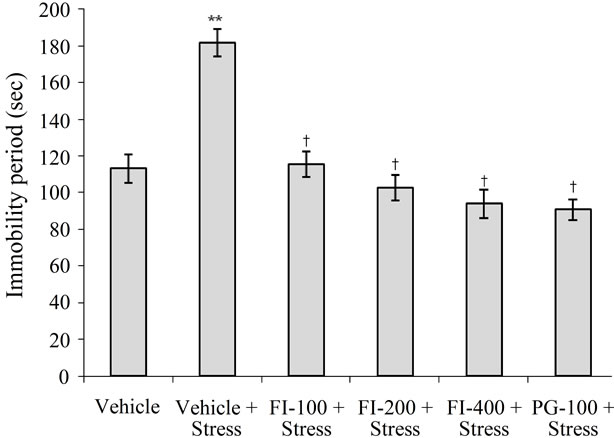
FI = Ethanolic extract of Fumaria indica, PG = Panax ginseng, n = 6 in each group, **p < 0.01 in comparison to Vehicle, †p < 0.05 in comparison to Vehicle + Stress.
Figure 1. Effect of Fumaria indica on chronic stress induced increase in immobility period.
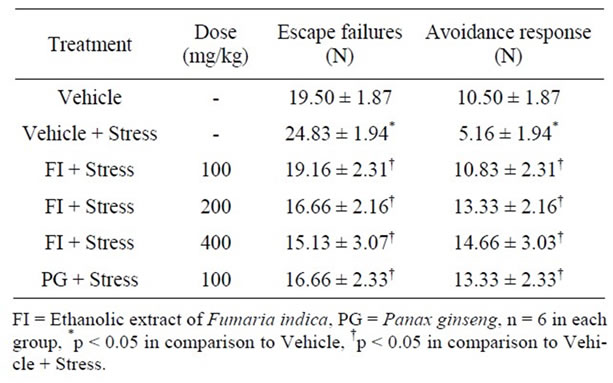
Table 4. Effect of Fumaria indica on chronic stress induced changes in learned helplessness test.
3.3.3. Active Avoidance Test
Number of avoidance responses of the vehicle treated stressed rats were significantly lower than those of the corresponding non-stressed ones. This stress-induced deficit in memory retention was dose dependently prevented by FI treatments. Dose-effect relationship of FI observed in this test was similar to those observed in other behavioral models using stress animals, and the magnitude of observed effect of PG (100 mg/kg/day) were as almost identical that of the highest FI doses (400 mg/kg/day). Results of this test are summarized in Figure 2.
3.3.4. Passive Avoidance Test
Mean step down latencies of the stressed and vehicle treated group was lower than that of the corresponding unstressed one. Observations summarized in Figure 3 demonstrate that stress-induced amnesia was significantly less pronounced in the FI, or PG treated groups. Efficacy of PG (100 mg/kg/day) in this test was almost equal to those of the higher two FI doses (200 and 400 mg/kg/day).
3.3.5. Sexual Behavior
In comparison to the non-stressed ones, sexual behavior of chronically stressed male rats was significantly depressed. The mean number of mounts of the vehicle treated stressed group was almost half of that of the vehicle treated control group (Figure 4). FI treatments dose dependently reversed this stress induced effect, and the observed magnitude of effect of PG (100 mg/kg/day) was almost identical to that of the highest FI dose studied (400 mg/kg/day).
3.4. Biochemical Observations
3.4.1. Plasma Corticosterone
As shown in Figure 5, chronic stress significantly increased the plasma corticosterone level (p < 0.001; in
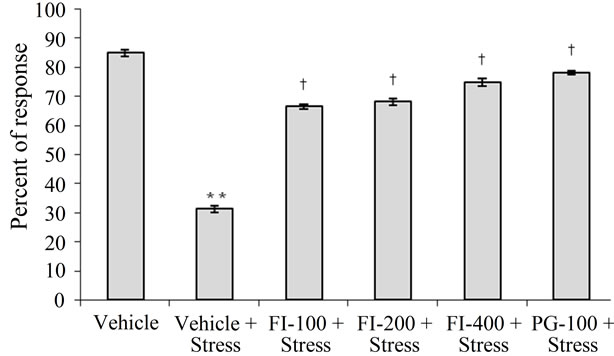
FI = Ethanolic extract of Fumaria indica, PG = Panax ginseng, n = 6 in each group, **p < 0.01 in comparison to Vehicle, †p < 0.05 in comparison to Vehicle + Stress
Figure 2. Effect of Fumaria indica on chronic stress induced memory deficits in active avoidance response in rats.
comparison to normal rats). This stress-induced effect was significantly suppressed, but not completely reversed, by FI or PG treatments. Mean plasma cortisone levels of all plant extracts treated groups were significantly higher than those of the vehicle treated unstressed group (p < 0.05 at least in all cases), and clear dose dependency of the FI effects was observed.
