Engineering
Vol. 3 No. 1 (2011) , Article ID: 3736 , 4 pages DOI:10.4236/eng.2011.31009
Functional Copolymer/Organo-MMT Nanoarchitectures. VIII. Synthesis, Morphology and Thermal Behavior of Poly(maleic anhydride-alt-acrylamide) -Organo-MMT Clays Nanohybrids*
Department of Chemical Engineering, Faculty of Engineering, Division of Nanoscience &Nanomedicine
Hacettepe University, Ankara, Turkey
E-mail: zmo@hacettepe.edu.tr
Received October 19, 2010; revised November 19, 2010; accepted December 13, 2010
Keywords: Functional Copolymer, Organoclays, Nanohybrid Composites, Thermal Behavior, Morphology
ABSTRACT
Functional copolymer–clay hybrids were synthesized by radical-initiated intercalative copolymerization of maleic acid (MA) and acrylamide (AAm) with 2,2’-azobis (2-methylpro-pionamidine) dihydrochloride as a water-soluble ionizable radical initiator in the presence of reactive (octadecyl amine (ODA)-MMT) and non-reactive (dimethyldodecyl ammonium (DMDA)-MMT) organoclays at 50oC in aqueous medium under nitrogen atmosphere. The monomers was dissolved in aqueous medium, as well as both used clay particles were easily dissolved and dispersed with partially swollen in deionized water, respectively. Structure, thermal behavior and morphology of the synthesized nanocomposites were investigated by FTIR, XRD, DSC-TGA, SEM and TEM analysis methods, respectively. It was demonstrated that intercalative copolymerization proceed via ion exchange between organoclays and carboxylic groups of monomers/polymers which essentially improved interfacial interaction of polymer matrix and clay layers through strong H-bonding. In case of intercalative copolymerization in the presence of ODA-MMT clay, similar improvement was provided by in situ hydrogen-bonding and amidolysis of carboxylic/anhydride groups from copolymer chains with primary amine group of ODA-MMT. The nanocomposites exhibit higher intercalation/exfoliation degree of copolymer chains, improved thermal properties and fine dispersed morphology.
1. Introduction
The conventional montmorillonite (Na+-MMT) clayfilled polymer microcomposites are disadvan-tageous for non-uniform distribution of the clay in matrix polymers due to hydrophilic origin of the inorganic clay which does not provide a better dispersion in most hydrophobic organic polymer phases. To improve the interfacial interaction between the clay galleries and polymer matrix and to prepare a polymer layered clay nanocomposite, many researchers utilized the organic derivatives of clays, which is easily prepared by surface modification of clay layers with organic surfactants through intercalation in situ processing [1-5]. Now, organoclays like well-investigated nanofillers have considerable attention from academic and industrial researchers to prepare the various polymer nanosystems and nanomaterials with improved properties and broad application areas. Unlike the pristine polymers and their clay-filled micro composites, polymer/organoclay nanocom-posites, containing the welldispersed nanosize particles, exhibit significantly improved thermal, mechanical, optical, barrier and physicochemical parameters [6-8]. Recently, many effective approaches and synthetic partway for the preparation of polymer/organoclay nanocomposites were developed. Althougth the most promising reaction to synthesize polymer/clay nanocomposites is the intercalative polymerization of functional monomers, such as styrene [9-10], N-vinyl carbazole [11], 4-vinylpyridine [12], methyl methacrylate [13-15], acrylonitrile [16] and N-n-butyl maleimide [17], the intercalative radical (co) polymerizetion of monomer systems in presence of mineral clay, especially in water solution, has been scarcely investigated. Examples are bulk copolymerization of styrenemethyl methacrylate [18-20], emulsion copolymerization of styrene-acrylonitrile [21], styrene-N-phenylmaleimide (PhMI) [22] and styrene-butyl methacrylate [23] monomer pairs. It was observed that the polymerization rate accelerated by the addition of the clay in reaction medium; this addition also significantly influenced on the structure and properties of the prepared polymer nanocomposites [19,24]. Liu et al. [24-26] investtigated the bulk, solution and emulsion copolymerization of styrene with methyl methacrylate and PhMI (at 1:9 molar PhMI/styrene feed ratio) in the presence of organo (cetyltrimethyl ammonium bromide)-modified MMT or Na+-MMT in order to prepare nanocomposites with good dispensability of clay. Wang et al. [27,28] showed that a comparison of organic solution, emulsion, suspension and bulk polymerization along the nanocom-posites may also prepared by melt in situ reactive blending of monomer/polymer/clay mixtures. Nguen and Baird [29] reported the different methods and types of polymers using for preparation of polymer nanocomposites, the structure and properties of layered silicates, and the most common techniques used for characterization of nanocomposites. In the last decade, the alternating, random and graft copolymers of maleic maleic and acrylic monomers widenly utilized for the preparation of polymer/organo-silicate (or silica) hybrid materials through melt compounding by reactive extrusion and sol-gel methods. However, interlamellar water solution copolymerization of maleic anhydride (MA)/acid (MAc) with acrylamide (AAm) via pre-intercalated MAc... organoclays (O-MMTs) complexes as a green processing, as well as copolymer/organoclay compositionnano-structure-property relationships have not been investigated.
