Journal of Modern Physics
Vol.4 No.2(2013), Article ID:27831,7 pages DOI:10.4236/jmp.2013.42029
Thermophysical Properties of NaCl, NaBr and NaF by γ-Ray Attenuation Technique
1Department of Physics, Kakatiya University, Warangal, India
2Central Instrumentation Centere (CIC), Kakatiya University, Warangal, India
Email: ngopikrishna2012@yahoo.com
Received November 14, 2012; revised December 16, 2012; accepted December 27, 2012
Keywords: Linear Attenuation Coefficient; Density; Thermal Expansion; γ-Ray Densitometer
ABSTRACT
The γ-ray densitometer has been designed and fabricated in our laboratory and carried out studies on temperature dependent γ-ray attenuation and thermo physical properties of NaCl, NaBr and NaF. The linear attenuation coefficients (μl) for the pellets of NaCl, NaBr and NaF as a function of temperature have been determined. The coefficients of temperature dependence of density have been reported. The variation of density and thermal expansion of NaCl, NaBr and NaF in the temperature range of 300 K - 1000 K have been studied and compared with results available in the literature. The temperature dependence of density and thermal expansion has been represented by linear equations. Volume thermal expansion coefficients have been reported.
1. Introduction
Density and Thermal expansion are fundamental thermo physical properties of solids. The study of temperature dependence of these properties is very important in understanding the temperature variation of other properties like elastic constants, refractive indices, dielectric constants, thermal conductivity, diffusion coefficients and other heat transfer dimensionless numbers. Thermal expansion of solids is of technical importance as it determines the thermal stability and thermal shock resistance of the material. In general the thermal expansion characteristics decide the choice of material for the construction of metrological instruments and in the choice of container material in nuclear fuel technology. Number of methods have evolved for the determination of density and thermal expansion of solids at high temperature like Archimedean method, pycnometry, dilatometry, electromagnetic levitation, Method of maximal pressure in gas bubble, method of sessile drop, hydrostatic weighing, high temperature electrostatic levitation and gamma ray densitometry. Thermal expansion studies on alkali halides have been reported by several workers using X-ray diffraction [1-3], dilatometry [4,5], Fabrey-Perot interference method [6] and by other theoretical models [7- 14]. Using γ-ray attenuation technique W. D. Drotning [15] measured thermal expansion of isotropic solid materials at high temperatures. He studied thermal expansion of Aluminum and type 303 stainless steel at high temperatures and such studies have been extended by him to study the thermal expansion of metals and glasses in the condensed state [16]. The γ-radiation attenuation technique for the determination of thermo physical properties in the condensed state offers several advantages over other methods at high temperatures. This is possible because the γ-ray is not in any kind of physical or thermal contact with the material and hence the thermal losses are also reduced and in addition eliminates sample and probe compatibility problem.
As NaCl, NaBr and NaF are isotropic solids; we extended, for the first time, the γ-ray attenuation technique, to carry out the studies on temperature variation of γ-ray attenuation and thermo physical properties of NaCl, NaBr and NaF. In this communication, we report the temperature dependence of linear attenuation coefficient of γ-radiation, density and thermal expansion of NaCl, NaBr and NaF in the temperature range 300 K - 1000 K. In order to carry out this work, we have designed and fabricated a γ-ray densitometer and a programmable temperature controlled furnace (PTC) which can reach up to a temperature of 1300 K in our laboratory. The data obtained in the present work for coefficient of linear thermal expansion of NaCl, NaBr and NaF as a function of temperature have been compared with experimental and theoretical data available in literature.