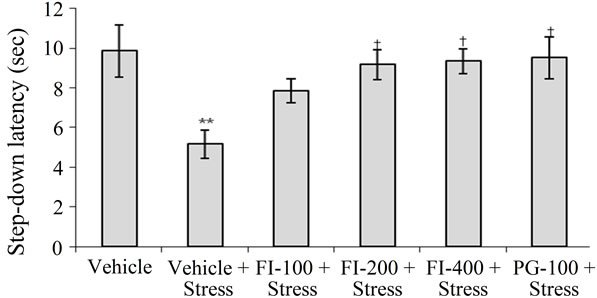
FI = Ethanolic extract of Fumaria indica, PG = Panax ginseng, n = 6 in each group, **p < 0.01 in comparison to Vehicle, †p < 0.05 in comparison to Vehicle + Stress.
Figure 3. Effect of Fumaria indica on chronic stress induced memory deficits in passive avoidance response in rats.
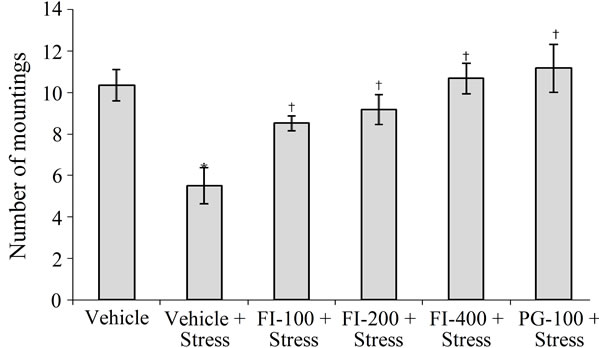
FI = Ethanolic extract of Fumaria indica, PG = Panax ginseng, n = 6 in each group, *p < 0.05 in comparison to Vehicle, †p < 0.05 in comparison to Vehicle + Stress.
Figure 4. Effect of Fumaria indica on chronic stress induced suppression of sexual behavior in male rats.
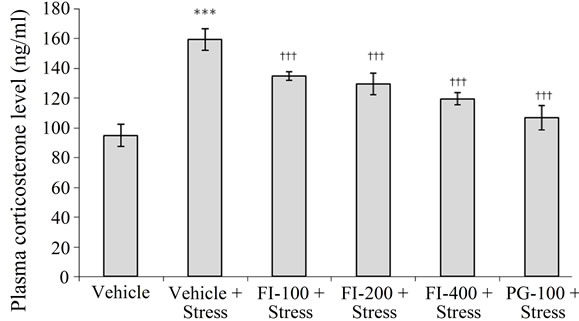
FI = Ethanolic extract of Fumaria indica, PG = Panax ginseng, n = 6 in each group, ***p< 0.001 in comparison to Vehicle, †††p < 0.001 in comparison to Vehicle + Stress
Figure 5. Effect of Fumaria indica on chronic stress induced plasma corticosterone level in rats.
3.4.2. Anti-Oxidant Activity
Concentrations of lipid peroxides (as judged by MDA concentrations) and glutathione (GSH) and of the enzymatic activities of superoxide dismutase (SOD) and catalase (CAT) quantified in the brain homogenates of different treatment groups are summarized in Table 5. Mean brain homogenate MDA level of the vehicle treated stressed group was significantly higher than that of the corresponding unstressed group. This stress induced elevation of lipid peroxides was significantly lower in all herbal extracts treated groups (p < 0.001 in all cases). The estimated MDA levels for the PG (100 mg/kg/day) and the two higher dose (200 and 400 mg/kg/day) FI treated groups were statistically not significantly different from that of the vehicle treated unstressed group.