Synthesis and characterization of some selective functional copolymer/organo-silicate hybrid nanocomposites prepared in organic solvents were a subject our previous publications [30-32]. In this work, we have synthesized functional polymer silicate layered nano-composites by the radical-initiated intercalative (co)-polymerization in the presence of water soluble reactive octadecyl amine (ODA-MMT) and partially water-swollen dimethyldidodecyl amonium (DMDA-MMT) as organic derivatives of MMT clay in water at 60oC under nitrogen atmosphere. The polymer/clay hybrid structure-composition-property (thermal behavior and morphology) relations were investigated by FTIR, XRD, DSC-TGA, SEM and TEM analysis methods. General scheme of synthetic routes for the preparation of nanostructural hybrids by the interlamellar copolymerization through interfacial monomer/organoclay complex-formation can be represented as follows Figure 1:
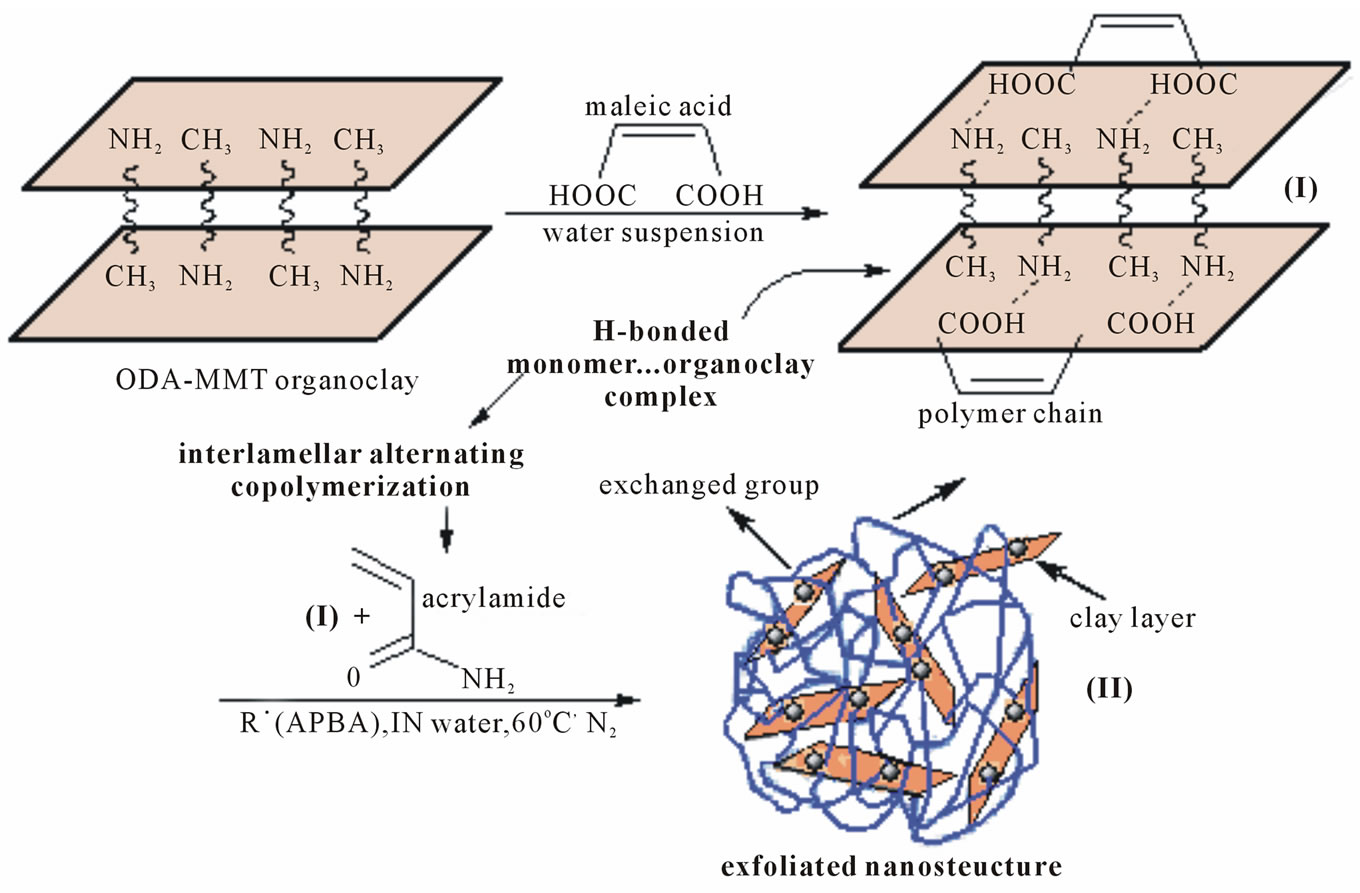
Figure 1. Schematic representation of synthetic partway for the preparation of functional copolymer/organoclay nanostructural hybrids through radical intercalative polymerization in aqueous solution.
2. Experiment
2.1. Materials
MA monomer (Fluka, Switzerland) was purified by recrystallization from anhydrous benzene solution and sublimation in vacuum. AAm monomer (Aldrich-Sigma, Germany) was purified before use by distillation under modernate vacuum. 2,2’-Azobis(2-methylpropion amidine) dihydrochloride (ABPA) an ionizable water-soluble radical initiator (Fluka) was recrystallized twice before use from methanol. Octadecyl amine (ODA)-montmorillonite (MMT) organoclay (Nanomer 1.30 E, Nanocor Co.) supplied from Aldrich, having the following average characteristics: content of octadecyl amine 25-30%; particle size 8-10 µm, bulk density 0.41 g/cm3, crystallinity 52.8% (by XRD), glass-transition temperature (Tg) 108.4°C (by DSC for the surface alkyl (C12) amine fragment), temperature of decomposition for Td(onset) 275°C and Td(max) 380°C (by TGA). Dimethyldidodecyl ammonium (DMDA-MMT, Viscobent SB-1) was purchased from Bensan (Enez, Turkey) having the following average parameters: specific surface area 43.6 m2.g-1, specific mesopore volume 0.14 cm3.g-1, content of N 1.12% and C 32.56%, crystallinity 58.2% (by XRD), glass-transition temperature (Tg) 158.6°C (by DSC for the surface alkyl ammonium fragment), temperature of decomposition for Td(onset) 238oC and Td(max) 361°C (by TGA). All other solvents and reagents were of analytical grade and used without purification.
2.2. Synthesis Procedures
The poly(MAc-alt-AAm)/ODA-MMT and poly(MAcalt-AAm)/DMDA-MMT hybrids were synthesized by intercalative copolymerization of equimolar mixture (1:1) of MAc monomer with AAm comonomer in the presence of ABPA (0.25 wt.%) as a radical initiator and reactive and non-reactive organoclays (5 wt.%) at 60 ± 0.1°C in deionized water under nitrogen atmosphere by intensive mixing in carousel type of micro reactor up to the formation homogeneous solution (for ODA-MMT) and fine dispersion (for DMDA-MMT). Then prepared mixture treated by large amount of methanol at 20°C by intensive mixing up to full precipitation of powder product, which is isolated by filtration, and drying under vacuum.
2.3. Characterization
FT-IR spectra of the copolymers (KBr pellet) were recorded with FT-IR Nicolet 510 Spectrometer in the 4000-400 cm-1 range, where 30 scans were taken at 4 cm-1 resolution.
The X-ray powder diffraction (XRD) patterns were obtained form a Rigaku D-Max 2200 powder diffractometer. The XRD difractograms were measured at 2q, in the range 1-50o, using a CuKa incident beam (l = 1.5406 Å), monochromated by a Ni-fiter. The Bragg equation was used to calculate the interlayer spacing (d): nl = 2dsinq, where n is the order of reflection, and q is the angle of reflection. Crystallinity of the nanocomposites was calculated using the Equations (1) and (2) [33]:
cc = o∫∞s2Io(s)ds /o∫∞s2I(s)ds (1)
where s is the magnitude of the reciprocal-lattice vector which is given by s = (2sinq)/l (q is one-half the angle of deviation of the diffracted rays from the incident X-rays and l is the wavelength); I(s) and Ic(s) are the intensities of coherent X-ray scattering from both crystalline and amorphous regions and from only crystalline region of polymer sample, respectively, and d is interlayer spacing.
cc (%) = Wc/( Wc + Wa).10-2 (2)
where, Wc and Wa are the areas of the crystalline and amorphous portions in the X-ray diffractogram, respectively.
Intercalation/exfoliation degree (ED) was calculated according to the following equation:
ED (%) = Ie/( Ie + Io).10-2 (3)
where, Ie (or Ic) and Io are the intensity of the diffraction peaks associated with the exfoliated (or intercalated) and non-exfoliated structures at corresponding 2q values.
The surface morphology of nanocomposites was examinated using a scanning electron microscope (SEM) (JSM-6400 JOEL SEM with scale: 1 and 10 mm, x104 and an accelaration voltage 20 kV). All speciments were freeze-dried and coated with a thin layer of gold before testing. Transmission electron microscopy (TEM) images were obtained on a Jeol JEM-2100F transmission electron microscope with an acceleration voltage of 200 kV and emission current 146 mA. This method allowed performs a higher resolution image due to the decrease in wavelength. An electron gun emits an electron beam which moves through a condenser aperture and then bombards the specimen. The TEM specimens were cut at room temperature using an ultramicrotome. Thin specimens, 50-80 nm, were collected in a trough filled with water and placed on 200 mesh copper grids.
Thermogravimetric (TGA) and differential scanning calorimetric (DSC) analyses were performed in a Thermal TGA-DTA Analyzer (Shimadzu DTG-60H, Japan) and Shimadzu DSC-60 Analyzer (Kyoto, Japan), under nitrogen atmosphere at a heating rate of 10°C/min.