2. Theory
The technique of γ-ray attenuation method is based on the fundamental equation
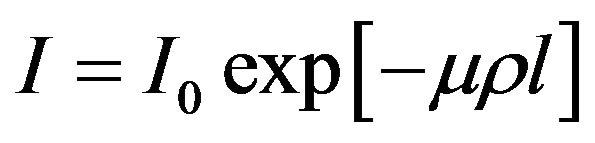 (1)
(1)
where I0, the intensity of γ-ray before passing through the sample, I, the intensity of γ-ray after passing through the sample, µ, the mass attenuation coefficient of the sample, ρ, the density of the sample and l, the thickness of the sample. It is clear from Equation (1) that any change in the temperature of the solid is accompanied by change in it’s density causing a change in the measured intensity. The density and thermal expansion of the materials studied in the present work have been determined following the method suggested by Drotning [15]. The relation between coefficient of volumetric thermal expansion (αρ) and coefficient of linear thermal expansion (α1) is given by
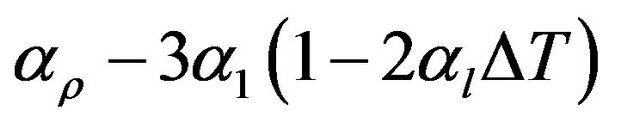 (2)
(2)
where αρ and αl are mean values over a temperature interval. 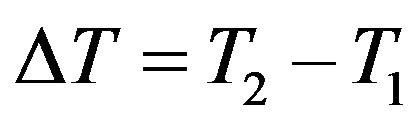 such that
such that
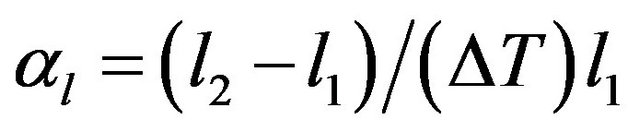 and
and 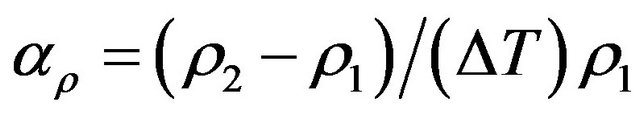
where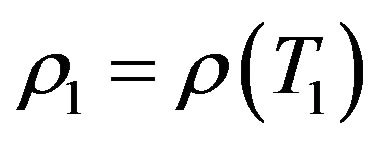 ,
, 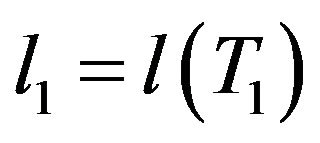 etc.
etc.
Rewriting Equation (2) as
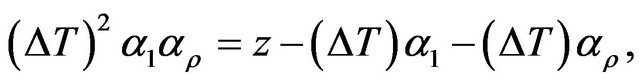 (3)
(3)
where z is defined by
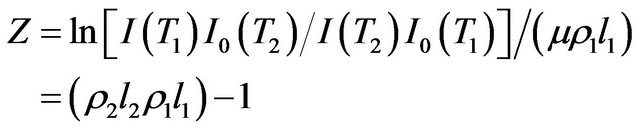 (4)
(4)
Substituting for αρ from Equation (2) gives
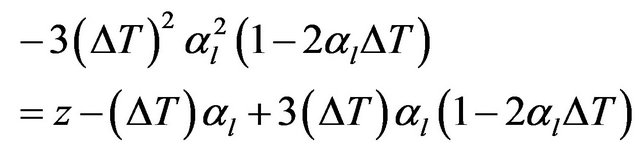 (5)
(5)
which can be rewritten as
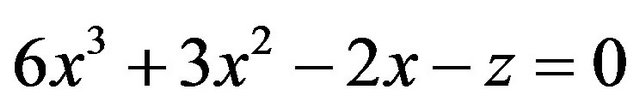 (6)
(6)
where
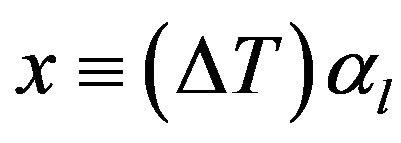 (7)
(7)
The intensities of γ-radiation with sample I and without sample I0 are recorded at every temperature. At room temperature T1, thickness of the sample l1 is measured and using Equation (1) µ is determined. Further measurements of I and I0 at different temperatures enable the determination of z by Equation (4) and hence x can be found from the solution of Equation (6). From the value of x, mean linear thermal expansion (αl) can be determined as a function of temperature.
3. Experimental
3.1. γ-Ray Densitometer
The experimental setup used for determination of density of materials utilizing γ-ray attenuation technique is called a γ-ray densitometer. A programmable temperature controlled furnace with sample inside the air tight quartz tube is introduced in the γ-radiation path allowing the beam to pass through the sample and to the detector without any interruption. The temperature of the sample is varied to study the attenuation at various temperatures. The block diagram of γ-ray densitometer is shown in Figure 1.