Stress induced elevation of MDA level in the vehicle treated group was accompanied by lower levels of GSH, SOD and CAT (in comparison to the vehicle treated unstressed group). Analogous were also the cases for the two lower dose (100 and 200 mg/kg/day) FI treated groups. However, the mean values of all these three parameters in PG and all FI treated groups were significantly lower than those of the vehicle treated stressed group (Table 5). The observed dose dependency of FI for its antioxidative activity was quite similar to those encountered in behavioral studies in stressed animals.
3.4.3. Blood Cell Cytokines
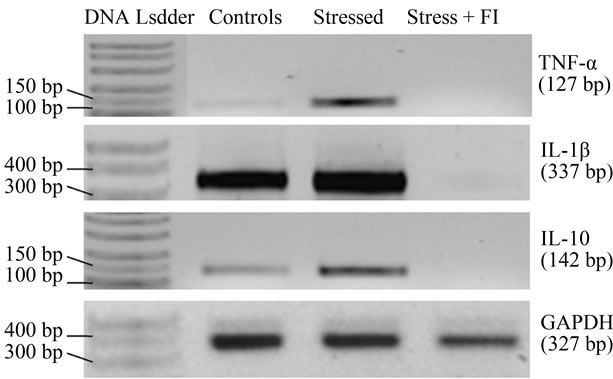
As shown in the gel picture (Figure 6(a)), stressful situation used in this study up regulated the expression of both pro-inflammatory (TNF-α, IL-1β) and anti-inflammatory (IL-10) cytokines in WBC of stressed animals. It is apparent from Figure 6(b), that this up-regulation was more prominent for TNF-α (6.7 fold) followed by IL-10 (3.2 fold) and IL-1β (1.4 fold). Surprisingly though, using the analytical method, no cytokine expression could be detected in the parallely run sample of an animal from the FI (100 mg/kg/day) treated group (Figure 6(a)). Although these observations suggest that down regulation of cytokines could be involved the mode of action FI, further more detailed experiments using more sensitive analytical methods will be necessary for confirming this possibility.
3.5. Dose Finding Experiments in Unstressed Animals
Critical analysis of the dose effect relationship of FI in animal behavioral models revealed that its maximal possible efficacy in most of them can be achieved after its 200 mg/kg/day dosing schedule, and that after 100 mg/kg/day
 (a)
(a)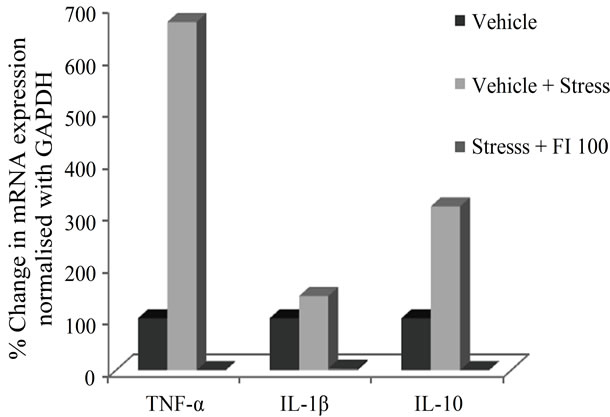 (b)
(b)
Figure 6. (a) Gel picture showing expression of various cytokines and house keeping gene GAPDH, in different experimental conditions; (b) Effect of Fumaria indica on mRNA expression of various cytokines in chronic stressed rats.
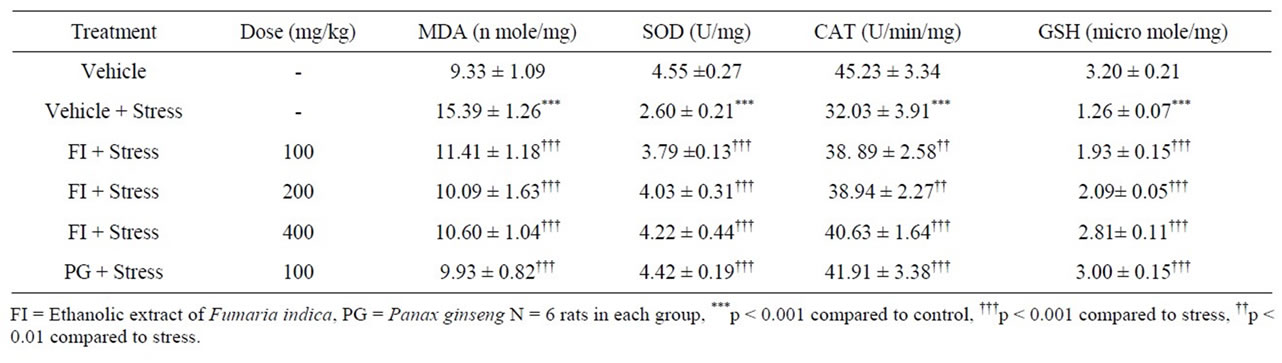
Table 5. Effect of Fumaria indica on anti-oxidant status of rat brain in foot-shock induced chronic stress.