3. Results and Discussion
3.1. Chemical and Physical Structures
We are investigated the influences of different organic modified MMT on the chemical and physical structures and properties of the copolymer/clay nanocomposites. Structures of synthesized poly(MAc-alt-AAm)/organoclay nanocomposites were confirmed by FTIR spectroscopy Figure 2.
Results of comparative spectral analysis of the pristine Na+-MMT clay, organoclays and copo-lymer/organoclays nanocomposites indicate the following changes of the characteristic bands in FTIR spectra of nanocomposites: 1) because the interlayer molecular complexes, new characteristic bands at 2531 cm-1 (stretching of –COOH in the complex), 1725 cm-1 (fairly broad acid C=O stretching in –COO– ), 1670 cm-1 (shifted to high region NH2 deformation), 1420 cm-1 (–H3N+ antisym. deformation) which can be related to complexed –COO– . +NH3– linkages, 2) the absorption bands at 3248 cm-1 (NH2 sym. stretching), 2926 and 2853 cm-1 (CH2 stretching), 800 cm-1 (NH2 wagging), 1468 and 723 cm-1 (CH2 deformation and CH2 rocking in –(CH2)n– methylene long chain) are associated with alkyl amine fragment in –(CH2)n–N+.–OOC– complex, 3) 1370 and 1364 cm-1 (CH3 deformation in –C(CH3)2– and 4) characteristic bands at 3632 and 3394 cm-1 (Si–OH and OH from water molecules in pristine clay, respectively), 1200-1000 cm-1 (strong broad Si–O–Si vibration), 916 cm-1 (Si-O stretching), 523 (Al-O stretching) and 463 (Si-O bending) of MMT silicate layers. Band at 1509 cm-1 associated with symmetric stretching vibration of –COO– group.
The formation of new characteristic amide-II band at 1655 cm-1, and significantly decreasing the intensity of broad carboxylic band at 1725 cm-1 and 2580 cm-1 band corresponding to the carboxylic groups in –NH3+. –OOC– complexes indicate the chemically and physically interactions between functional copolymer chains and organic modified silicate layers.
XRD patterns of pristine MMT, organoclays (ODAMMT and DMDA-MMT), PAAm/MMT and poly (MAc-alt-AAm)/organoclays nanocomposites are shown in Figure 3, and obtained XRD parameters were summarized in Table 1.
The copolymer/organoclay nanosystems synthesized by interlamellar copolymerization predomina-ntly exhibit semicrystalline structure and better intercalation/exfoLiation in situ processes. The formation of crystalline phases significantly depends on the nature of organoclay used in nanocomposites. The average basal d-spacing of pristine MMT clay significantly increases from 14.5 Å to 34.6 Å and 2.42 Å after surface modification of clay by ODA and DMDA surfactants, respectively. This in situ process is simultaneously accompanied by decreasing the 2q values, which indicated the intercalation of organic surfactants into the silicate galleries on MMT through cationic arrangement. Observed relatively higher values of d-spacing for the copolymer/organoclay systems at lower angle than that of the organoclays indicates the realization of intercalation and exfoliation (predominantly) processes and formation of semicrystalline nanostructures. Distance between silicate layers (d(001)- spacing) essentially increase from 10.96 and 16.98 Å for the ODAand DMDA-MMT clays to16.87 (Dd = (dc-do) = 5.91 Å) and 27.14 Å (Δd = 10.16 Å) for the reactive and non-reactive organoclaycontaining nano-composites, respectively (Table 1). Simultaneously shift of 2q to
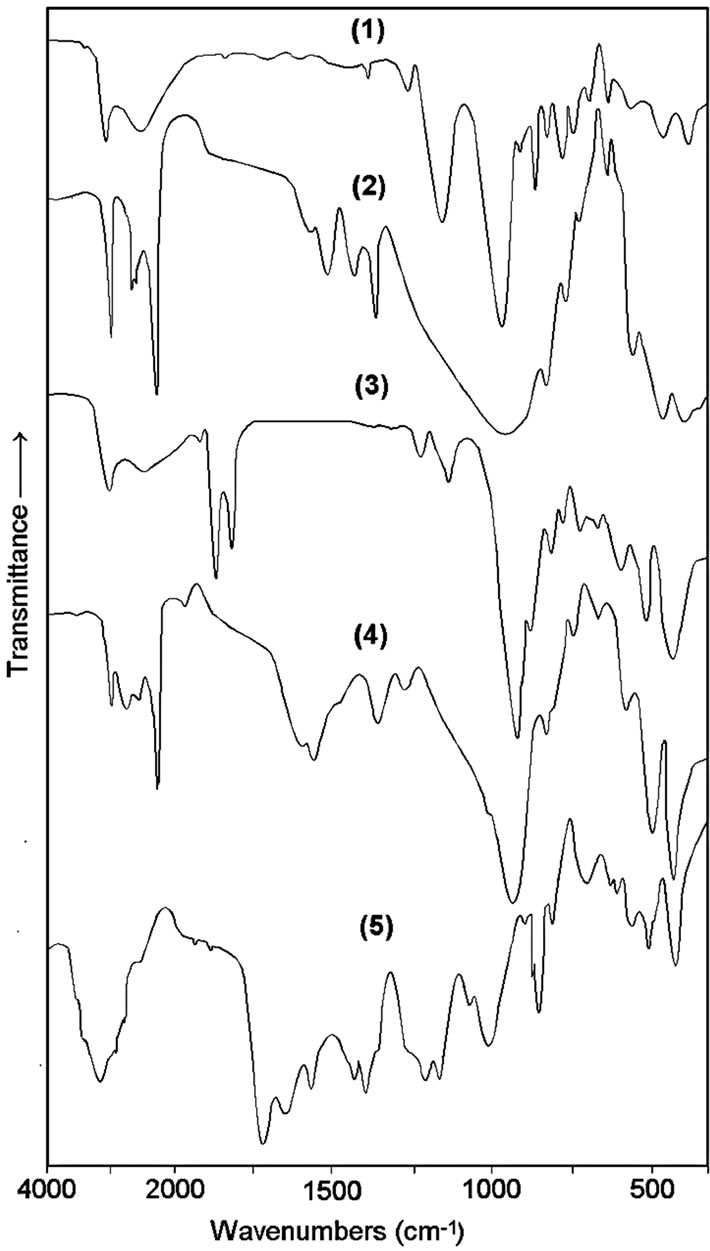
Figure 2. FTIR spectra of (1) MMT clay, (2) ODA-MMT,(3) DMDA-MMT, (4) poly(MAc-alt-AAm)/ODA-MMT and (5) poly (MAc-alt-AAm)/DMDA-MMT nanocomposites.