3.1.1. Stage-1
Consists of source housing, programmable temperature control (PTC) furnace with sample holder inside the air tight quartz tube, lead and stainless steel collimators, detector housing. The γ-radiation detector used in our study is a sodium iodide-thallium activated detector. The 3 inch diameter and 3 inch thick crystal is integrally coupled to a 3 inch diameter photo multiplier tube (PMT). The PMT has a 14 pin base and can be mounted on two types of PMT preamplifier units. The one used in our study is a coaxial in-line pre-amplifier. The detector has a resolution of 8.5% for 137Cs.
3.1.2. Stage-2
Consists of amplifier which is fed to multi-channel analyzer where γ-ray spectrum is analyzed.
3.1.3. Stage-3
Consists of PC with Nets win Software and printer to calculate and to store the data. PTC furnace is an electric muffle furnace consisting temperature sensors along with programmable logic controllers (PLC) used for monitoring and recording the temperature. Type K thermocouple is used in the furnace for wide operating temperatures. The operation of furnace is monitored with the help of electronic panel. The cross sectional view of γ-ray densitometer is shown in Figure 2.
The samples studied in the present work were in the form of pellets. The pellets were prepared with fine powder of NaCl, NaBr and NaF with a diameter of 20 mm with varying thicknesses with a die set by applying hydraulic press pressure. NaCl, NaBr and NaF pellets were sintered at a temperature of 800 K for densification. The pellet was then firmly mounted on the round sample holder made of flat stainless steel strip inserted into an air tight quartz tube. The precise sample temperature was measured using a thermocouple sensor. The thermocouple sensor tip was mounted on the sample holder ensuring a perfect physical contact with the sample for recording

Figure 1. Block Diagram of γ-ray densitometer.
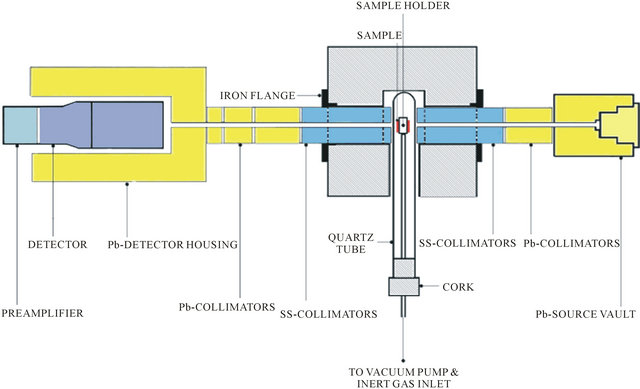
Figure 2. Cross-section view of γ-ray densitometer.
precise sample temperature. The sample holder along with the sample and thermocouple was slid through a cork into an air tight quartz tube and was fixed firmly. A diffusion pump was then connected to the sample holder tube for evacuation. For inert atmosphere, argon gas was introduced into the quartz tube through the sample holder tube. Then the quartz tube assembly along with the sample was slid into the programmable temperature controlled (PTC) furnace and fixed at appropriate position ensuring a perfect alignment of sample with collimation on either sides. The PTC furnace was programmed in such a way that the furnace temperature is increased by 25 K in every step starting from room temperature, and stabilizes there for a certain length of time. At each temperature the γ-ray counts of 137Cs with sample (I) and without sample (Io) were detected and recorded using a multichannel analyzer. Measurement of γ-ray attenuation counts at every step of temperature was repeated a minimum of nine times. The γ-ray counts were recorded while heating and cooling the sample. This procedure was repeated until the desired temperature range was covered in each case.
4. Results and Discussion
The results obtained for the temperature dependence of the linear attenuation coefficient (μl), density (ρ) and the coefficient of linear thermal expansion (α) of NaCl, NaBr and NaF are summarized in Table 1. The measurements have been carried out in solid phase only. The experimental data obtained in the present work for the density have been fit to a linear equation of the form
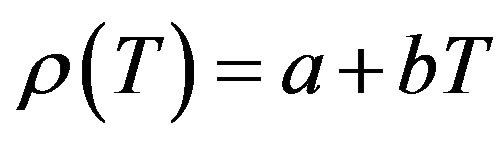 (8)
(8)
Since the measurements have been made in the limited temperature range the coefficient of volumetric thermal expansion (CVTE) was calculated using the equation
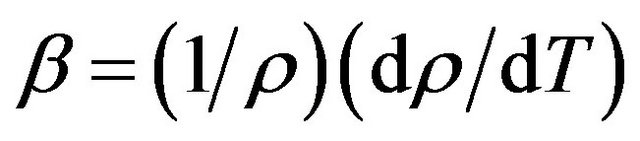 (9)
(9)
where  is the first derivative of density with respect to the absolute temperature which is determined from Equation (8). The variation of linear attenuation coefficient and variation of density with temperature of NaCl, NaBr and NaF have been shown in Figures 3 and 4 respectively. The mass attenuation coefficients (µ) of of NaCl, NaBr and NaF are found to be 7.428 × 10−3 m2∙kg−1, 7.164 × 10−3 m2∙kg−1 and 7.289 × 10−3 m2∙kg−1 respectively these results agree well with the calculated values 7.434 × 10−3 m2∙kg−1, 7.187 × 10−3 m2∙kg−1 and 7.367 × 10−3 m2∙kg−1 respectively from National Institute of Standards and Technology (NIST-X-COM).