more than 50% of its efficacy is achieved. Consequently, some efforts were made to more precisely define its minimum effective doses in a few behavioral models using unstressed animals and its lower doses. During such experiments no effects of single oral doses (20, 60 or 180 mg/kg) of FI were observed in forced swim test in rats and mice for antidepressants, or in novelty induced feeding and social interaction tests in rats for anxiolytics (results not shown). However, after its 7 repeated daily dose (180 mg/kg/day) significant anxiolytic like efficacy was observed in both the mentioned rat models for anxiolytics. Thus it becomes apparent that antidepressants like effects of FI observed in chronically stressed animals is mainly due to its modulating effects on stress induced pathologies.
4. Discussion
Homeostatic survival mechanism of all living organisms regulates their internal environment, and helps maintain a stable healthy condition. Imbalance in this survival mechanism is caused or followed by stress induced responses involving diverse neuro-endocrine and biochemical mechanism. Stomach ulcers are ultimate responses in variety of stress triggered health conditions and it has been proposed that stress induces hyperactivity of paraventricular nucleus (PVN) in the hypothalamus. Since gastric mucosal blood flow is an important part of defensive mechanism of gastric mucosa, its disturbance results in genesis of ulcers. In our study, FI or PG treatments significantly reduced the incidences and severities of ulcers caused by chronic unpredictable stress. But these treatments only partially compensated for the accompanying body weight losses. Alterations in adrenal gland and spleen weights, as well as body weight loses are some other consequences of chronic stress [29]. Although both FI and PG had only partial beneficial effects on stress induced body weight loses, they completely reversed the stress induced adrenal hypertrophy and spleen hypotrophy. Hypertrophy of adrenal gland is an adaptive mechanism for compensating increased corticosterone demand of the body necessary for stress alleviation mechanism.
In our earlier studies, even after highest dose of FI tested (400 mg/kg/day), no antidepressant like activity of FI was observed in unstressed animals [10]. On the contrary, even the lowest dose of FI (100 mg/kg/day) reversed completely the exaggerated depressive responses observed in chronically stressed animals, and after its higher doses its efficacy increased only slightly. Thus, it seems reasonable to assume that its 100 mg/kg/day dose is sufficient for counteracting the behavioral abnormalities induced by the chronic stressful condition used, and that biological mechanism involved in its mode of action are those vulnerable to chronic unpredictable stress.
Unlike its antidepressants like activity, anxiolytic and cognitive function improving effects of FI were observed also in non stressed animals [10-12]. The reported results of the dose finding experiments during this study reaffirm that its anxiolytic like efficacy can be observed after its repeated daily doses only. Thus, it seems reasonable to assume that repeated daily FI treatments likely to primarily modulates the biological mechanisms involved in anxiety, and that its observed beneficial effects on memory function could be the consequences of its anxiolytic like activity. It has been reported that protopine, i.e. one of the major bioactive alkaloidal components of Fumaria indica, which by virtue of its inhibitory effects on serotonin and noradrenaline transporters could have beneficial against cognitive functions and depression [9]. In light of the recent report demonstrating adaptive translocation of serotonin transporters after unpredictable chronic mild stress [30], it could as well be that the antidepressants like effects of FI observed in stress animals is due to the effects of low dose protopine on the serotonin transporters in a new location. Moreover it is possible that its bioactivities are modulated by other components of FI. Further efforts are necessary for clarifying these possibilities.
Antioxidative and other therapeutically interesting bioactivities of fumaric acid has been known since long, and during more recent years several clinical trials have demonstrated immunomodulatory and neuronal functions modulating effects of its esters [31]. Our observations revealed that elevated levels of lipid peroxides, and the lower ones of GSH, CAT and SOD, in chronically stressed rats were all reversed by FI treatments. These observations might indicate that fumaric acid and its conjugates present in the extract (ca 0.8% in total) could as well be involved in these effects of FI.