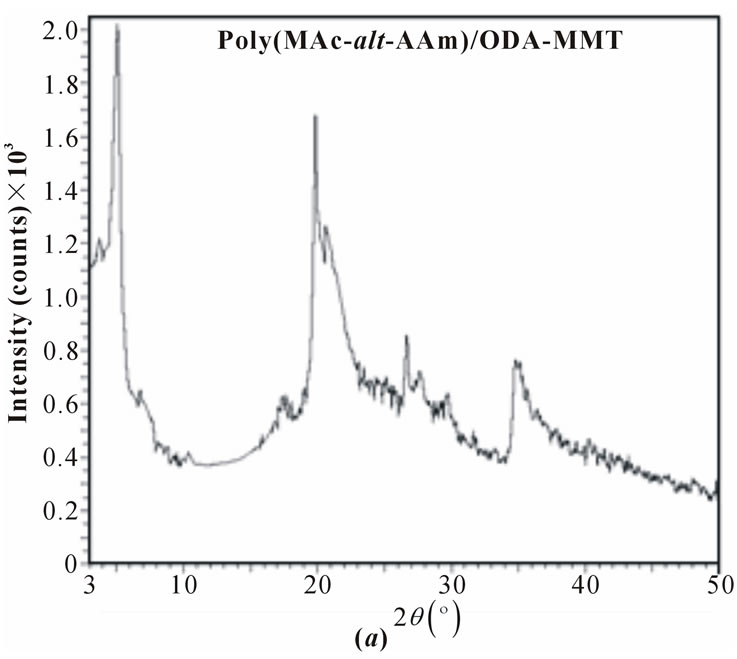
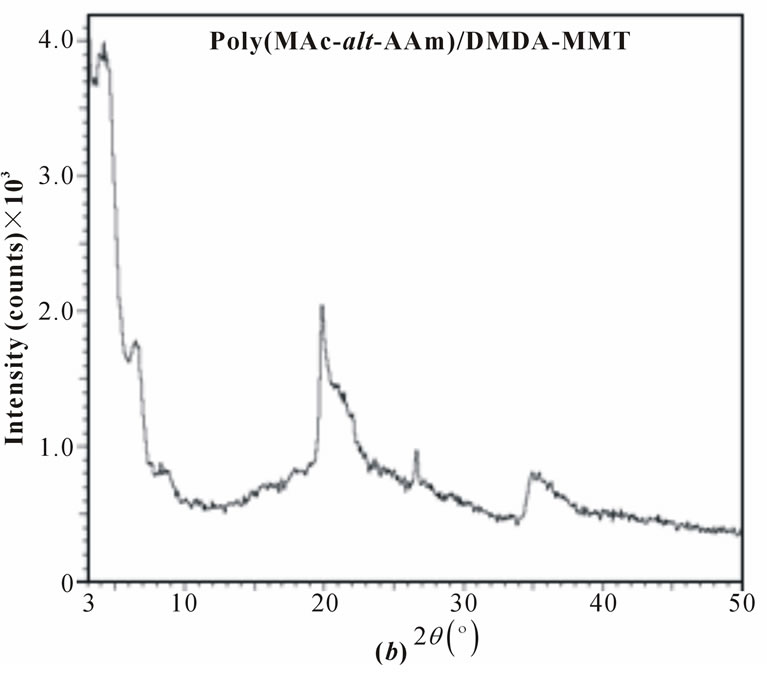
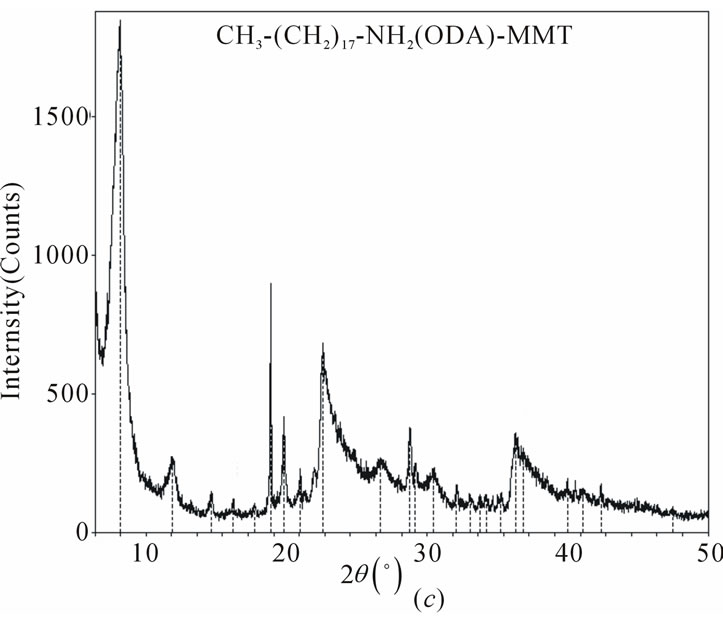
Figure 3. XRD patterns of (a) and (b) copolymerorganoclays and (c) pristine organoclay.
lower region in the XDR spectra was observed. Calculated values of the exfoliation degree (ED) for both the copolymer/organoclay nanocomposites show that copolymer/DMDA-MMT system exhibits relatively high degree of exfoliation (80.7%) than that of the nanocomposite prepared in the presence of reactive organoclay (69.9%).
3.2. Composition–Morphology Relations
It is well known that SEM method is important in the investigation of surface morphology-structure-property relationships of polymer composites and polymer/organo-silicate nanocomposites on various length scales and provides very direct evidence of intercalation and exfoliation of the polymer chains into silicate galleries [34]. SEM images indicated that surface morphology of the copolymer/organoclay nanocomposites essentially depends on the type of used organo-clay. Sem images of synthesized nanocomposites were illustrated in Figure 4.
In the case of reactive organoclay (ODA-MMT), relatively fine distributed morphologies were observed. Agreeing with these images, the dispersed phases are visibly reduced in the non-reactive organoclay (DMDAMMT) nanosystems. This observed fact can be explained by chemical in situ processing through amine/acid reactions which are carried out in nano scale between silicate galleries.
TEM is required to image the clay platelets and fully identify the type of nanocomposites that has been produced. To further confirm the dispersion of organoclay fragments in the matrix copolymer, TEM investigations are also required. For the preparing TEM specimens of the copolymer/organoclay nanocomposite, the focused ion beam (FIB) technique with a sort time of exposition (10-20 s) was used. According to Monticelli et al.[35], TEM analysis method turn out to be less challenging when exfoliated nanocomposites are studied with respect to the characterization of intercalated nanostructure systems, where clay structure undergoes a modification and
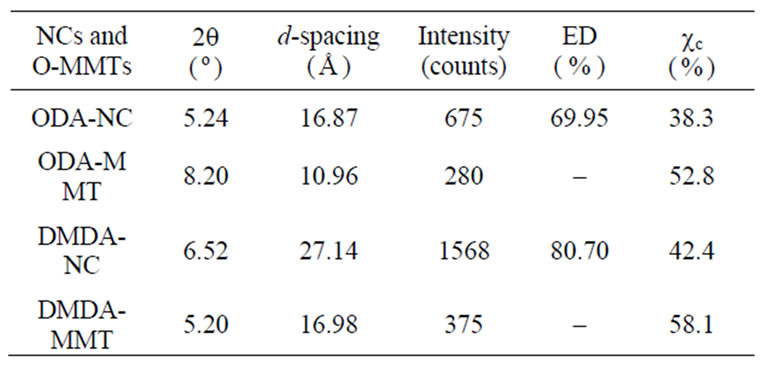
Table 1. XRD parameters and crystallinity (cc) of copolymer-organoclay hybrid nanocomposites (NC).
