Health
Vol.5 No.1(2013), Article ID:26522,8 pages DOI:10.4236/health.2013.51009
Modulation of peroxisomes abundance by argan oil and lipopolysaccharides in acyl-CoA oxidase 1-deficient fibroblasts
![]()
1Université de Bourgogne, Laboratoire BioPeroxIL EA7270, Dijon, France; *Corresponding Author: malki@u-bourgogne.fr
2Laboratoir de Biochimie et Neurosciences, Faculté des Sciences et Techniques, Université Hassan I, Settat, Morocco
3Université de Bourgogne, Centre de Recherche INSERM, Dijon, France
4Laboratoire de Recherche sur les Lipoprotéines et l’Athérosclérose, Faculté des Sciences Ben M’sik, Université Hassan IIMohammedia, Casablanca, Morocco
5INSERM and HMNO, CBP, CHRU Lille, Lille, France
Received 25 October 2012; revised 30 November 2012; accepted 8 December 2012
Keywords: Acyl-CoA Oxidase 1; Argan Oil; LPS; PGC-1α; Peroxisome Proliferation; P-NALD; PPARα
ABSTRACT
Pseudo-neonatal adrenoleukodystrophy (P-NALD) is a neurodegenerative disorder caused by acylCoA oxidase 1 (ACOX1) deficiency with subsequent impairment of peroxisomal fatty acid β- oxidation, accumulation of very long chain fatty acids (VLCFAs) and strong reduction in peroxisome abundance. Increase in peroxisome number has been previously suggested to improve peroxisomal disorders, and in this perspective, the present work was aimed at exploring whether modulation of peroxisomes abundance could be achieved in P-NALD fibroblasts. Here we showed that treatment with the natural Argan oil induced peroxisome proliferation in P-NALD fibroblasts. This induction was independent on activations of both nuclear receptor PPARα and its coactivator PGC-1α. Lipopolysaccharides (LPS) treatment, which caused inflammation, induced also a peroxisome proliferation that, in contrast, was dependent on activations of PPARα and PGC-1α. By its ability to induce peroxisome proliferation, Argan oil is suggested to be of potential therapeutic use in patients with P-NALD.
1. INTRODUCTION
Alteration of peroxisome biogenesis in the neurodegenerative pseudo-neonatal adrenoleukodystrophy (P-NALD;
OMIM 264470) is correlated to the underlying mutation in acyl-CoA oxidase 1 (ACOX1) gene, which encodes the first and rate-limiting enzyme in peroxisomal β-oxidation pathway [1,2]. In this single-enzyme deficiency, the metabolic defect impairs peroxisomal acyl-CoAs β- oxidation and, because causing accumulation of very long chain fatty acids (VLCFAs), evokes biochemically the Zellweger syndrome [1-3]. However, in contrast to P-NALD, Zellweger syndrome underlying defect addresses one of the many peroxin genes required for proper peroxisome biogenesis and maintenance [2]. Peroxinencoding gene defect leads to deficient import of peroxisomal matrix proteins, which precedes the defect in peroxisomal fatty acid β-oxidation and accumulation of VLCFAs [2]. In P-NALD disorder, peroxisome biogenesis appears to be secondarily altered, as reported in fibroblasts and hepatocytes from patients, and this alteration consists of a reduced number of peroxisomes per cell and an increase in peroxisome size [1,2].
Singularly, peroxisome abundance is the result of a cellular balance between peroxisome biogenesis and peroxisome proliferation. Accordingly, substantial data have been collected on the plasticity of peroxisomes in adapting their morphology, enzyme content, and abundance to environmental stresses [2,4,5]. Peroxisomes biogenesis involves sequentially budding of peroxisomal membranes from endoplasmic reticulum components, import of peroxisomal matrix enzymes and fission of the organelles [6, 7]. As for mitochondria, the biogenesis of peroxisomes relies heavily on transcriptional coactivator Peroxisome Proliferator-Activated Receptor-Coactivator-1α (PGC-1α) which controls the expression of several key genes involved in peroxisomal biogenesis (i.e. peroxines) and metabolism [8]. In contrast to peroxisome proliferationwhich is under the strict control of nuclear receptor PPARα [9,10], forced expression of PGC-1α by adenofection into human and rodent cells is sufficient to drive peroxisomal remodeling and biogenesis in a PPARα-independent manner [8].