is the first derivative of density with respect to the absolute temperature which is determined from Equation (8). The variation of linear attenuation coefficient and variation of density with temperature of NaCl, NaBr and NaF have been shown in Figures 3 and 4 respectively. The mass attenuation coefficients (µ) of of NaCl, NaBr and NaF are found to be 7.428 × 10−3 m2∙kg−1, 7.164 × 10−3 m2∙kg−1 and 7.289 × 10−3 m2∙kg−1 respectively these results agree well with the calculated values 7.434 × 10−3 m2∙kg−1, 7.187 × 10−3 m2∙kg−1 and 7.367 × 10−3 m2∙kg−1 respectively from National Institute of Standards and Technology (NIST-X-COM).
4.1. NaCl
The density of NaCl decreases from a value of 2165 kg∙m−3 at 300 K to a value of 1904 kg∙m−3 at 1000 K a
Table 1. Variation of linear attenuation coefficient, density and coefficient of linear thermal expansion of NaCl, NaBr and NaF with temperature.
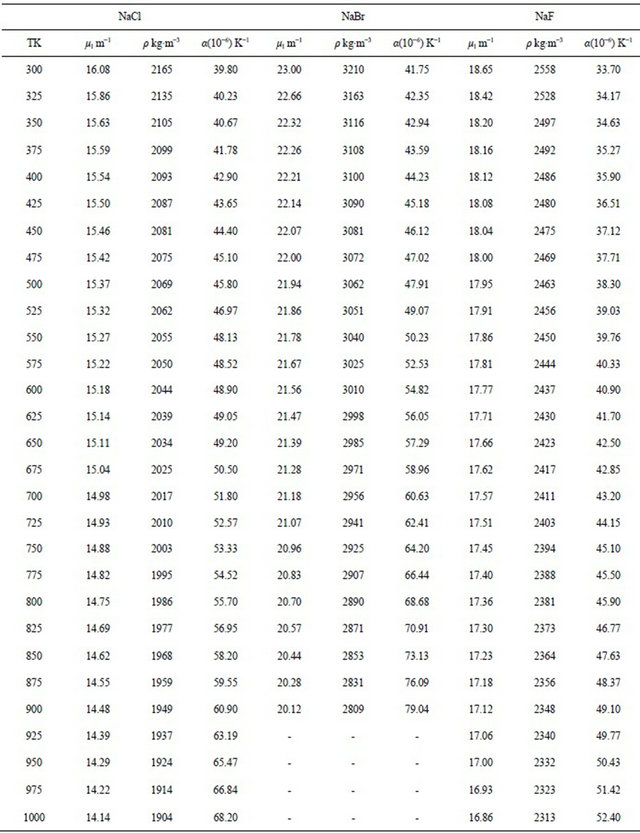
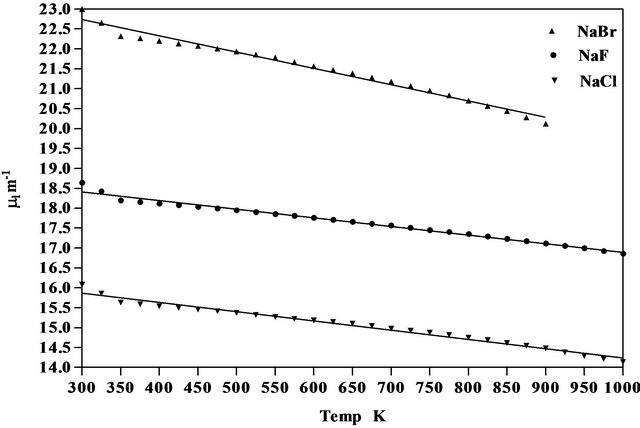
Figure 3. Variation of linear attenuation coefficient of NaCl, NaBr and NaF with temperature.