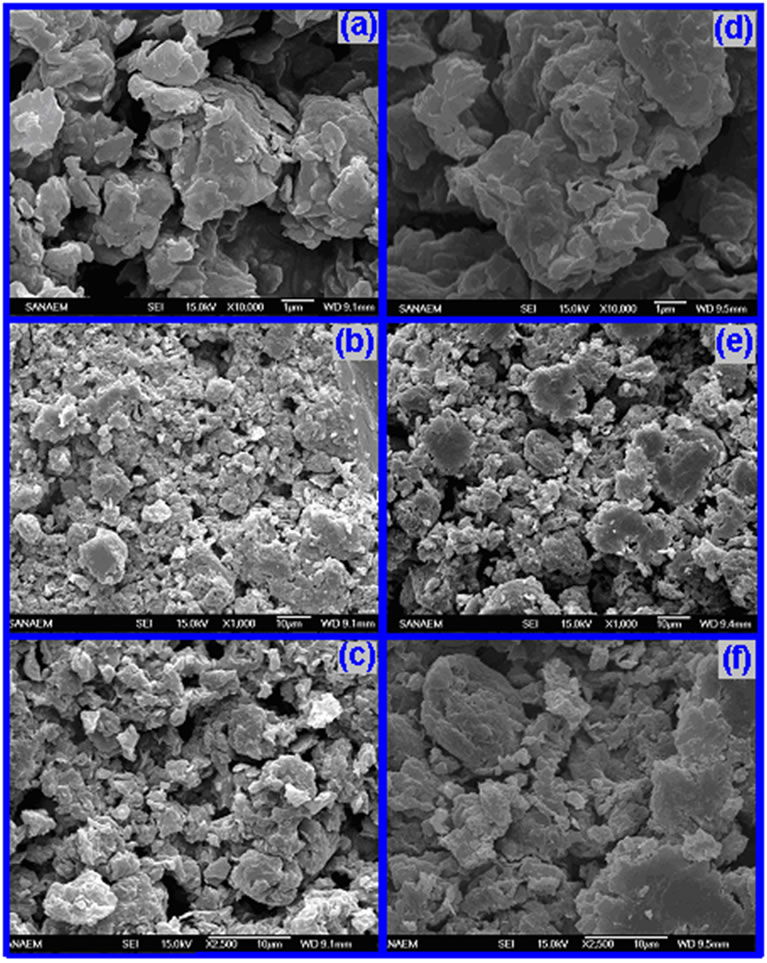
Figure 4. SEM images of copolymer/ODA-MMT nanocomposite: (a) 1 mm x 10.000, (b) 10 mm x 1000 and (c) 10 mm x 2500; copolymer/DMDA-MMT nanocomposite: (d) 1 mm x 10.000, (e) 10 mm x1000 and (f) 10 mm x 2500 magnification.
decomposition under electron beams, which is supported by the disappearance of some layers and the toning down of others. The TEM images are illustrated in Figure 5. The TEM observations show that the formation of an exfoliated nano-morphology between silicate galleries. The presence of single and partially agglomerated (black points in TEM images associated with intercalated structures) clay layers dispersed in copolymer matrix indicates the formation of individual dispersion of delaminated clay platelets, and therefore a predominantly exfoliated nanostructure. Relatively homogenous phase distribution was observed for nanocomposites prepared in the presence of ODA-MMT clay Figure 5 which is reasonable agreement with SEM analysis results.
3.3. Thermal Behavior
The results of differential scanning calorimetric (DSC) and thermal gravimetric analyses (TGA) of pristine Na+-MMTand its organic derivatives, as well as synthesized nanocomposites were illustrated in Figures 6-7, and summarized in Table 2.
All samples were undergoing to thermo treatment at 110°C for 2 h before performing the thermal analysis. DSC curves of organoclay indicate that both the organoclays exhibit glass-transition temperatures (Tg) at 158.6 and 108.4°C, respectively (Figures 6(a-b)). These observed transitions may be attributed to the alkyl amine (or ammonium) surfactants. While nonorganic pristine Na+-MMT (Figure 6(c)) is not exhibits a glass-transition. All three type of clays show very broad endo-peaks
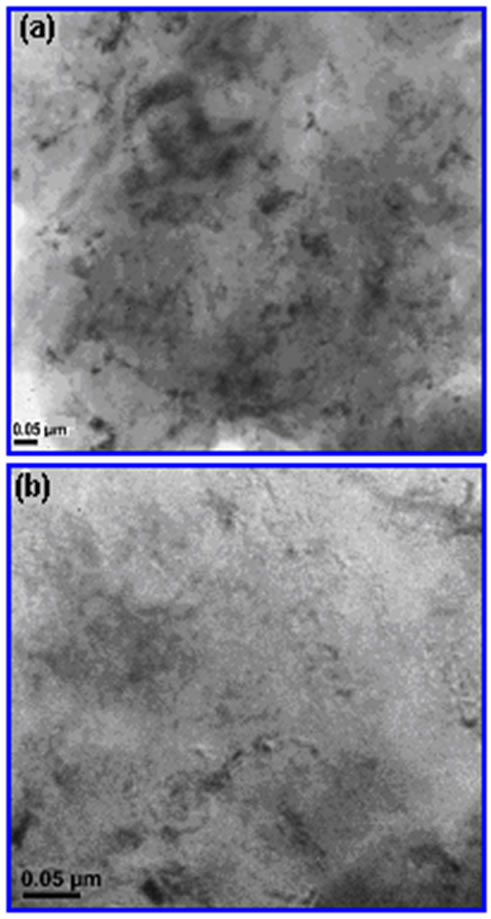
Figure 5. TEM images of poly (AAm-co-MAc)/ODAMMT nanocomposite. Scale: (a) 40 kV x 500 and (b) 40 kV x 1000.
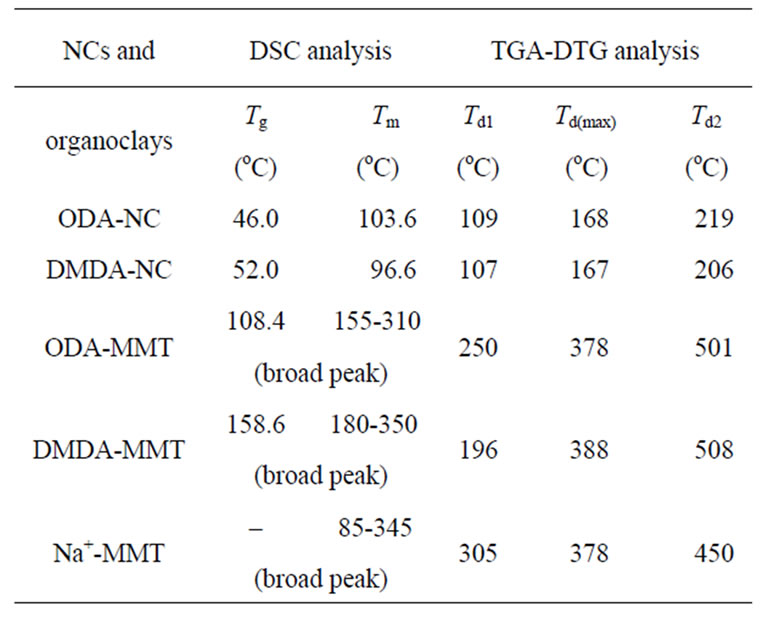
Table 2. Thermal behavior of functional copolymer– organoclay nanocom-posites (NC), organoclays and pristine clay.