Alongside, peroxisome proliferation and induction of peroxisomal fatty acid β-oxidation may be induced by structurally diverse synthetic ligands referred to as peroxisome proliferators and by several saturated fatty acids and their polyunsaturated forms [9-13]. Ligand-dependent activation of nuclear receptor PPARα leads to its heterodimerization with RXRα. This PPARα/RXRα complex binds to PPARα-response elements (PPRE) of target genes which encompass diverse genes coding for mitochondrial and peroxisomal enzymes involved in fatty acid β-oxidation pathways [4,5,10]. Mice lacking Acox1 exhibit a severe liver steatosis with achronic hepatic endoplasmic reticulum stress and, importantly, these mice present with a sustained activation of PPARα [14,15]. Interestingly also, only regenerated hepatocytes lacking steatosis show a massive peroxisome proliferation, which is dependent on the activation of PPARα [14,15]. The particular phenotype of this Acox1 null mouse model, i.e. sustained PPARα-driven peroxisome proliferation, can be reversed by restoring the expression of the human ACOX1b isoform [16]. Thus, peroxisome abundance and PPARα activation seem to be correlated to the level of ACOX1 activity.
In a previous work, we have shown that P-NALD patient-derived deficient fibroblasts exhibited a strongly reduced number and enlarged size of peroxisomes [3]. Although no apparent genotype-phenotype correlation has been established in P-NALD [17], residual VLCFA β- oxidation, due to a branched chain-ACOX2 non-specific activity, reached an average of 25% in P-NALD fibroblasts [3,17]. Earlier study had suggested that increase in peroxisome number might have a favorable effect on peroxisomal biogenesis disorders [18]. Regarding the key role of peroxisome biogenesis in the development of peroxisomal disorders, this study was designed to determine whether modulation of peroxisomes abundance could be achieved in P-NALD fibroblasts. For this purpose, we tested the natural Argan oil, attending the role of unsaturated fatty acids in the activation of PPARα. Indeed, Argan oil is a rich source in unsaturated fatty acids, when compared to olive oil, with a higher unsaturation index 120.4 versus 108.3 for olive oil (supplementary Table S1 and Figure S1). On the other hand, we challenged P-NALD fibroblasts with lipopolysaccharides (LPS), which have been reported to be modulators of peroxisome proliferation [19]. In the present work, the abilities of these approaches (Argan oil and LPS) to modulate or not the peroxisomal population of P-NALD fibroblasts have been studied.
2. MATERIAL AND METHODS:
2.1. Cell Culture and Argan Oil Treatment
Skin fibroblasts were cultured as described elsewhere [17] and handled according to national and institutional guidelines. Skin fibroblasts were maintained in EMEM medium (Lonza) containing fetal bovine serum (10%), sodium pyruvate (1 mM) with penicillin and streptomycin (1%) at 37˚C with 5% CO2. The virgin Argan oil used in this work was obtained from the Aklim area in the northeast of Morocco. For fibroblasts treatment, Argan oil was solubilized in ethanol and Lipopolysaccharides (LPS Sigmaaldrich; 1 mg/mL) were dissolved in phosphate buffer saline. Final concentration of ethanol (vehicle) in the culture medium was 0.2%. For fibroblasts treatment, the final concentration of Argan oil and LPS whereas indicated in figure legends.
2.2. Immunostaining and Morphometry
Fluorescence microscopy was achieved as previously described [20]. To perform immunofluorescence staining, cells were seeded at 2 × 105 cells/cm2 on 12 mm glass coverslips, which have been introduced into the wells of micro-plates containing 1 ml of medium culture. After 48 h of culture, cells were fixed with 2% paraformaldehyde for 5 min at room temperature, washed three times with PBS, pre-incubated with FACS permeabilizing solution (BD-Biosciences) for 5 min at room temperature, and incubated with blocking buffer (PBS, 0.05% saponin (Sigma-Aldrich), 10% goat serum (PAN™ Biotech GmbH) for 20 min at room temperature. Following washing in PBS, cells were incubated at room temperature for onehour with 1/100 dilution of L-PBE primary antibodies (rabbit polyclonal antibodies directed against rat L-PBE) in PBS containing 10% goat serum, washed twice in PBS, and then incubated for 30 min with a 488-Alexa goat anti-rabbit at 1/300. After extensive washings with PBS, slides were mounted, and digital images acquisitions were collected with an SP2 AOBS confocal laser microscope (Leica, Wetzlar, Germany) equipped for epifluorescence microscopy. The number of peroxisomes were evaluated from confocal microscopy image stacks with Velocity 3D Image Analysis® (PerkinElmer) and Image J (National Institute of Mental Health, Bethesda, Maryland, USA) softwares.
