Health
Vol.6 No.1(2014), Article ID:41698,7 pages DOI:10.4236/health.2014.61009
Scaling up of trachoma mapping in Salima District, Central Malawi
![]()
1Department of Ophthalmology, College of Medicine, University of Malawi, Blantyre, Malawi; *Corresponding Author: khumbokalua@yahoo.com
2Blantyre Institute for Community Ophthalmology, Lions Sight First Eye Hospital, Blantyre, Malawi
3John Hopkins Research Project, University of Malawi, College of Medicine, Blantyre, Malawi
4Malawi Liverpool Welcome Trust (MLWT), College of Medicine, Blantyre
5World Health Organisation, Lilongwe, Malawi
6Ministry of Health, Lilongwe, Malawi
7London School of Hygiene and Tropical Medicine, London, UK
Received 15 November 2013; revised 26 December 2013; accepted 6 January 2014
ABSTRACT
Background: A number of suspected endemic districts with Trachoma have not been mapped in Malawi, and this contributes to delays for scaling up trachoma control activities. Objectives: To determine the prevalence of trachoma and associated risk factors in one of the suspected endemic districts (Salima District) in central Malawi and to generate information to guide policy decisions. Methods: A population-based survey conducted in randomly selected clusters in Salima District (population 418,672), central Malawi. Children aged 1 - 9 years and adults aged 15 and above were assessed for clinical signs of trachoma. Results: In total, 884 households were enumerated within 36 clusters. A total of 2765 persons were examined for ocular signs of trachoma. The prevalence of trachomatous inflammation, follicular (TF) among children aged 1 - 9 years was 17.1% (95% CI 14.9 - 19.4). The prevalence of trachoma trichiasis (TT) in women aged 15 years and above was 1.3% (CI 0.7 - 2.3), while the prevalence in men was zero. The presence of a dirty face and lack of sanitation were significantly associated with trachoma follicular (P < 0.001). Conclusion: Prevalence rate of trachoma follicles (TF) in Central Malawi exceeds the WHO guidelines for the intervention with mass antibiotic distribution (TF > 10%), and warrants the trachoma SAFE (Surgery, Antibiotics, Face washing and Environmental hygiene) control strategy to be undertaken in Salima District.
Keywords:Malawi; Trachoma; Prevalence; Risk Factors; Blindness; Trichiasis; Follicles
1. INTRODUCTION
Trachoma is an infectious disease triggered by the bacterium Chlamydia trachomatis, mostly by the serotypes A, B, Ba and C [1,2] which have all been observed in the Gambia [3]. Repeated infections can eventually lead to eyelids thickening and developing scars resulting in the eyelashes turning inwards and rubbing on the cornea [4], causing abrasions and ulceration, eventually leading to visual loss and blindness. The World Health Organization (WHO) in 1993 categorized the clinical features of trachoma in five stages using the WHO simplified grading system [5,6] as follows: Trachomatous inflammation follicular (TF), Trachomatous inflammation intense (TI), Trachoma Scarring (TS), Trachomatous Trichiasis (TT) and Corneal Opacity (CO).
Trachoma is still considered the leading cause of preventable blindness in sub-Saharan Africa especially in countries that have poor environmental sanitation, inadequate water supply and poor socio-economic status [7-9]. Worldwide, despite improvements in trachoma control over the recent years, trachoma still contributes to 2.9% of all global causes of blindness and is the 8th commonest blinding disease [10,11]. There are still 8.2 million people living with trichiasis globally and an estimated 40.6 million cases of active disease [7]. Blinding trachoma remains a problem where living conditions facilitate continuous transmission of Chlamydia trachomatis among family members [12]. Indeed trachoma is among the many neglected tropical diseases (NTD’s) that are associated with poverty and with together produce a disease burden almost as great as that associated with human immunodeficiency virus/AIDS, tuberculosis, or malaria [13]. It is estimated that neglected tropical diseases contribute to approximately 500,000 deaths per year and further result in an additional 57 million disability adjusted years (DALY’s) [14,15].