 (a)
(a)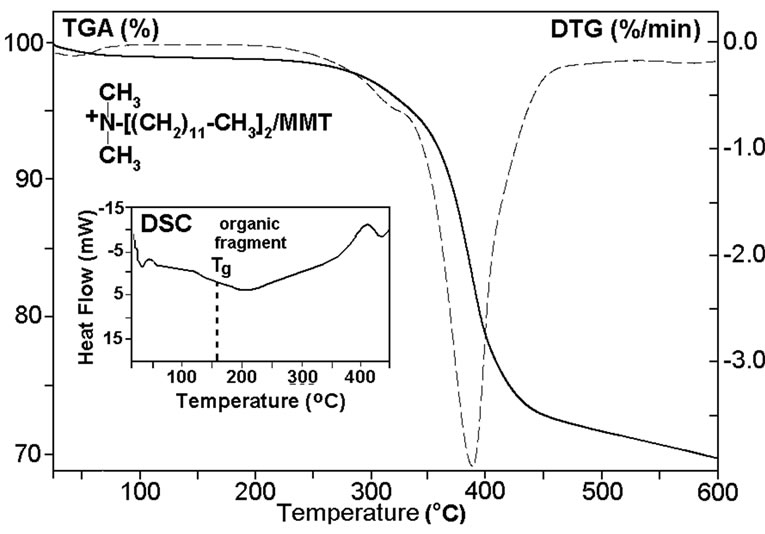 (b)
(b)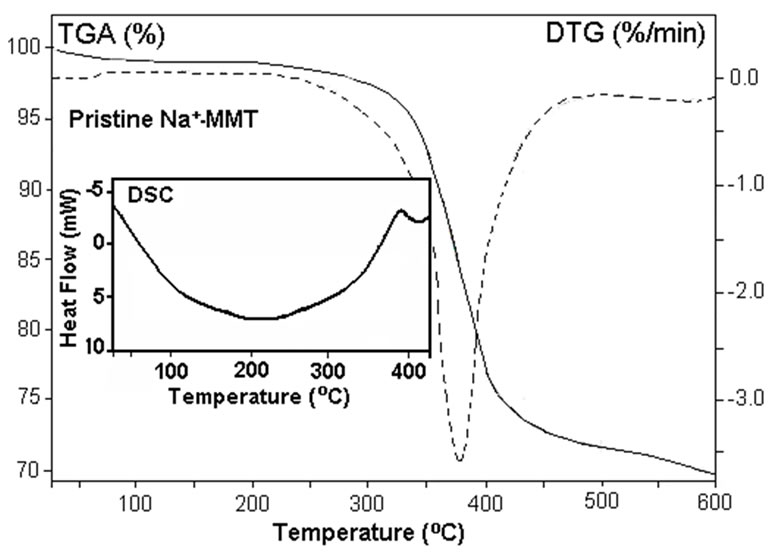 (c)
(c)
Figure 6. DSC-TGA-DTG thermograms of (a) ODA-MMT and (b) DMDA-MMT organoclays, and (c) pristine Na+- MMT clay. Heating rate is 10oC/min.
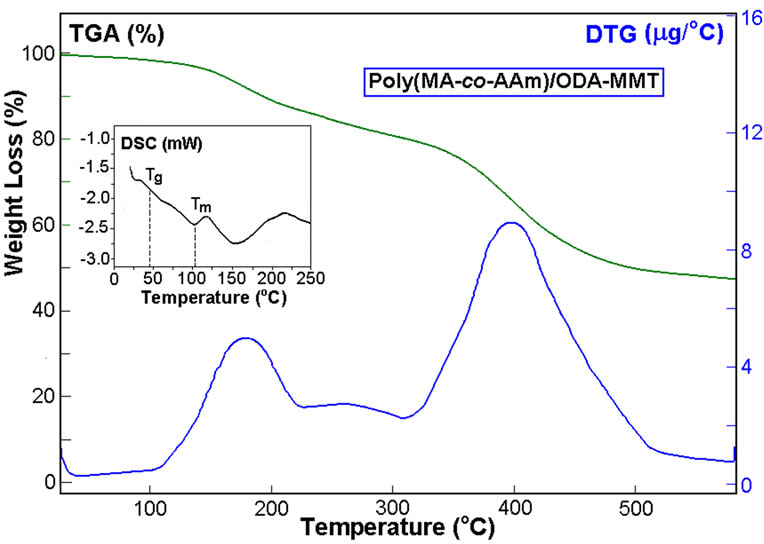
around 155-350°C which are associated with melt transitions of their different inorganic crystalline structures. It was found that both the nanocomposites exhibit semicrystalline behavior with lower glass-transition temperatures (Tg) and high melt transition temperatures (Tm). These parameters visible depend on the nature of the organic modified clays. TGA-DTG thermograms of nanocomposites were performed under a nitrogen atmosphere. TGA curves show two step thermal degradation of nanocomposites which accompanied by chemical reactions. Appearance of broad endo-peak around 125-200°C in DSC curves of nanocomposites, which accompanied by weight loss around 12-15% (TGA curves), can be explained by anhydridization of free carboxylic groups formed during intercalative copoly-merization in aqueous medium via easily hydrolysis of MA monomer before chain growing reactions. However, TGA curves indicated that the copolymer/DMDA-MMT nanocomposite at above mention temperature region exhibits relatively high weight loss in comparison with those for the reactive ODA-MMT organoclay containing nanocomposite. Calculated amount of eliminated H2O via anhy-
 (a)
(a)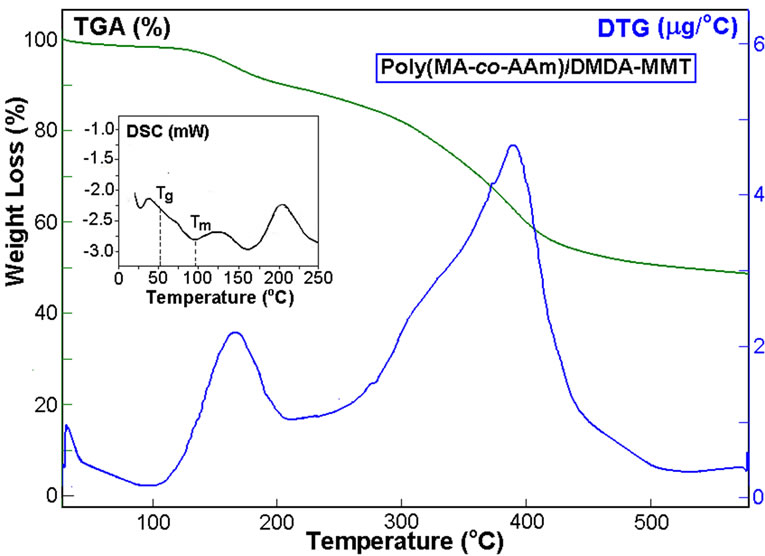 (b)
(b)
Figure 7. DSC-TGA-DTG curves of (a) poly (MAc-altAAm)/ODA-MMT and (b) poly (MAc-alt-AAm)/DMDAMMT nanohybrids. Heating rate as in Figure 6.
dridization reaction is 10.4% for the reactive organoclay containing nanosystems that reasonable agree with founded weight loss (8.8%) from TGA curve (Figure 7(a)). While for the non-reactive organoclay containing nanosystems this value is found to be 13.0% (Figure 7(b)). This observed difference can be explained by preceding the additional amidolysis of the free carboxylic groups with the primary amine groups of ODA-MMT in the isothermal conditions. It is known that the other alternating copolymers of MA may be undergoing to reversible hydrolysis-anhydridization reactions in aqueous solution and in solid state thermo treatment, respectively [36].