2.3. Quantitative PCR Analysis
Cells were harvested with 0.25% trypsin/EDTA and washed twice with PBS. Total RNA from fibroblasts was extracted using the RNeasy Mini kit (Qiagen) following the manufacturer’s instructions. cDNA was generated by reverse transcription using Moloney Murine Leukemia Virus Reverse Transcriptase (Promega) according to the manufacturer’s protocol and analyzed by quantitative PCR using the GoTaq® qPCR Master Mix (Promega), and a Step One Plus Real-Time PCR System (Applied Biosystem). The Beacon Designer Software (Bio-Rad) was used to determine primer sequences specific of PPARα: (forward 5’AGAGTGGGCTTTCCGTGTC3’ and reverse 5’GGACTCAACAGTTTGTGGCA-3’), PGC-1α (forward 5’AATCCGTCTTCATCCACAGG3’ and reverse 5’GGTGCAGTGACCAATCAGAA3’), L-PBE (forward 5’AAGAAGGACTACAGAAAGCTGTA3’ and reverse 5’CCCAGTGTAAGGCCAAATGT3’), PEX11α (forward 5’GGTAATGAAGCTCAAGAAACTGGAG3’ and reverse 5’TGCTCTGCTCAGTTGCCTGT3’), and ribosomal 36B4 (forward 5’ATCTGCTTGGAGCCCACAT3’ and reverse 5’GCGACCTGGAAGTCCAACTA3’). Subsequent PCR reactions were carried out in duplicate in a final volume of 12.5 µL containing 6.25 µL of MESA Green qPCR Mastermix (Eurogentec), 2.5 µL of cDNA and forward and reverse primers at 300 nM. The PCR enzyme (Taq DNA polymerase) was heat-activated at 95˚C for 10 min, and the DNA was amplified for 40 cycles at 95˚C for 15 s, 60˚C for 30 s, and 72˚C for 30 s, followed by a melting curve analysis to control the absence of nonspecific products. For each transcript, the amplification efficiency was determined by the slope of the standard curve generated from two fold serial dilutions of cDNA. Gene expression was quantified using cycle to threshold (Ct) values and normalized by the ribosomal 36B4 reference gene. To this end, the quantitative expression of PPARα and PGC-1α was determined according to 2–∆Ct with ∆Ct = (Ct of the gene studied) – (Ct of the b-Actin gene).
2.4. Statistical Analysis
Statistical analyses to compare two experimental groups were performed with, an unpaired, two-tailed, Student-t test (Excel software) for calculating the probability values and data were considered statistically different at a P-value of 0.05 or less.
3. RESULTS
3.1. Argan Oil and LPS Increase Peroxisome Population in P-NALD-Fibroblasts
Peroxisome abundance was assessed in P-NALD fibroblasts by immunofluorescence staining. Typical punctuated fluorescence pattern of peroxisomes was shown in control fibroblasts using the antibody against L-peroxisomal bifunctional enzyme (L-PBE) (Figures 1(a) and (b)). When compared to control fibroblasts, P-NALD cells exhibited a few number of large peroxisomes per cell (Figures 1(b) and (c)) and the peroxisome density (peroxisome/µm2) was decreased by 60% (Table 1). To modulate peroxisome abundance in P-NALD fibroblasts, we