The strategy recommended for control of trachoma is known as SAFE, an acronym standing for Surgery for Trichiasis, Antibiotic for active disease, Facial cleanliness promotion to reduce transmission from person to person by flies and Environmental improvement which reduces transmission of the infection. The WHO aims to achieve Global Elimination of Blinding Trachoma by the year 2020 (GET2020) through implementation of the SAFE strategy [16]. Trachoma is suspected to be highly endemic in Malawi even though there have been only a few populations based on studies done [17-19]. Lack of district level prevalence data exists in many other countries suspected of having endemic trachoma [20]. Some evidence suggests that prevalence of trachoma can be reduced over a period of time even if only certain parts of the SAFE are implemented [18]. There is therefore a need to conduct population-based studies of trachoma in areas where the prevalence was assessed previously and some trachoma control activities implemented afterwards. It is necessary to document the changes over a period of time and give guidance as to whether there is still a need to implement the recommended SAFE strategy in an integrated manner.
We sought to determine the prevalence of trachoma in Salima, one of the previously reported highly trachoma endemic districts in central Malawi, with Trachoma Follicular prevalence rates of 60% reported [21].
2. METHODS
Ethical approval was obtained from the National Health Sciences Research Committee (NHSRC) based in Lilongwe and the district health administrative offices (DHO) in Salima, Malawi. Upon explanation of the purpose of the study, written informed consent was obtained from all subjects who participated in the study. Where the participants were illiterate, information was read to them and consent was obtained by using the thumb print on the consent form. For minors informed consent was obtained from the guardians. According to the extrapolation of the Malawi National Statistical office (NSO) population census results of 2008, Salima hada population of 418672.
Figure 1 shows map of Malawi and where Salima is situated.
The district lies on the rift valley lakeshore, adjacent the western side of Lake Malawi, with a latitude of 13˚45'55" South and a longitude: 34˚23'53" East.
Some residents along the shore have their houses built as close as 50 metres away from the lake, and they draw drinking from the lake and wash and bath directly in the lake. Most of the lowland soils are made up of sand and clay and are seasonally waterlogged, making it impossible to construct pit latrines and garbage disposal pits. Since there is no bush for people to help themselves (due to heavy deforestation), it is not uncommon for adults to urinate and defecate in the sand while children defecate in the open space a few metres away from the houses. Plenty of flies are often seen along the lakeshore in Salima, as sanitation and hygiene is generally poor. Socially, men are usually polygamist and their main occupation is fishing. On the other hand women spend long time travelling to highlands to fetch firewood for cooking.

Figure 1. Map of Malawi showing the survey site.
In regard to eyecare, the district has a referral general hospital where an ophthalmic clinical officer (OCO), a clinically trained ophthalmic technician is based and regularly conducts eye clinics at the district hospital and also conducts outreach eye clinics at various health centres located within the community. The Ophthalmic clinical officer is trained to do trichiasis surgeries within the district hospital using the WHO recommended bilamelar tarsal plate rotation. However, coverage for trichiasis surgery is very low (<50 cases per year), and this has been partly attributed to lack of surgical equipment and supplies. Health promotion on primary eye care, including hygiene and environmental control is regularly done by community health workers (Health Surveillance Assistants) who have been trained and are fully based in the community. Trachomatous infections are currently treated with tetracycline eye ointment within the health centers and at the district hospitals.
The study was a cross-sectional household based survey designed to obtain district level prevalence estimates for trachoma, Trachoma Follicular (TF) and Trachoma Intense (TI), collectively constituting active trachoma— in children aged 1 - 9 years, Trachoma trichiasis (TT) in adult men and women aged 15 years and above, and to assess the predisposing risk factors. The study was conducted between April and July 2012.
3. SAMPLE SIZE
To calculate the sample size, the World Health Organisation (WHO) recommended methodology for sample size calculation for trachoma surveys [22] was used. Using the following parameters and an assumed prevalence of TF/TI of 20%, with the lowest expected rate being 16%, a significance level of 5%, [confidence of 95%], and a design effect of 4; the number of children aged 1 - 9 years and adults aged 15 years and above required for this study was 1537 children. With an estimated average of 1.9 children per household in Malawi, and an assumed response rate of 90%, a total of 890 households were needed. Accordingly, 36 clusters (villages) per district were selected through probability proportionate to size sampling (PPS), using census data from the 2008 as the sampling frame, in which subsequently 25 households per cluster were sampled, and all children (aged 1 - 9 years) and adults in those households were examined. A list was produced of the villages and their respective population sizes. A column was created with the cumulative population across the enumeration areas and the total population was divided by the number of clusters (36) required to derive the sampling interval. The first cluster was selected by multiplying the sampling interval with a random number between 0 and 1, the resulting number was traced in the cumulative population column, and the first cluster was taken from the corresponding village. Consecutive clusters were identified by adding the sampling interval to the previous number. The second stage of selecting 25 households within the cluster was done randomly using a known list of all households within the cluster (collected by a community health worker), and a computer programme choosing 25 randomly selected households from the list.