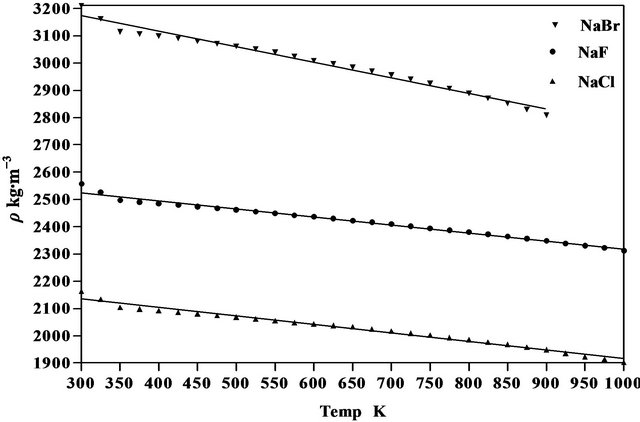
Figure 4. Variation of density of NaCl, NaBr and NaF with temperature.
decrease of about 12.06%. The temperature dependence of density is a negative linear function of temperature. For NaCl the temperature dependence of density is represented by linear equation
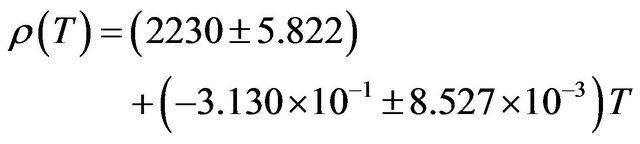 (10)
(10)
The coefficient of temperature dependence of density is −0.3130 kg∙m−3∙K−1 and the coefficient of volume thermal expansion is 1.446 × 10−4 K−1 in the temperature range 300 K - 1000 K. The thermal expansion increases linearly with temperature and the results on thermal expansion in the temperature range from 300 K to 1000 K have been analyzed by least squares method and is represented by the linear equation
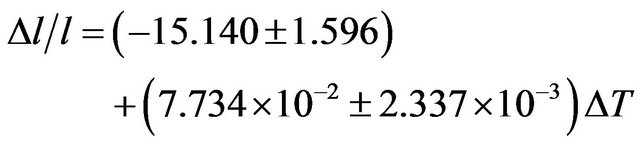 (11)
(11)
4.2. NaBr
The density of NaBr decreases from a value of 3210 kg∙m−3 at 300 K to a value of 2809 kg∙m−3 at 900 K a decrease of about 12.49%. The temperature dependence of density is a negative linear function of temperature. For NaBr the temperature dependence of density is represented by linear equation
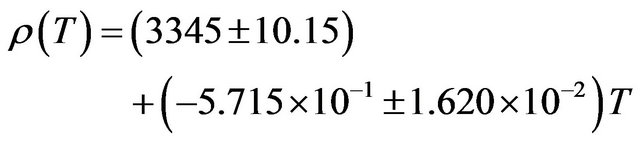 (12)
(12)
The coefficient of temperature dependence of density is −0.5715 kg∙m−3∙K−1 and the coefficient of volume thermal expansion is 1.78 × 10−4 K−1. The thermal expansion increases linearly with temperature and the results on thermal expansion in the temperature range from 300 K to 900 K have been analyzed by least squares method and is represented by the linear equation
 (13)
(13)
4.3. NaF
The density of NaF decreases from a value of 2558 kg∙m−3 at 300 K to a value of 2313 kg∙m−3 at 1000 K a decrease of about 9.58%. The temperature dependence of density is a negative linear function of temperature. For NaF the temperature dependence of density is represented by linear equation
 (14)
(14)
The coefficient of temperature dependence of density is −0.2962 kg∙m−3∙K−1 and the coefficient of volume thermal expansion is 1.16 × 10−4 K−1 in the temperature range 300 K - 1000 K. The thermal expansion increases linearly with temperature and the results on thermal expansion in the temperature range from 300 K to 1000 K have been analyzed by least squares method and is represented by the linear equation
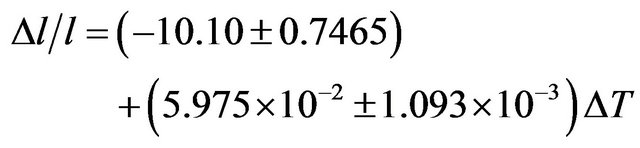 (15)
(15)
The results obtained for the coefficient of thermal expansion in the present work agree well with the values reported from X-ray studies, dilatometry and theoretical models [17-22] as seen from Figures 5-7 of NaCl, NaBr and NaF respectively. However, the results on variation of density and linear attenuation coefficient of NaCl, NaBr and NaF with temperature are not available from other methods for comparison.