It remains certain that antidepressant and memory enhancing effects of FI observed in chronically stressed rats are accompanied by its protective effects against deregulations of oxidative status in the brains due to unpredictable chronic stress. Involvement of oxidative processes in the etiologies of anxiety, depression, and other psychopathologies have been known since long [32], and beneficial effects of a few naturally occurring antioxidants against such disorders have also been clinically demonstrated [33]. It has been reported indeed that inhibittion of nuclear factor NF-kB activity could be a feasible means for ameliorating stress-induced behavioral perturbations [34], and that fumaric acid conjugates suppress activities of nuclear factors [35]. Therefore, it is not unlikely that fumarates present in FI could as well be its major bio-active ingredient. If such would indeed be the case, it can be expected that FI also could lead to down regulation of cytokine expressions. Consequently, we estimated expressions of pro-inflammatory cytokines TNF- α and IL-1β and anti-inflammatory cytokines IL-10 in circulating WBCs in peripheral blood of vehicle or FI treated stressed animals. Results of the experiments revealed that all cytokines (pro-inflammatory cytokines TNF-α and IL-1β and anti-inflammatory cytokines IL-10) expressions in a vehicle treated stressed rats were much higher than those of a vehicle treated unstressed one, and that the stress-induced effects were completely suppressed by the lowest dose (100 mg/kg/day) of FI. IL-10 level is known to peak up later in inflammation and suppress the pro-inflammatory cytokines like TNF-α and IL- 1β which peak up shortly after inflammation but no such observation could be made in our case as samples were not collected at multiple time points. Although, these observations could indicate possible involvement of cytokines and inflammatory processes in the modes of actions of FI, further studies with lower doses and more sensitive methods are warranted.
Observations reported in this communication were made in the realm of a recently initiated project directed toward identifying promising Ayurvedic herbs potentially useful for helping patients with mental health problems. Selections of plants and pharmacological models for this project are made after thorough literature survey on available preclinical information on readily acquirable Ayurvedic plants commonly used in multi-herbal preparations commercialized as ayurvedic remedies. Initially, several older reports revealing psychopharmacological activities of protopine and other Fumaria indica alkaloids triggered our interest in screening FI for its potential antidepressant and anxiolytic like activities. These efforts soon revealed that it possesses anxiolytic and memory facilitating activities and that it is inactive in several behavioral models commonly used for identifying antidepressants [10-12]. Encouraged by its observed big safety margin [36,37] and presence of higher contents of fumaric acid and its conjugates in the extract, we became interested in testing its therapeutic potentials against psychological comorbidities often encountered in aged or chronically ill patients. Observation reported in this paper strongly suggest that Fumaria indica is another herbal adaptogen specially useful for combating co-morbid anxiety and cognitive dysfunctions commonly encountered in a vast majority of patients suffering from diverse chronic diseases caused by systemic inflammations.
Our observations clearly demonstrate protective effects of FI against chronic stress induced ulcer and diverse other pathologies including those involving the malfunctions of the central nervous system. Although further dose response and mechanistic studies are necessary for proper development of the tested extract, it remains certain that its pharmacologically active doses will be lower than 100 mg/kg, and that repeated daily doses of the extract will be necessary for therapeutic purposes. Since Fumaria indica is a wildly growing weed offering such a broad spectrum of therapeutic possibility, its further pharmacological exploration could lead to more affordable and effective therapies for the more economically underprivileged populations. In case such efforts would eventually lead to the identification of fumaric acid and its conjugates as its psychoactive constituents, this know how could be used for identifying novel pharmacological targets and mechanisms involved in diverse co-morbidities often encountered in a vast number of chronically ill patients. It cannot be overemphasized that Fumaria indica is a cheap as well as safe source for fumarates with known therapeutically interesting immune function modulating properties, and the list of therapeutic potentials of fumaric acid and its conjugates is still growing. For example, during the course of our efforts, a report [38] pointing out therapeutic potential of fumaric acid esters against Huntington’s disease appeared. Thus, the observations reported in this communication can be considered as preliminary experimenttal evidences to our conviction that fumaric acid, or its esters, present in FI could as well be involved in its observed adaptogenics like effects.