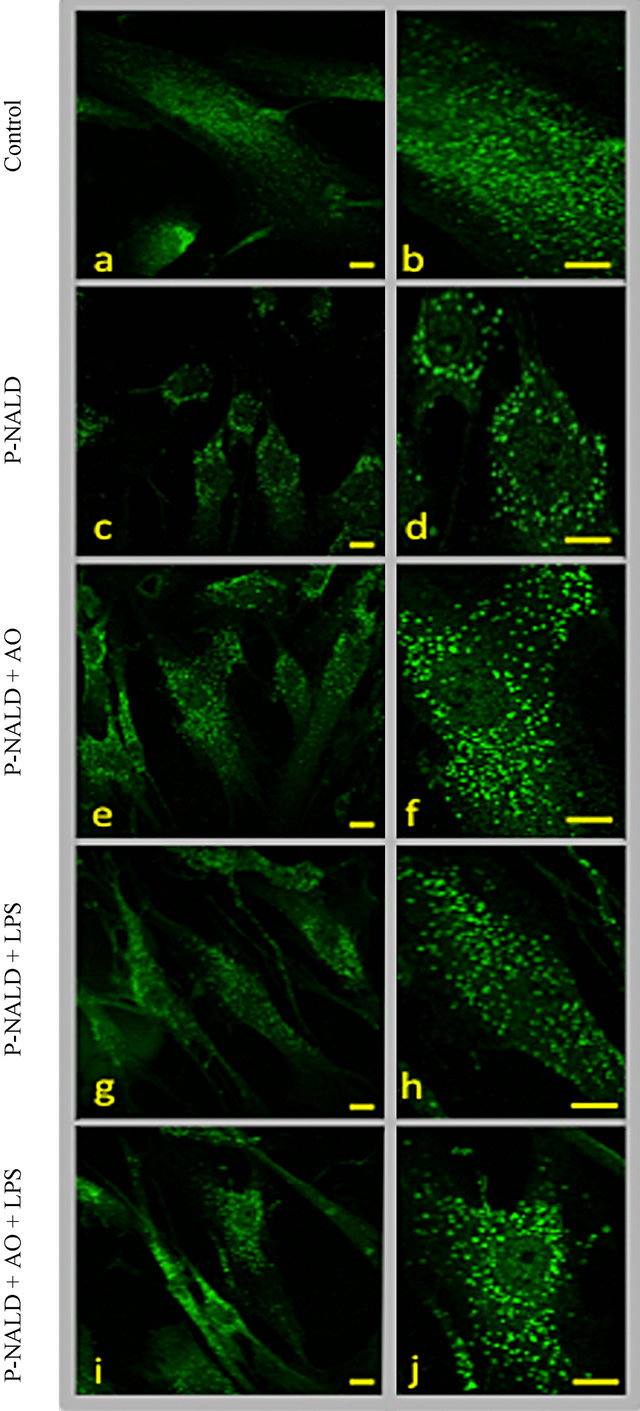
Figure 1. Argan oil and LPS treatments induce peroxisome proliferation in P-NALD fibroblasts. Typical punctuated fluorescent pattern of peroxisomes in control fibroblasts are illustrated in (a) and (b) panels. In comparison, P-NALD fibroblasts show a reduced number of peroxisomes which present with an enlarged size ((c) and (d) panels). P-NALD fibroblasts were treated with Argan oil at 56 µg/mL (e), (f), LPS at 0.2 µg/mL (g), (h) or concomitantly by Argan oil at 56 ng/mL and LPS at 0.2 µg/mL (i) (j) during 48 h. Fibroblasts were seeded at 2 × 105 cells/cm2 on 12-mm glass cover slips and immunofluorescence staining was performed as described by Baarine et al. [20]. L-PBE Immunostaining was performed in order to visualize peroxisomes using anti-L-PBE antibodies (1/100). Microscope images magnifications were ×100 and bar = 10 µm.
Table 1. Effect of Argan oil and LPS on peroxisome density in P-NALD fibroblasts.
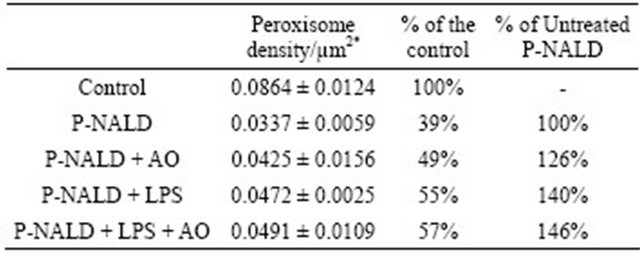
*Values are mean ± SD.
used the natural Argan oil, rich in unsaturated fatty acids, as an activator of PPARα. 48 hours Argan oil exposure showed an increase in peroxisome population in these P-NALD fibroblasts (Figures 1(d) and (e)) and peroxisome density was increased by 26% compared to untreated P-NALD fibroblasts (Table 1). On the other hand, LPS has been shown to modulate peroxisome β-oxidation in fibroblasts. So we treated P-NALD fibroblasts with LPS for 48 hours. Figures 1(f) and (g) shows that LPS treatment provoked also an increase in peroxisomes population of about 40% in term of density (Table 1). Intriguingly, co-treatment with both Argan oil and LPS has no additional effect on the increased peroxisomes population obtained by each treatment alone (Figures 1(i), (j) and Table 1). These results indicate that different exogenous compounds, such as Argan oil or LPS, may modulate peroxisome abundance in a non-additive way.
3.2. Roles of PPARα and PGC-1α in the Increase of Peroxisome Population in P-NALD Fibroblasts by Argan Oil and LPS
Due to the strong implication of PPARα and PGC-1α in peroxisome proliferation and in peroxisome biogenesis respectively [4,5,8], we evaluated first the levels of their transcripts in control and P-NALD fibroblasts by real-time PCR. Figures 1(a) and (b) showed that the expression levels of PPARα mRNA were significantly reduced in P-NALD fibroblasts by 20% compared to the control. The PGC-1α mRNAs level was strongly induced by 4 fold in these P-NALD/ACOX1-deficient cells. As we showed before [3], higher gene expression of IL-6 cytokine (10 folds) underlined the strong inflammatory status in the ACOX1-deficient P-NALD fibroblasts (Figure 1(e)).