5. Conclusions
The pellets were prepared with fine powder of NaCl,
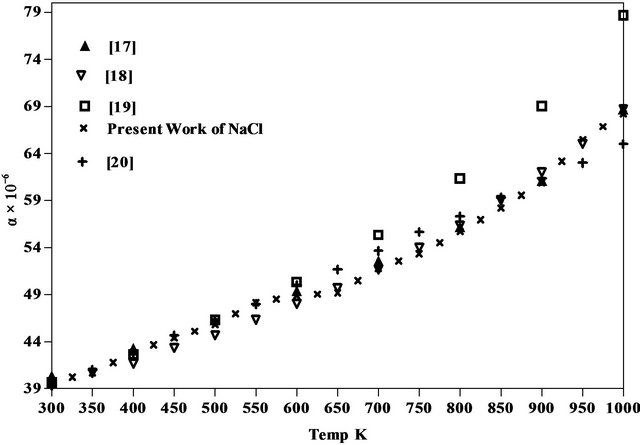
Figure 5. Comparison of coefficient of linear thermal expansion of NaCl with available data.
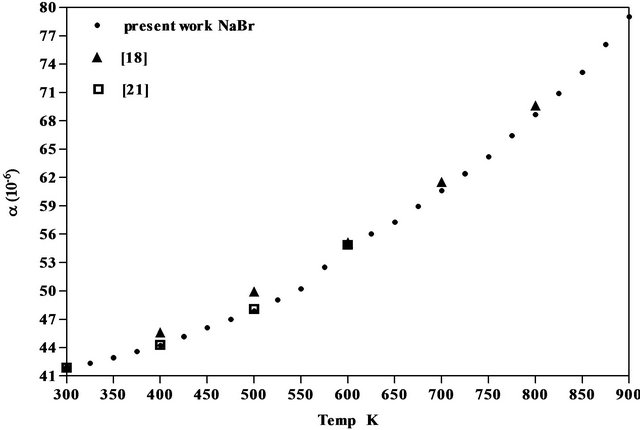
Figure 6. Comparison of coefficient of linear thermal expansion of NaBr with data available.
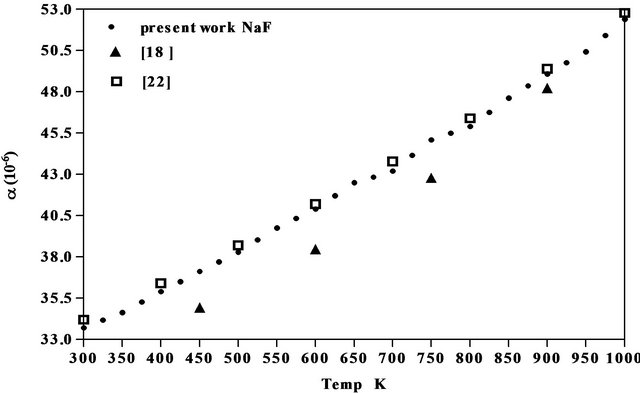
Figure 7. Comparison of coefficient of linear thermal expansion of NaF with data available.
NaBr and NaF with a diameter of 20 mm with varying thicknesses with a die set by applying hydraulic press pressure. The γ-ray attenuation measurements have been made using a γ-ray densitometer designed and fabricated in our laboratory. The results on the variation of linear attenuation coefficient, density and linear thermal expansion with temperature of these pellets have been reported and these variations have been represented by linear equations. The results on these pellets by using γ-ray attenuation technique are being reported for the first time.
6. Acknowledgements
The authors thank University Grants Commission (UGC), New Delhi for the financial assistance through Special Assistance Programme (SAP) No. F. 530/8/DRS/2009 (SAP-1).