5. Conclusion
Our findings strongly suggest that Fumaria indica could be an economical source for obtaining a pharmacologically and phyto-chemically better standardized herbal adaptogen potentially useful for combating co-morbid mental health problems arising from uncontrollable environmental stress. Apart from protopine and other psychoactive alkaloids, fumaric acid and its conjugates could also be involved in stress alleviating effects of hydro alcoholic extracts of the herb. In any case, oxidative mechanisms seem to be involved in the observed effects of such extracts.
6. Acknowledgements
Authors are thankful for financial assistance provided by the Indian Council of Medical Research, Government of India, New Delhi. Thanks are also due to Mr. S. K. Chauhan, Research Scholar, Department of Molecular and Human Genetics, Faculty of Science, Banaras Hindu University, Varanasi for his technical assistance in cytokines assay.
REFERENCES
- E. M. Williamson, “Major Herbs of Ayurveda,” Churchill Livingstone, Churchill, 2006.
- A. H. Gilani, K. H. Janbaz and M. S. Akhtar, “Selective Protective Effect of the Extract from Fumaria parviflora on Paracetamol-Induced Hepatotoxicity,” General Pharmacology, Vol. 27, No. 6, 1996, pp. 979-983. doi:10.1016/0306-3623(95)02140-X
- M. S. Akhtar, Q. M. Khan and T. Khaliq, “Effect of Euphorbia prostrate and Fumaria indica in Normoglycemic and Alloxan-Treated Hyperglycemic Rabbits,” Planta Medica, Vol. 50, No. 2, 1984, pp. 140-142. doi:10.1055/s-2007-969653
- C. V. Rao, A. R. Verma, P. K. Gupta and M. Vijaykumar, “Anti-Inflammatory and Anti-Nociceptive Activities of Fumaria indica Whole Plant Extract in Experimental Animals,” Acta Pharmaceutica, Vol. 57, No. 4, 2007, pp. 491-498. doi:10.2478/v10007-007-0039-z
- M. Tripathi, B. K. Singh, C. Mishra, S. Raisuddin and P. Kakkar, “Involvement of Mitochondria Mediated Pathways in Hepatoprotection Conferred by Fumaria parviflora Lam. Extract against Nimesulide Induced Apoptosis in Vitro,” Toxicology in Vitro, Vol. 24, No. 2, 2010, pp. 495-508. doi:10.1016/j.tiv.2009.09.011
- S. J. Wasu and B. P. Muley, “Antioxidant Activity of Fumaria officinalis Linn and Its Study on Ethanol Induced Immunosupression,” Research Journal of Pharmacy and Technology, Vol. 2, No. 2, 2009, pp. 405-408.
- Y. C. Tripathi, M. Rathore and M. Kumar, “On the Variation of Alkaloidal Contents of Fumaria indica at Different Stages of Life Span,” Ancient Science of Life, Vol. 13, 1994, pp. 271-273.
- A. Rathi, A. K. Srivastava, A. Shirwaikar, A. K. S. Rawat and S. Mehrotra, “Hepatoprotective Potential of Fumaria indica Pugsley Whole Plant Extracts, Fractions and Isolated Alkaloid Protopine,” Phytomedecine, Vol. 15, 2008, pp. 470-477. doi:10.1016/j.phymed.2007.11.010
- L. F. Xu, W. J. Chu, X. Y. Qing, S. Li, X. S. Wang, G. W. Qing, J. Fei and L. H. Guo, “Protopine Inhibits Serotonin Transporter and Noradrenalin Transporter and Has the Antidepressant-Like Effect in Mice Models,” Neuropharmacology, Vol. 50, No. 8, 2006, pp. 934-940. doi:10.1016/j.neuropharm.2006.01.003
- G. K. Singh and V. Kumar, “Neuropharmacological Screening and Lack of Antidepressant Activity of Standardized Extract of Fumaria indica: A Preclinical Study,” Electronic Journal of Pharmacology and Therapy, Vol. 3, 2010, pp. 19-28.
- G. K. Singh and V. Kumar, “Anxiolytic Activity of Standardized Extract of Fumaria indica,” Annals of Neurosciences, Vol. 16, 2009, p. 81.
- G. K. Singh and V. Kumar, “Memory Enhancing and Anti-Amnestic Activity of Standardized Extract of Fumaria indica: An in-Vivo Study,” International Symposium on Brain Aging and Dementia: Basic and Translational Aspects, Varanasi, 29-30 November 2010.