4. DATA COLLECTION
For data collection, 6 teams of paired ophthalmic clinical officers underwent a one week certification training session conducted by two experts on grading and trachoma surveys (KK & RB). Prior to the training, a baseline exercise involving a set of 50 digital eyelid photographs derived from studies in Pret studies in Gambia [23] were shown to the trainees with grade explanations and advice on grading. After training, a further set of 50 slides were projected and shown to the trainees, and they were asked to grade as taught during the training. Differences among trainees were examined and all queries were resolved. Trainees then went to the field for a pilot study where they were observed examining and grading children and adults. After the field exercise, trainees were assessed using a final set of 50 digital photographs that were projected and graded independently by the trainer. A final Kappa score was obtained by comparing the grades of each trainee with the trainers (KK & RB). For certification all trainee examiners were required to reach a Kappa value of 0.61 - 0.8.
Data was collected using 3 generic WHO standard forms for trachoma survey (village, household and ocular examination questionnaires). On arrival in the field, the first questionnaire was administered to village headman, the second questionnaire to the head of the household and the third questionnaires to all members of household. The village level questions focused on health facilities within the village, sources of water that the villages used and whether they had committees to oversee water issues in the villages, and schools that could be used as sources of health promotion in the area.
Each eye was examined for presence of trachoma using a 2.5× magnifying loupe (Binomag plastic, Zabby’s, India). Other data collected included the environmental factors such as availability of functional latrines, presence of solid waste or animal pens, distance to water source and hygienic condition of children faces (clean/ unclean). A clean face was defined as absence of obvious ocular and nasal discharge.
Information on the questionnaires was double entered into Epidata software 3.1 at a central location, validated, cleaned and imported into STATA version 10.0 for analysis, which took account of the cluster design. All tests were two-tailed and an alpha level of 0.05 for statistical significance was selected. Bivariate analyses were performed to compare differences using the Chi-square test and univariate logistic regression (taking clustering/study design into account) was used for the risk factor analysis.
5. RESULTS
A total of 884 households out of the planned 890 households were enumerated, giving an overall response rate of 99%. Overall a total of 2781 persons were enumerated and among these, 2749 (98.8%) people participated in ocular examination. Among these 1095 were children aged 1 - 9 years and 1258 were adults aged 15 and above.
Table 1 shows the baseline characteristics (sex and age range) of the study participants and Table 2 shows availability of household characteristics that were studied.
The prevalence rates of Trachoma Follicular (TF) and Trachoma Intense (TI) in children aged 1 - 9 years and Trachoma Scarring (TS), Trachoma Trichiasis (TT) and Cornea Opacity (CO) in adults aged 15 years are shown in Table3 The prevalence of trachomatous inflammation, follicular (TF) among children aged 1 - 9 years was 17.1% (95% CI 14.9 - 19.4).
The trend of prevalence of trachoma follicles (TF) among children aged 1 - 9 years by age and by sex is shown in Figure 2 and Table 4 respectively: The prevalence of TF tended to reach peak between two and four years. Prevalence of TF was significantly higher in girls than boys (19.4% versus 14.6%), P = 0.034, chi square.
Figure 3 shows a child with Active Trachoma evidenced by presence of ocular and nasal discharge, while Figure 4 shows scarring of the upper lid (TS) as a result of Chronic Recurrent Trachoma infections.
The prevalence of trachoma trichiasis (TT) in women aged 15 years and above was 1.3% (CI 0.7 - 2.3), while the prevalence in men was zero. Trachoma follicular (TF) was significantly correlated with absence of a “clean face” (P < 0.001) and absence of a toilet facility (P < 0.001), but not with presence of solid waste, animal pens and distance to water source (Table 5).
Table 1. Baseline characteristics of the study participants.
Table 2. Availability of household characteristics.
*Trachoma Follicles (TF), Trachoma Intense (TI), in children aged 1 - 9 years; Trachoma scarring (TS), Trachoma Trichiasis (TT) and in adults aged 15 years and above. **95% confidence Intervals.