Treatment with Argan oil revealed only a slight decrease of PPARα mRNA level in control fibroblasts, while this treatment has no effect on the levels of PPARα and PGC-1α gene expressions in P-NALD fibroblasts (Figures 1(a) and (b)). To assess PPARα and PGC-1α coactivation, we tested the expression of their respective specific target genes L-PBE and PEX11α [4,5,8]. L-PBE encodes the peroxisomal bifunctional enzyme, a known marker of PPARα-dependent peroxisome proliferation, and PEX11α encodes a peroxin involved in PGC-1α-dependent peroxisome biogenesis. Downregulation of LPBE and PEX11α mRNAs, in P-NALD versus control fibroblasts, accounted for the reduced peroxisome biogenesis in P-NALD fibroblasts (Figures 2(c) and (d)). However, treatment with Argan oil has no effect on the expression or activation of PPARα and PGC-1α mRNAs in P-NALD fibroblasts. Surprisingly, in Figures 1(e) and (f), we showed that Argan oil treatment increased peroxisome population in P-NALD cells (Figures 1(b) and (d)). Thus peroxisome abundance can be increased in P-NALD fibroblasts independently of PPARα and PGC-1α activations.
As we showed recently, P-NALD fibroblasts present a high inflammatory status [3] and this was confirmed here in Figure 1 (C, insert), showing that P-NALD/ACOX1 deficient fibroblasts exhibit a 10-fold induction of interleukine-6 (IL-6) mRNAs level due to the activation of IL-1 pathway [3]. Whether PPARα and PGC-1α mRNA expressions were regulated in this inflammatory context was unknown. So we treated both control and P-NALD fibroblasts with LPS in the absence or in the presence of Argan oil. Figure 2 shows that LPS had no effect on PPARα and PGC-1α mRNA levels in control fibroblasts, while LPS alone induced significantly PPARα (1.5-fold) and PGC-1α (1.5-fold) mRNAs levels in P-NALD fibroblasts when compared to untreated cells. The incidence of the LPS-induced PPARα and PGC-1α mRNAs was shown by the induction of their target genes, L-PBE (1.5- fold) and PEX11α (2.2-fold). Surprisingly, co-treatment of P-NALD fibroblasts with LPS and Argan oil had a higher effect on the expression of both PPARα and PGC-1α mRNA, showing, in comparison to untreated cells, an increase in the mRNA levels by 1.75-fold for PPARα and 3.75-fold for PGC-1α respectively (Figures 2(a) and (b)). These increases impacted also the expressions of L-PBE (1.3 folds) and PEX11α (2 folds) in LPStreated P-NALD fibroblasts (Figures 2(c) and (d)). LPS treatment exacerbated the expression of IL-6 mRNAs (30-fold), revealing aggravated inflammatory status in P-NALD fibroblasts (Figure 2(e)). However, in control fibroblasts, even in the inflammatory conditions, raised by LPS treatment as shown by a 20-fold increase of IL-6 mRNA (Figure 2(e)), co-treatment with LPS and Argan oil had no effect on PPARα and PGC-1α mRNA levels. Thus, changes in the expression of PPARα and PGC-1α mRNAs seem to be not correlated to the level of inflammation in the control fibroblasts. By contrast, in the P-NALD fibroblasts, aggravation of the inflammatory context is concomitant to a net induction of PPARα and PGC-1α mRNAs.

Figure 2. Differential effect of Argan oil on the expressions of PPARα and PGC-1α in P-NALD fibroblasts. Real-time PCR was used to quantify the mRNA levels of PPARα (a), PGC-1α (b), L-PBE (c), PEX11α (d) and IL-6 (e) genes after a 48 h exposure of P-NALD fibroblasts to argan oil. Fibroblasts were seeded at 5 × 105 cells per 10 cm plate in duplicate. Control fibroblasts were treated with vehicle, 0.2% ethanol (Ctrl), Argan oil (AO) at 56 µg/mL, LPS at 0.2 µg/mL (LPS), or Argan oil at 56 µg/mL plus LPS at 0.2 µg/mL (AO + LPS). All real-time PCR reactions were performed in duplicate. All values are means ± SEM from two experiments and are normalized to control. Statistical significance was determined by unpaired two-tailed Student’s test. Symbols (*) correspond to a statistical significance, (p < 0.01 for ***, p < 0.02 for **, p < 0.05 for *), compared with the control (*).
4. DISCUSSION
P-NALD disorder is characterized by a defect in peroxisomal fatty acid β-oxidation, due to ACOX1 deficiency, and subsequent accumulation of VLCFAs with a strong reduction in peroxisome abundance [1,2]. This biochemical status is shared in common with other peroxisomal biogenesis disorders, in which, however, defect in peroxisome biogenesis is linked to mutation in a peroxin involved in the import machinery [2]. Thus, increase in peroxisome number has been stipulated to have favorable effect on peroxisomal biogenesis disorders [18]. Using immunofluorescence microscopy, we showed here an increase in peroxisome population in P-NALD fibroblasts by Argan oil, which is rich in polyunsaturated fatty acids particularly oleic (45%) and linoleic (34%) acids. The latter, is a precursor of arachidonic acid. Accordingly, it has been shown that polyunsaturated fatty acids, in particular arachidonic acid, induce the formation of tubular peroxisomes in HepG2 cells [21]. Thus, the availability of linoleic acid in Argan oil-treated P-NALD fibroblasts probably increased the number of peroxisomes with a rounded shape and a normal size comparable to control fibroblasts. This is in the opposite of what we observed in untreated P-NALD fibroblasts, which have reduced number of peroxisomes with enlarged size. The shape of these proliferating peroxisomes, under Argan oil treatment, resembles more to the one obtained under 4-phenyl butyrate treatment in fibroblasts with peroxisomal biogenesis disorders [18,22].