- K. S. Rao and S. H. Mishra, “Antihepatotoxic Activity of Monomethyl Fumarate Isolated from Fumaria indica,” Journal of Ethnopharmacology, Vol. 60, No. 3, 1998, pp. 207-213. doi:10.1016/S0378-8741(97)00149-9
- D. Moharregh-Khiabani, R. A. Linker, R. Gold and M. Stangel, “Fumaric Acid and Its Esters: An Emerging Treatment for Multiple Sclerosis,” Current Neuropharmacology, Vol. 7, No. 1, 2009, pp. 60-64. doi:10.2174/157015909787602788
- HPTLC-Fingerprint Atlas of Ayurvedic Single Plant Drugs Mentioned in Ayurvedic Pharmacopoeia Vol-III and IV. http://www.whoindia.org/LinkFiles/Traditional_Medicine_HPTLC_Finger_Print_Atlas_of_Medicinal_Plants.pdf
- I. Armando, A. P. Lemoine, E. T. Segura and M. B. Barontini, “The Stress-Induced Reduction in Monoamine Oxidase (MAO) Activity Is Reversed by Benzodiazepines: Role of Peripheral Benzodiazepine Receptors,” Cellular and Molecular Neurobiology, Vol. 13, No. 6, 1993, pp. 593-600. doi:10.1007/BF00711559
- O. P. Tiwari, S. K. Bhattamisra, P. N. Singh and V. Kumar, “Adaptogenic Anti-Stress Activity of Standardized Extract of Marselia Minuta L,” Pharmacologyonline, Vol. 1, 2009, pp. 290-299.
- R. D. Porsolt, A. Bertin and M. Jalfre, “Behavioral Despair in Mice: A Primary Screening Test for Antidepressants,” Archives Internationales de Pharmacodynamie et de Therapie, Vol. 229, No. 2, 1977, pp. 327-336.
- M. H. Thiebot, P. Martin and A. J. Puech, “Animal Behavioral Studies in the Evaluation of Antidepressant Drugs,” British Journal of Psychiatry, Vol. 160, No. 15, 1992, pp. 44-50.
- V. Kumar, P. N. Singh and S. K. Bhattacharya, “AntiStress Activity of Indian Hypericum perforatum Linn,” Indian Journal of Experimental Biology, Vol. 39, 2001, pp. 344-349.
- A. P. Sen and S. K. Bhattacharya, “Effect of Selective Muscarinic Receptor Agonists and Antagonists on Active-Avoidance Learning Acquisition in Rats,” Indian Journal of Experimental Biology, Vol. 29, 1991, pp. 136- 139.
- S. Morishita, M. Shoji, Y. Oguni, Y. Hirai, C. Sugimoto and C. Ito, “Effects of Crude Drugs Derived from Animal Sources on Sexual and Learning Behaviour in Chemically Stressed Mice,” Phytotherapy Research, Vol. 7, No. 1, 1993, pp. 57-63. doi:10.1002/ptr.2650070114
- O. H. Lowry, N. J. Rosenborough, A. L. Farr and R. J. Randal, “Protein Measurement with Folin Phenol Reagent,” The Journal of Biological Chemistry, Vol. 193, 1951, pp. 265-275.
- H. Ohkawa, N. Ohishi and K. Yagi, “Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction,” Analytical Biochemistry, Vol. 95, No. 2, 1979, pp. 351-358. doi:10.1016/0003-2697(79)90738-3
- P. Kakkar, B. Das and P. N. Viswanathan, “A Modified Spectrophotometric Assay of Superoxide Dismutase,” Indian Journal of Biochemistry & Biophysics, Vol. 21, 1984, pp. 130-132.
- A. K. Sinha, “Colorimetric Assay of Catalase,” Analytical Biochemistry, Vol. 47, No. 2, 1972, pp. 389-394. doi:10.1016/0003-2697(72)90132-7
- D. J. Jollow, J. R. Mitchell, N. Zampaghone and J. R. Gillete, “Bromobenzene Induced Oxide as the Hepatotoxic Intermediate,” Pharmacology, Vol. 11, 1974, pp. 161-169.
- V. Kumar, A. K. Jaiswal, P. N. Singh and S. K. Bhattacharya, “Anxiolytic Activity of Indian Hypericum perforatum Linn: An Experimental Study,” Indian Journal of Experimental Biology, Vol. 38, 2000, pp. 36-41.