6. DISCUSSION
This study was conducted to determine the prevalence of trachoma infections (TF/TI) among children aged 1 - 9 years, the prevalence of trachoma scaring (TS), trachoma trichiasis (TT) and cornea opacity (CO) among adult men and women aged 15 years and above, and associated risk factors for trachoma in Salima District. This was the second population based study on Trachoma in the mentioned district, even though the methodology of the previous study [21] was not the recommended WHO methodology.
The prevalence of TF tended to reach peak between two and four years.

Figure 2. Trends of prevalence of TF in children of age 1 - 9 years.
Table 4. Prevalence of trachoma follicles (TF) by sex.
There was significantly more TF in girls than boys (P = 0.034, chi square 4.48).

Figure 3. A child with active trachoma evidenced by presence of ocular and nasal discharge.
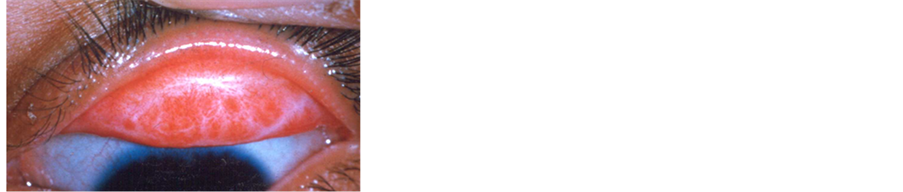
Figure 4. Scarring of the upper lid as a result of chronic recurrent trachoma infections.
The results of this study show that a trachoma follicular infection (TF) is still a disease of public health importance despite a marked reduction in prevalence in
*Correlation is significant at the 0.05 level.
Salima over a 10-year period (from 60% to 17.1%). It is important to note that the differences in methodology can partly explain the difference. The earlier study selected only clusters from highly suspected endemic sub-areas within the district while this study randomly selected clusters within the entire district. It is therefore more likely that the earlier study would have found a much higher prevalence. However, the observations in change of prevalence over time are in agreement with a study by Hoechsmann et al. (14) in another area in Malawi, who reported a decline in Trachoma over a 20 year period in an area where no SAFE had been implemented.
The prevalence of Trachoma Follicular (TF) found among children aged 1 - 9 years in both districts exceeds the WHO guidelines for the intervention with mass antibiotic distribution (TF > 10%), and warrants the SAFE strategy to be undertaken to address the issue of trachoma in Salima District. An interesting but challenging observation is the observed increase in trachoma follicular infections in girls than boys, and the absence of cases of trichiasis (TT) in men, while the prevalence in women aged 15 years and above is above threshold 1.3% (CI 0.7 - 2.3). It is not clear why the TF infections should be more common in girls than boys, however considering that TT in children has been associated with gender with girls having more TT than boys [24], this would imply that there would initially be more TF in girls to cause progression of trachoma. There are possibly two explanations for trichiasis to be more common in women than men [25]; firstly women spend more time with children than men hence get exposed more to infections, and secondly women access eye surgical services less than men [26]. The observation that only a few trichiasis surgeries were being done, did not support the hypothesis that women had more trichiasis because they were accessing services less than men. There are possibly still other reasons that are not clearly understood as to why girls should get more TF than boys and women more TT than men, and reasons may be related and need to be explored through other studies. For the mass drug administration, the findings that there was no trichiasis in men raises questions as to whether the MDA should be limited to women and children or include men as well. There is need for more operational research to justify reasons for giving Azithromycin drugs to men in Salima.
In regards to risk factors, although lack of water and long distance to water source is associated with active trachoma [12], it is not surprising that distance to a water source was not a major factor in the transmission of trachoma in Salima, as the district lies along the lake shore. However despite there being plenty of water, there was still trachoma and this deserves discussion. Trachoma TF is most likely due to poor sanitation (absence of permanent toilets) in Salima. It was observed during the survey (by RB & KK) that the so called traditional pit latrines were deliberately constructed swallow for fear of reaching the water table and these attracted a lot of flies, in addition to attraction from feces of children which were only a few metres within houses. The residents are aware of the need to have good toilets; however the soil and local materials available do not support the construction of recommended toilets. This is a major challenge in implementing SAFE, and unless some innovative ways are achieved, complete SAFE cannot be fully implemented, and trachoma will remain a problem in this area. It should be noted that with care, certain activities of the SAFE strategy (antibiotic distribution) may be successfully integrated with control of other diseases [27]. One major limitation of the study is that diagnosis of active Trachoma infections (TF/TI) is based on the clinical diagnosis, but we do not know how well a positive diagnosis of TF/TI in this setting this correlates to serological presence of chlamydia. In future it would be advisable to test for presence of infections and correlate to the clinical findings.