In our hand, the moderate peroxisome proliferation induced by Argan oil seems to be PPARα-independent. This is underlined by the absence of induction of L-PBE gene expression, a PPARα-target gene. The existence of PPARα-independent peroxisome proliferation has been described in PPARα null mice treated with fenofibrate or 4-phenyl butyrate, the well-known PPARα ligands [23, 24]. Furthermore, the PPARα-independent induction of peroxisomal protein encoding gene in mice seems to be tissue-specific as this independency phenomenon is only partial in hepatocytes and total in fibroblasts [23]. Thus, the implication of other compounds present in Argan oil, such as sterols and/or polyphenols, cannot be excluded. Hence, phytol-enriched diet has been shown to induce several peroxisomal proteins in PPARα null mice [25]. In addition, the coactivator PGC-1α, as the mRNA expression of its target gene PEX11α is not augmented, does not promote this peroxisome proliferation. Nevertheless, induction of peroxisome biogenesis relies on the induction of several peroxin genes in a PGC-1α-dependent manner and this can be achieved independently of PPARα via a yet uncharacterized transcription factor [8]. Accordingly, evidence of the participation of other PPAR isotypes in peroxisome proliferation induction has been reported elsewhere [26]. However, absence of the induction of PPARα-target gene, L-PBE, which is highly correlatable with peroxisome proliferation [27], attests for the existence of other pathway activated by Argan oil.
Earlier studies on the effect of LPS on peroxisome functions have shown that these endotoxins induced peroxisomal proteins, affected peroxisomal membrane composition and reduced the yield of peroxisome fraction in rat liver [28,29]. In rat C6 glial cells, LPS strongly diminished the activity of ACOX1 and the oxidation of VLCFAs [30]. Nonetheless, to our knowledge, the effect of LPS in ACOX1 deficiency context has not been studied yet. Here we showed that LPS induced peroxisomes population in P-NALD fibroblasts. This induction is associated with the activation of both PPARα and PGC-1α only in P-NALD fibroblasts, as shown by the increase of their target genes (i.e. L-PBE and PEX11α). Thus, the action of LPS seems to be correlated to the ACOX1 activity level and/or, at least partially, to the aggravated inflammatory context in P-NALD-treated cells. However, the induced expression of PPARα and PGC-1α by the co-treatment with both LPS and Argan oil is not associated to an additional activation of their target genes or induction of peroxisome proliferation. By contrast, LPS were associated, in cytokines-dependent manner, to the induction of oxidative stress, inhibition of PPARα activiity, and peroxisomal dysfunction in developing rat oligodendrocytes [31]. Apparent dissimilarity with our results may be linked to the peroxisomal dysfunction, as in P-NALD fibroblasts the chronic cytokine induction is mainly correlated to ACOX1 deficiency without any LPS treatment [3]. Thus, the metabolic context may account for the differential cell response. Accordingly, recent data reported that LPS molecules triggered the cellular energy metabolic reprogramming through depression of PGC-1α activity [32]. In addition, numerous studies have reported such differential cell adaptation in term of peroxisome morphology, abundance and enzymes content; and in term of PPARα activation as well [3,4,8,9,16,23, 28,31].
In conclusion, the present study showed that Argan oil is able to stimulate peroxisome proliferation in P-NALD fibroblasts in the absence of PPARα and PGC-1α activetion, while LPS, which aggravate the inflammatory status in P-NALD cells, induced peroxisome proliferation and activation of PPARα and PGC-1α.
5. ACKNOWLEDGEMENTS
Financial support for this study was received from grants from the Action Intégrée of the Comité Mixte Inter-universitaire Franco-Marocain (CMIFM, AIMA/10/238, EGIDE) from the PHC volubulis program, Ministère des Affaires Etrangères, the Conseil Régional de Bourgogne, the Ministère de l’enseignement et de la Recherche.
![]()
![]()
REFERENCES
- Poll-The, B.T., et al. (1988) A new peroxisomal disorder with enlarged peroxisomes and a specific deficiency of acyl-CoA oxidase (pseudo-neonatal adrenoleukodystrophy). American Journal of Human Genetics, 42, 422-434.