According to Cai et al. [37], thermal stability of polymer nanocomposites strongly depends on the nanocomposites morphology. The silicate clay layers act as a superior thermal isolator and as a mass-transport barrier to the volatile products eliminated during the thermal degradation process which are important factors for increasing the thermal stability and flammability of polymer nanocomposites. We observed that the nanocomposites (containing 5 wt.% organoclay) exhibit decomposition temperatures (Tdmax) 115 and 410°C. The observed better thermal stability may be attributed to the hindrance of the diffusion of the volatile degradation products due to the presence of a combination of intercalated and exfoliated nanostructures in studied nanosystems.
The TEM and XRD results indicate that the poly (MAc-alt-AAm)/ODA-MMT (or DMDA-MMT) clay nanocomposites have a mixed nanomorphology containing predominantly exfoliated structure and partially intercalated structure (ED 69.9% for copolymer/ODAMTT nanosystems) due to this reactive nanosystems`high thermal stability (26.7% of weight loss at 400oC) (Table 2). While the nanocomposites with higher degree exfoliation (80.7% for copolymer/ DMDA-MMT nanosystems) exhibit relatively less thermal stability (32.6% of weight loss at 400°C). Thus, nanocomposites containing approximately 70/30 mixture of intercalated/ exfoliated structures show higher thermal stability than nanocomposite having significant exfoliated structure. Similar effect was observed by the other researchers in a study of poly (etherimide) [37], polypropylene/polystyrene [38] and poly (styrene-co-acrylonitrile) [39] silicate layered nanocomposites which are prepared in the nonaqueous medium.
Thus, evaluated effect of reactive and non-reactive organoclays on the intercalated/exfoliated nanostructural ratios in the nanocomposites prepared in water solution by heterogeneous in situ copolymerization method can be described as a new approach in macromolecular engineering. Process of nanostructure forming in these systems is controllable and can be utilized using a wide range of conventional water-soluble functional polymers for the fabrication of engineering hybrid materials such as nanoadhesives, nanofilms and nanocoatings.
4. Conclusions
We have developed a facile and effective strategy for the design and synthesis of polymer/organo-silicate nanoarchitectures by an interlamellar copolymerization method in aqueous medium. Unlike the known methods, the novel approach involves the use of deionized water as reaction medium, water-soluble radical initiator and monomers to prepare nanohybrid systems with well exfoliated copolymer/silicate layered nanoarchitectures with fine dispersed morphology and high performance thermal properties. It was demonstrated that thermal stability of nanocomposites depends on the ratio of intercalated/exfoliated structures. Nanocomposites containing a combination of intercalated (partially) and exfoliated (predominantly) nanostructures exhibit higher thermal stability as compared with those for predominantly exfoliated nanocomposites. This strategy can also be broadly utilized to other water-soluble hydrophilic/hydrophobic functional monomer systems for the preparation of polymer/silicate hybrid nanomaterials. Another interesting aspect of the new approach is the utilization of the synthesized carboxyl/amide functionalized copolymer/ organo-MMT hybrids as reactive compatibilizer/nanofillers for the immiscible thermoplastic polymer blends and their in situ melt processing by a reactive extrusion system and in-line nanocoating technology to improve the barrier properties by surface modification of the conventional polymer multilayered packaging thin films. These aspects will be a subject of our investigations in the nearest future.
5. Acknowledgements
The authors thank the Turkish Scientific and Technology Research Council (TÜBİTAK) for the financial support of this work through project TBAG-HD/249.
6. REFERENCES
- S. B. Hendricks, “Base Exchange of the Clay Mineral Montmorillonite for Organic Cations and Its Dependence upon Absorption due to Wander-Waals Forces,” Journal of Physical Chemistry, Vol. 45, 1941, pp. 65-81. doi:10. 1021/j150406a006
- A. Weiss, “Organic Derivatives of Mica-Type Layer Silicates,” Angewandte Chemie International Edition, Vol. 2, No. 3, March 1963, pp. 134-144. doi:10.1002/anie. 196301341
- B. K. G. Theng, “The Chemistry of Clay-Organic Reactions,” Wiley, New York, 1974.
- A. C. D. Newman, “Chemistry of Clays and Clay Materials,” Mineralogical Society Monograph, No. 6, Wiley, New York, 1987.
- Y. Komori and K. Kuroda, “Polymer-Layered Silicate Nanocomposies,” Wiley, New York, 2000.
- M. Alexandre and P. Dubois, “Polymer-Layered Siliate Nanocomposites: Preparation, Properties and Uses of a New Class of Materials,” Material Science and Engineering R, Vol. 28, No. 1, 2000, pp. 1-63. doi:10.1016/ S0927-796X(00)00012-7
- S. S. Ray and M. Okamoto, “Polymer/Layered Silicate Nanocomposites: A Review from Preparation to Processing,” Progress in Polymer Science, Vol. 28, No. 11, 2003, pp. 1539-1641. doi:10.1016/j.progpolymsci. 2003.08.002
- S. C. Tjong, “Structural and Echanical Properties of Polymer Nanocomposites,” Material Science and Engineering R, Vol. 53, No. 3-4, 2006, pp. 73-197. doi:10. 1016/j.mser.2006.06.001
- Q. H. Zeng, D. Z. Wang and G. Q. Lu, “Synthesis of Polymer–Montmorillonite Nanocomposites by in Situ Intercalative Polymerization,” Nanotechnology, Vol. 13, No. 5, 2002, pp. 549-553. doi:10.1088/0957-4484/13/5/3 01
- G. D. Liu, L. C. Zhang, D. G. Zhao and X. G. Qu, “Bulk Polymerization of Styrene in the Presence of Organomodified Montmorillonite,” Journal of Applied Polymer Science, Vol. 96, No. 4, May 2005, pp. 1146-1152. doi:10. 1002/app.21559
- M. Biswas and S. S. Ray, “Preparation and Evaluation of Composites from Montmorillonite and Some Heterocyclic Polymers. 1: Poly (N-Vinyl Carbazole)-Montmorillonite Nanocomposite System,” Polymer, Vol. 39, 1998, pp. 6423- 6428. doi:10.1016/S0032-3861(97)10366-4
- H. Z. Friedlander, “Spontaneous Polymerization in and on Clay,” American Chemical Society (ACS Division), Polymer Chemistry Reprints, Vol. 4, 1963, pp. 300-306.