7. CONCLUSION
In conclusion, the prevalence of trachoma found in Salima District confirms that trachoma is still a disease of public health importance, but the distribution is different as earlier seen in other parts of Malawi [19], with TT present only in women and TF more in girls than boys. Implementation of the full SAFE strategy is warranted, even though paradoxically the presence of plenty of water along the lakeshore poses different challenges in sanitation.
There are still many districts that are suspected to be endemic for trachoma in Malawi and it is important that funds can be identified and mapping can be completed as soon as possible, to enable the SAFE strategy to be implemented in confirmed endemic districts well in time in order to achieve the goal of eliminating trachoma by the year 2020.
ACKNOWLEDGEMENTS
We would like to acknowledge the contributions by the following individuals and organisations during the survey period: Misheck Nyirenda, for the overall logistical support provided during the survey, the Ministry of Health task force for the Trachoma survey, World Health Organisation for financial support for the survey, District Health management teams for Salima and all the data collection team members and the community health workers that took part in the survey.
REFERENCES
- Bailey, R. (2007) rRNA-based tests for chlamydial infection in trachoma. British Journal of Ophthalmology, 91, 271. http://dx.doi.org/10.1136/bjo.2006.105270
- Wang, S.P. and Grayston, J.T. (1991) Serotyping of Chlamydia trachomatis by indirect fluorescent-antibody staining of inclusions in cell culture with monoclonal antibodies. Journal of Clinical Microbiology, 29, 1295-1298.
- Mabey, D.C., Forsey, T. and Treharne, J.D. (1987) Serotypes of Chlamydia trachomatis in The Gambia. Lancet, 2, 452. http://dx.doi.org/10.1016/S0140-6736(87)90984-6
- Bowman, R.J., Jatta, B., Cham, B., Bailey, R.L., Faal, H., et al. (2001) Natural history of trachomatous scarring in The Gambia: Results of a 12-year longitudinal follow-up. Ophthalmology, 108, 2219-2224. http://dx.doi.org/10.1016/S0161-6420(01)00645-5
- WHO (2004) WHO simplified trachoma grading system. Community Eye Health, 17, 68.
- Thylefors, B., Dawson C.R., Jones, B.R., West, S.K. and Taylor, H.R. (1987) A simple system for the assessment of trachoma and its complications. Bulletin of World Health Organisation, 65, 477-483.
- Mariotti, S.P., Pascolini, D. and Rose-Nussbaumer, J. (2009) Trachoma: Global magnitude of a preventable cause of blindness. British Journal of Ophthalmology, 93, 563-568. http://dx.doi.org/10.1136/bjo.2008.148494
- Burton, M.J. (2007) Trachoma: An overview. British Medical Bulletin, 84, 99-116. http://dx.doi.org/10.1093/bmb/ldm034
- Hu, V.H., Harding-Esch, E.M., Burton, M.J., Bailey, R.L., Kadimpeul, J., et al. (2010) Epidemiology and control of trachoma: Systematic review. Tropical Medicine & International Health, 15, 673-691. http://dx.doi.org/10.1111/j.1365-3156.2010.02521.x
- Resnikoff, S., Pascolini, D., Mariotti, S.P. and Pokharel, G.P. (2008) Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bulletin of the World Health Organization, 86, 63-70. http://dx.doi.org/10.2471/BLT.07.041210
- Burton, M.J. and Mabey, D.C. (2009) The global burden of trachoma: A review. PLOS Neglected Tropical Diseases, 3, e460. http://dx.doi.org/10.1371/journal.pntd.0000460
- West, S.K., Munoz, B., Lynch, M., Kayongoya, A., Mmbaga, B.B., et al. (1996) Risk factors for constant, severe trachoma among preschool children in Kongwa, Tanzania. American Journal of Epidemiology, 143, 73-78. http://dx.doi.org/10.1093/oxfordjournals.aje.a008659
- Reddy, M., Gill, S.S., Kalkar, S.R., Wu, W., Anderson, P.J., et al. (2007) Oral drug therapy for multiple neglected tropical diseases: A systematic review. JAMA, 298, 1911- 1924. http://dx.doi.org/10.1001/jama.298.16.1911
- Utzinger, J. and de Savigny, D. (2006) Control of neglected tropical diseases: Integrated chemotherapy and beyond. PLOS Medicine, 3, e112. http://dx.doi.org/10.1371/journal.pmed.0030112
- Molyneux, D.H., Hotez, P.J. and Fenwick, A. (2005) “Rapid-impact interventions”: How a policy of integrated control for Africa’s neglected tropical diseases could benefit the poor. PLOS Medicine, 2, e336. http://dx.doi.org/10.1371/journal.pmed.0020336
- Mariotti, S.P., Pararajasegaram, R. and Resnikoff, S (2003) Trachoma: Looking forward to Global Elimination of Trachoma by 2020 (GET 2020). American Journal of Tropical Medicine and Hygiene, 69, 33-35.