- Wanders, R.J. and Waterham, H.R. (2006) Peroxisomal disorders: The single peroxisomal enzyme deficiencies. Biochimica et Biophysica Acta, 1763, 1707-1720. doi:10.1016/j.bbamcr.2006.08.010
- El Hajj, H.I. et al. (2012) The inflammatory response in acyl-CoA oxidase 1 deficiency (pseudoneonatal adrenoleukodystrophy). Endocrinology, 153, 2568-2575. doi:10.1210/en.2012-1137
- Cherkaoui-Malki, M., et al. (2012) Hepatic steatosis and peroxisomal fatty acid beta-oxidation. Current Drug Metabolism, 13, 1412-1421. doi:10.2174/138920012803762765
- Reddy, J.K. (2004) Peroxisome proliferators and peroxisome proliferator-activated receptor alpha: Biotic and xenobiotic sensing. American Journal of Pathology, 164, 2305-2321. doi:10.1073/pnas.1009176107
- Delille, H.K., Alves, R. and Schrader, M. (2009) Biogenesis of peroxisomes and mitochondria: Linked by division. Histochemistry and Cell Biology, 131, 441-446.
- Girzalsky, W., Saffian, D. and Erdmann, R. (2010) Peroxisomal protein translocation. Biochimica et Biophysica Acta, 1803, 724-731.
- Bagattin, A., Hugendubler, L. and Mueller, E. (2010) Transcriptional coactivator PGC-1α promotes peroxisomal remodeling and biogenesis. Proceedings of the National Academy of Sciences, 107, 20376-20381.
- Cherkaoui-Malki, M., et al. (2001) Identification of novel peroxisome proliferator-activated receptor alpha (PPARα) target genes in mouse liver using cDNA microarray analysis. Gene Expression, 9, 291-304.
- Vluggens, A. and Reddy, J.K. (2012) Nuclear receptors and transcription factors in the development of fatty liver disease. Current Drug Metabolism, 13, 1422-1435. doi:10.2174/138920012803762710
- Forman, B.M., Chen, J. and Evans, R.M. (1997) Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proceedings of the National Academy of Sciences, 94, 4312-4317. doi:10.1073/pnas.94.9.4312
- Gottlicher, M., et al. (1992) Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proceedings of the National Academy of Sciences, 89, 4653-4657. doi:10.1073/pnas.89.10.4653
- Issemann, I. and Green, S. (1990) Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature, 347, 645-650. doi:10.1038/347645a0
- Fan, C.Y., et al. (1998) Steatohepatitis, spontaneous peroxisome proliferation and liver tumors in mice lacking peroxisomal fatty acyl-CoA oxidase. Implications for peroxisome proliferator-activated receptor alpha natural ligand metabolism. Journal of Biological Chemistry, 273, 15639-15645. doi:10.1074/jbc.273.25.15639
- Huang, J., et al. (2011) Progressive endoplasmic reticulum stress contributes to hepatocarcinogenesis in fatty acyl-CoA oxidase 1-deficient mice. The American Journal of Pathology, 179, 703-713. doi:10.1016/j.ajpath.2011.04.030
- Vluggens, A., et al. (2010) Reversal of mouse Acyl-CoA oxidase 1 (ACOX1) null phenotype by human ACOX1b isoform [corrected]. Laboratory Investigation: A Journal of Technical Methods and Pathology, 90, 696-708.
- Ferdinandusse, S., et al. (2007) Clinical, biochemical, and mutational spectrum of peroxisomal acyl-coenzyme A oxidase deficiency. Human Mutation, 28, 904-912. doi:10.1002/humu.20535
- Wei, H., et al. (2000) Pharmacological induction of peroxisomes in peroxisome biogenesis disorders. Annals of neurology, 47, 286-296. doi:10.1002/1531-8249(200003)47:3<286::AID-ANA3>3.0.CO;2-B
- Paintlia, M.K., et al. (2008) Lipopolysaccharide-induced peroxisomal dysfunction exacerbates cerebral white matter injury: Attenuation by N-acetyl cysteine. Experimental Neurology, 210, 560-576. doi:10.1016/j.expneurol.2007.12.011
- Baarine, M., et al. (2009) Peroxisomal and mitochondrial status of two murine oligodendrocytic cell lines (158N, 158JP): Potential models for the study of peroxisomal disorders associated with dysmyelination processes. Journal of Neurochemistry, 111, 119-131. doi:10.1111/j.1471-4159.2009.06311.x
- Schrader, M., Krieglstein, K. and Fahimi, H. D. (1998) Tubular peroxisomes in HepG2 cells: Selective induction by growth factors and arachidonic acid. European Journal of Cell Biology, 75, 87-96. doi:10.1016/S0171-9335(98)80051-4
- Savary, S., et al. (2012) Fatty acids-induced lipotoxicity and inflammation. Current Drug Metabolism, 13, 1358- 1370. doi:10.2174/138920012803762729
- Gondcaille, C., et al. (2005) Phenylbutyrate up-regulates the adrenoleukodystrophy-related gene as a nonclassical peroxisome proliferator. Journal of Cell Biology, 169, 93-104. doi:10.1083/jcb.200501036
- Zhang, X., et al. (2006) Peroxisome proliferator-activated receptor alpha-independent peroxisome proliferation. Biochemical and Biophysical Research Communications, 346, 1307-1311. doi:10.1016/j.bbrc.2006.06.042
- Gloerich, J., et al. (2005) A phytol-enriched diet induces changes in fatty acid metabolism in mice both via PPARα-dependent and -independent pathways. Journal of Lipid Research, 46, 716-726. doi:10.1194/jlr.M400337-JLR200
- DeLuca, J.G., et al. (2000) Evidence for peroxisome proliferator-activated receptor (PPAR)α-independent peroxisome proliferation: Effects of PPARγ/delta-specific agonists in PPARαlpha-null mice. Molecular Pharmacology, 58, 470-476.