- M. M. Ostrander, Y. M. Ulrich-Lai, D. C. Choi, N. M. Richtand and J. P. Herman, “Hypoactivity of the Hypothalamo-Pituitary-Adrenocortical Axis during Recovery from Chronic Variable Stress,” Endocrinology, Vol. 147, No. 4, 2006, pp. 2008-2017. doi:10.1210/en.2005-1041
- A. Baudry, S. Mouillet-Richard, B. Schneider, J. M. Launey and O. Kellermann, “MiR-16 Targets the Serotonin Transporter: A New Facet for Adaptive Responses to Antidepressants,” Science, Vol. 329, No. 5998, 2010, pp. 1537-1541. doi:10.1126/science.1193692
- R. Gold, R. A. Linker and M. Stangel, “Fumaric and Its Esters: An Emerging Treatment for Multiple Sclerosis with Antioxidative Mechanism of Action,” Clinical Immunology, Vol. 142, No. 1, 2012, pp. 44-48. doi:10.1016/j.clin.2011.02.017
- I. Hovatta, J. Juhila and J. Donner, “Oxidative Stress in Anxiety and Comorbid Disorders,” Neuroscience Research, Vol. 68, No. 4, 2010, pp. 261-275. doi:10.1016/j.neures.2010.08.007
- S. E. Lakhan and K. F. Vieira, “Nutritional and Herbal Supplements for Anxiety and Anxiety-Related Disorders: Systematic Review,” Nutrition Journal, Vol. 9, 2010, pp. 42. doi:10.1186/1475-2891-9-42
- R. K. Manchanda, A. S. Jaggi and N. Singh, “Ameliorative Potential of Sodium Chromoglycate and Diethylthiocarbamic Acid in Restraint Stress-Induced Behavioral Alterations in Rats,” Pharmacology Reports, Vol. 63, 2011, pp. 54-63.
- T. J. Stoof, J. Flier, S. Sampat, C. Nieboer, C. P. Tensen and D. M. Boorsma, “The Antipsoritic Drug Dimethyl Fumarate Strongly Suppresses Chemokine Production in Human Keratinocytes and Peripheral Blood Mononuclear Cells,” British Journal of Dermatology, Vol. 144, No. 6, 2011, pp. 1114-1120. doi:10.1046/j.1365-2133.2001.04220.x
- G. K. Singh and V. Kumar, “Acute and Sub-Chronic Toxicity Study of Standardized Extract of Fumaria indica in Rodents,” Journal of Ethnopharmacology, Vol. 134, No. 3, 2011, pp. 992-995. doi:10.1016/j.jep.2011.01.045
- G. K. Singh, S. K. Chauhan, G. Rai and V. Kumar, “Fumaria indica Is Safe during Chronic Toxicity and Cytotoxicity: A Preclinical Study,” Journal of Pharmacology and Pharmacotherapeutics, Vol. 2, No. 3, 2011, pp. 191- 192. doi:10.4103/0976-500X.83287
- G. Ellrichmann, E. Petrasch-Parwez, D. H. Lee, C. Reick, L. Arning, C. Saft, R. Gold and R. A. Linker, “Efficacy of Fumaric Acid Esters in the R6/2 and YAC128 Models of Huntington’s Disease,” PLoS ONE, Vol. 6, 2011, e16172. doi:10.1371/journal.pone.0016172
- M. L. Batista, J. C. Rosa, R. D. Lopes, F. S. Lira, E. Martins, A. S. Yamashita, P. C. Brum, A. H. Lancha, A. C. Lopes and M. Seelaender, “Exercise Training Changes IL-10/TNF-alpha Ratio in the Skeletal Muscle of Post-MI Rats,” Cytokine, Vol. 49, No. 1, 2010, pp. 102-108. doi:10.1016/j.cyto.2009.10.007
- N. Tanda, H. Ohyama, M. Yamakawa, M. Ericsson, T. Tsuji, J. McBride, A. Elovic, D. T. Wong and G. R. Login, “IL-1 Beta and IL-6 in Mouse Parotid Acinar Cells: Characterization of Synthesis, Storage, and Release,” American Journal of Physiology, Vol. 274, No. 1, 1998, pp. 147-156.
NOTES
*Conflict of interest statement: All authors declare that they have no conflict of interests.
#Corresponding author.
†Retired Head of Pharmacology Research Laboratories, Dr. Willmar Schwabe GmbH & Co. KG., Karlsruhe, Germany.