- Tielsch, J.M., West Jr., K.P., Katz, J., Keyvan-Larijani, E., Tizazu, T., et al. (1988) The epidemiology of trachoma in southern Malawi. American Journal of Tropical Medicine and Hygiene, 38, 393-399.
- Hoechsmann, A., Metcalfe, N., Kanjaloti, S., Godia, H., Mtambo, O., et al. (2001) Reduction of trachoma in the absence of antibiotic treatment: Evidence from a population-based survey in Malawi. Ophthalmic Epidemiology, 8, 145-153. http://dx.doi.org/10.1076/opep.8.2.145.4169
- Kalua, K., Chirwa, T., Kalilani, L., Abbenyi, S., Mukaka, M., et al. (2010) Prevalence and risk factors for trachoma in central and southern Malawi. PLoS One, 5, e9067. http://dx.doi.org/10.1371/journal.pone.0009067
- Polack, S., Brooker, S., Kuper, H., Mariotti, S., Mabey, D., et al. (2005) Mapping the global distribution of trachoma. Bulletin of the World Health Organization, 83, 913-919.
- Myatt, M., Limburg, H., Minassian, D. and Katyola, D. (2003) Field trial of applicability of lot quality assurance sampling survey method for rapid assessment of prevalence of active trachoma. Bulletin of the World Health Organization, 81, 877-885.
- WHO (2006) Trachoma control: A guide for programme managers. Published jointly by the World Health Organization, the London School of Hygiene and Tropical Medicine, and the International Trachoma Initiative. Geneva. WHO Library Cataloguing-in-Publication Data.
- Stare, D., Harding-Esch, E., Munoz, B., Bailey, R., Mabey, D., et al. (2011) Design and baseline data of a randomized trial to evaluate coverage and frequency of mass treatment with azithromycin: The partnership for rapid elimination of trachoma (PRET) in Tanzania and The Gambia. Ophthalmic Epidemiology, 18, 20-29. http://dx.doi.org/10.3109/09286586.2010.545500
- Ngondi, J., Reacher, M.H., Matthews, F.E., Brayne, C., Gatpan, G., et al. (2009) Risk factors for trachomatous trichiasis in children: Cross-sectional household surveys in Southern Sudan. Transactions of the Royal Society of Tropical Medicine and Hygiene, 103, 305-314. http://dx.doi.org/10.1016/j.trstmh.2008.08.024
- Courtright, P. and West, S.K. (2004) Contribution of sexlinked biology and gender roles to disparities with trachoma. Emerging Infectious Diseases, 10, 2012-2016. http://dx.doi.org/10.3201/eid1011.040353
- Lewallen, S., Mousa, A., Bassett, K. and Courtright, P. (2009) Cataract surgical coverage remains lower in women. British Journal of Ophthalmology, 93, 295-298. http://dx.doi.org/10.1136/bjo.2008.140301
- Emerson, P.M., Ngondi, J., Biru, E., Graves, P.M., Ejigsemahu, Y., et al. (2008) Integrating an NTD with one of “The big three”: Combined malaria and trachoma survey in Amhara Region of Ethiopia. PLOS Neglected Tropical Diseases, 2, e197. http://dx.doi.org/10.1371/journal.pntd.0000197