- Jia, Y., et al. (2003) Overexpression of peroxisome proliferator-activated receptor-alpha (PPARα)-regulated genes in liver in the absence of peroxisome proliferation in mice deficient in both Land D-forms of enoyl-CoA hydratase/dehydrogenase enzymes of peroxisomal beta-oxidation system. Journal of Biological Chemistry, 278, 47232- 47239. doi:10.1074/jbc.M306363200
- Contreras, M.A., et al. (2000) Endotoxin induces structure-function alterations of rat liver peroxisomes: Kupffer cells released factors as possible modulators. Hepatology, 31, 446-455. doi:10.1002/hep.510310226
- Dhaunsi, G.S., Hanevold, C.D. and Singh, I. (1994) Impairment of peroxisomal beta-oxidation system by endotoxin treatment. Molecular and Cellular Biochemistry, 135, 187-193. doi:10.1007/BF00926522
- Khan, M., et al. (1998) Cytokine-induced accumulation of very long-chain fatty acids in rat C6 glial cells: Implication for X-adrenoleukodystrophy. Journal of neurochemistry, 71, 78-87. doi:10.1046/j.1471-4159.1998.71010078.x
- Paintlia, M.K., et al. (2008) Modulation of peroxisome proliferator-activated receptor-alpha activity by N-acetyl cysteine attenuates inhibition of oligodendrocyte development in lipopolysaccharide stimulated mixed glial cultures. Journal of Neurochemistry, 105, 956-970. doi:10.1111/j.1471-4159.2007.05199.x
- Schilling, J., et al. (2011) Toll-like receptor-mediated inflammatory signaling reprograms cardiac energy metabolism by repressing peroxisome proliferator-activated receptor gamma coactivator-1 signaling. Circulation: Heart Failure, 4, 474-482. doi:10.1161/CIRCHEARTFAILURE.110.959833
SUPPLEMENTARY DATA
Table S1. Comparison of individual fatty acid compositions of Argan and olive oils inferred from published and present data expressed in % of total oil fatty acid contents.

aBenzaria A. et al. Nutrition, 2006, 22: 628-637.
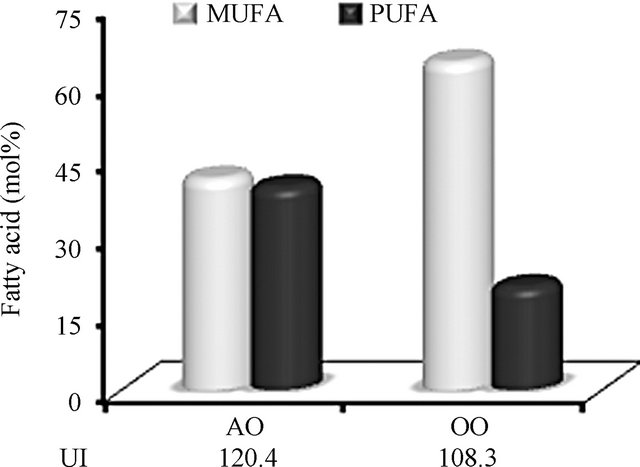
Figure S1. Comparison of monounsaturated fatty acids (MUFA) versus polyunsaturated fatty acids (PUFA) contents of argan oil and olive oil. Argan oil (AO) has an equilibrated mixed content in monoand polyunsaturated fatty acids contrarily to olive oil (OO). Unsaturation index (UI) is calculated as summed moles per 100 moles multiplied by the number of double bonds.