- D. H. Solomon and B. C. Loft, “Reactions Catalyzed by Minerals. Part III. The Mechanism of Spontaneous Interlamellar Polymerizations in Aluminosilicates,” Journal of Applied Polymer Science, Vol. 12, No. 5, 1968, pp. 1253- 1262. doi:10.1002/app.1968.070120523
- A. Nese, S. Sen, M. A. Tasdelen, N. Nugay and Y. Yagci, “Clay-PMMA Nanocomposites by Photoinitiated Radical Polymerization Using Intercalated Phenacyl Pyridinium Salt Initiators,” Macromolecular Chemistry and Physics, Vol. 207, No. 9, May 2006, pp. 820-825. doi:10.1002/ macp.200500511
- L. M. Stadtmueller, K. R. Ratinac and S. P. Ringer, “The Effects of Intragallery Polymerization on the Structure of PMMA–Clay Nanocomposites,” Polymer, Vol. 46, 2005, pp. 9574-9584. doi:10.1016/j.polymer.2005.08.036
- Y. Sugahara, S. Satokawa, K. Kuroda and C. Kato, “Evidence for the Formation of Interlayer Polyacrylonitrile in Kaolinite”, Clays and Clay Minerals, Vol. 36, No. 4, 1988, pp. 343-348. doi:10.1346/CCMN.1988.0360408
- H. M. Li and H. B. Chen, “Synthesis and Characterization of Poly (N-n-Butylmaleimide)–Clay Nanocomposites,” Material Letters, Vol. 57, No. 20, June 2003, pp. 3000-3004. doi:10.1016/S0167-577X(02)01420-9
- C. Vaysse, L. Guerlou-Demourgues, C. Delmas and E. Duguest, “Tentative Mchanisms for Acrylate Intercalation and in Situ Polymerization in Nickel-Based Layered Double Hydroxides,” Macromolecules, Vol. 37, No. 1, 2004, pp. 45-51. doi:10.1021/ma025882w
- C. Vaysse, L. Guerlou-Demourgues, E. Duguet and C. Delmas, “Acrylate Intercalation and in Situ Polymerization in Iron-, Cobalt-, or Manganese-Substituted Nickel Hydroxides,” Inorganic Chemistry, Vol. 42, No. 15, 2003, pp. 4559-4567. doi:10.1021/ic026229s
- L. Vieille, C. Taviot-Gueho, J. P. Besse and F. Leroux, “Hydrocalumite and Its Polymer Derivatives. 2. Polymer Incorporation versus in Situ Polymerization of Styrene-4-Sulfonate,” Chemistry of Materials, Vol. 15, 2003, pp. 4369-4376. doi:10.1021/cm031070i
- L. Vieille, E. M. Moujahid, C. Taviot-Gueho, J. Cellier, J. P. Besse and F. Leroux, “In Situ Polymerization of Interleaved Monomers: a Comparative Study between Hydrotalcite and Hydrocalumite Host Structures,” Journal of Physical Chemistry in Solids, Vol. 65, No. 2-3, March 2004, pp. 385-393. doi:10.1016/j.jpcs.2003.08.029
- F. Yu, K. Yao, L. Shi, W. Wan, Q. Zhong, Y. Fu and X. You, “Investigation of Acrylic Acid Polymerization in Novel Two-Dimensional Molecular Space with Regular Amino Groups of Layered Aminopropylsilica,” Chemistry of Materials, Vol. 19, No. 14, 2007, pp. 3412-3418. doi:10.1021/cm070325f
- E. M. Moujahid, M. Dubois, J. P. Besse and F. Leroux, “Role of Atmospheric Oxygen for the Polymerization of Interleaved Aniline Sulfonic Acid in LDH,” Chemistry of Materials, Vol. 14, No. 9, 2002, pp. 3799-3807
- M. M. Al-Esaimi, “Reaction Catalyzed by Montmorillonite: Polymerization of Methyl Methacrylate,” Journal of Applied Polymer Science, Vol. 64, No. 2, April 1997, pp. 367-372. doi:10.1002/(SICI)1097-4628(19970411)64:2< 367::AID-APP18>3.3.CO;2-W
- G. D. Liu, L. C. Zhang, C. H. Gao and X. G. Qu, “Copolymerization of styrene with N-phenylmaleimide in the presence of montmorillonite,” Journal of Applied Polymer Science, Vol. 98, 2005, pp.1932-1937. doi:10.1002/ app.22364
- G. D. Liu, L. C. Zhang, X. G. Qu, P. Liu, L. Yang and C. H. Gao, “Thermal Analysis of Solution Copolymers of Styrene with N-Phenylmaleimide,” Journal of Applied Polymer Science, Vol. 83, No. 2, January 2002, pp. 417- 422. doi:10.1002/app.10065
- D. Wang, J. Zhu, Q. Yao and C. A. Wilkie, “A Comparison of Variuos Methods for the Preparation of Polypropylene and Poly (Methyl Methacrylate) Clay Nanocomposites,” Chemistry of Materials, Vol. 14, No. 9, 2002, pp. 3837-3843. doi:10.1021/cm011656+
- D. Wang, J. Zhu and C. A. Wilkie, “In Situ Reactive Blending to Prepare Poly (Styrene-Clay and Polypropylene-Clay Nanocomposites),” Polymer Degradation and Stabilization, Vol. 80, No. 1, 2003, pp. 171-182. doi:10. 1016/S0141-3910(02)00399-3
- Q. T. Nguyen and D. G. Baird, “Preparation of Polymer-Clay Nanocomposites and Their Properties,” Polymers for Advanced Technologies, Vol. 25, No. 4, Winter 2006, pp. 270-285. doi:10.1002/adv.20079
- E. Söylemez, N. Çaylak and Z. M. O. Rzayev, “Functional Copolymer/Organo-Silicate Nanoarchitectures. III. Synthesis and Characterization of Poly (İtaconic Acid-coBMA)/Dodecyl Amine-MMT Nano-composites by İnterlamellar Copolymerization Method,” Express Polymer Letters, Vol. 2, 2008, pp. 639-654.
- Z. M. O. Rzayev, “Nanotechnology Methods in Polymer Engineering,” Proceedimgs of 4th Nanaoscience & Nanotechnology Conference, June 2007, Bilkent, p. 154.
- Z. M. O. Rzayev, A. Guner, E. Söylemez and S. Kavlak, “Functional Copolymer/Organo-MMT Nanoarchitectures. III. Dynamic Mechanical Behaviour of Poly (IA-coBMA)-Organo-MMT Clay Nanocomposites,” Polymers for Advanced Technologies, 2010. doi:10,1002/pat.1618.
- J. F. Rabek, “Experimental Methods in Polymer Chemistry: Physical Principles and Applications,” John Wiley & Sons, New York, 1980, p. 507.
- R. Adhikri and G. H. Michler, “Polymer Nanocomposites Characterization by Microscopy,” Polymer Reviews, Vol. 49, No. 3, 2009, pp. 141-180. doi:10.1080/15583720903 048094
- O. Monticelli, Z. Musina, S. Russo and S. Bals, “On the Use of TEM in Characterization of Nanocomposites,” Material Letters, Vol. 61, 2007, pp. 3446-3450. doi:10. 1016/j.matlet.2006.11.086
- H. K. Can, A. L. Doğan, Z. M. O. Rzayev, A. H. Uner and A. Güner, “Synthesis and Antitumor Activity of Poly (3,4-Dihydro-2H-Pyran-Co-Maleicanhydride-Co-Vi-Nylacetate),” Journal of Applied Polymer Science, Vol. 96, 2005, pp. 2352-2359. doi:10.1002/app.21660
- Y. Cai, Y. Hu, J. Xiao, L. Song and W. Fan, “Morphology, Thermal and Mechanical Properties of Poly (Styrene-Acry-Lo-Nitrile) (SAN)/Clay Nanocomposites from Organic-Modified Montmorillonite,” Polymer-Plastics Technology and Engineering, Vol. 46, No. 5, 2007, pp. 541-548. doi:10.1080/03602550701298655
- J. W. Gilman, C. L. Jakson, A. B. Morgan, et al., “Flammability Properties of Polymer-Layered-Silicate Nanocomposites: Polypropylene and Polystyrene Nanocomposites,” Chemistry of Materials, Vol. 12, No. 7, 2000, pp. 1866-1873. doi:10.1021/cm0001760
- J. Lee, T. Takekoshi and E. P. Giannelis, “Fire Retardant Polyetherimide Nanocomposites,” Material Society Symposium Proceedings, Vol. 457, 1997, pp. 513-518.
NOTES
*Sponsors: SUPERFILM Package Ind. & Com. Inc.(Gaziantep,TR) and POLIMIKS Varnish Chem. Ind. & Com. Ltd. (Istanbul,TR). Authors thank for the financial supports via MS and PhD bursars.