REFERENCES
- M. E. Straumanis and A. Levins, “The Thermal Expansion of Bismuth by X-Ray Measurements,” Journal of Inorganic and General Chemistry, Vol. 238, 1938, p. 175.
- K. K. Srivastava and H. D. Merchant, “Thermal Expansion of Alkali Hallides above 300 K,” Journal of Physics and Chemistry of Solids, Vol. 34, No. 12, 1973, pp. 2069- 2073. doi:10.1016/S0022-3697(73)80055-1
- V. T. Deshpande, “Thermal Expansion of Sodium Flouride and Sodium Bromide,” Acta Crystallographica, Vol. 14, 1961, p. 794. doi:10.1107/S0365110X61002357
- G. K. White, “The Thermal Expansion of Alkali Hallides at Low Temperatures,” Proceedings of the Royal Society of London A, Vol. 286, No. 1405, 1965, pp. 204-217. doi:10.1098/rspa.1965.0139
- G. K. White and J. G. Collins, “The Thermal Expansion of Alkali Halides at Low Temperatures. II. Sodium, Rubidium and Caesium Halides,” Proceedings of the Royal Society of London A, Vol. 333, No. 1593, 1973, pp. 237- 259. doi:10.1098/rspa.1973.0060
- P. P. M. Meincke and G. M. Graham, “The Thermal Expansion of Alkali Halides,” Canadian Journal of Physics, Vol. 43, No. 10, 1965, pp. 1853-1866.
- A. M. Sherry and M. Kumar, “Analysis of Thermal Expansion for Alkalihalide Crystals Using the Isobaric Equation of State,” Journal of Physics and Chemistry of Solids, Vol. 52, No. 9, 1991, pp. 1145-1148. doi:10.1016/0022-3697(91)90047-4
- M. Kumar and S. P. Upadhyay, “Analysis of the Thermal Expansion Coefficient and It’S Temperature Dependence for Alkali Halides,” Physical Status Solidi (B), Vol. 181, No. 1, 1994, pp. 55-61.
- K. Wang and R. R. Reeber, “Thermal Expansion of Alkali Halides at High Pressure: NaCl as an Example,” Physics and Chemistry of Minerals, Vol. 23, No. 6, 1996, pp. 254-360. doi:10.1007/BF00199501
- M. Kumar and S. P. Upadhyay, “Pressure Dependence of Thermal Expansivity for Alkali Halides,” Journal of Physics and Chemistry of Solids, Vol. 54, No. 6, 1993, pp. 773-777. doi:10.1016/0022-3697(93)90140-M
- J. F. Vetelino, K. V. Namjoshi and S. S. Mitra, “ModeGruneisen Parameters and Thermalexpansion Coefficient of NaCl, CsCl, and Zinc-Blende-Type Crystals,” Journal of Applied Physics, Vol. 1, 1973, pp. 5141-5144.
- L. M. Thomas and J. Shanker, “Temperature Dependence of Elastic Constants and Thermal Expansion Coefficient for NaCl Crystals,” Physica Status Solidi (B), Vol. 195, No. 2, 2006, pp. 361-366.
- Z.-H. Fang, “Temperature Dependence of Inter Atomic Separation for Alkali Halides,” Physica Status Solidi (B), Vol. 241, No. 13, 2004, pp. 2886-2892.
- C. H. Nie, S. Y. Huang and W. Huang, “Temperature Dependence of Anderson Gruneisen Parameter for NaCl,” Applied Physics Research, Vol. 2, No. 1, 2010.
- W. D. Drotning, “Thermal Expansion of Solids at High Temperatures by the Gamma Attenuation Technique,” Review of Scientific Instruments, Vol. 50, No. 12, 1979, Article ID: 121567.
- W. D. Drotning, “Thermal Expansion of the Group IIb Liquid Metals Zinc, Cadmiumand Mercury,” Journal of the Less-Common Metals, Vol. 96, 1984, pp. 223-227. doi:10.1016/0022-5088(84)90198-X
- P. D. Pathak and N. G. Vasavada, “Thermal Expansion of NaCl, KCl and CsBr by X-Ray Diffraction and the Law of Corresponding States,” Acta Crystallographica Section A, Vol. 26, Part 6, 1970, pp. 655-658. doi:10.1107/S0567739470001602
- K. Sunil and B. S. Sharma, “Thermoelastic Properties of Alkali Halides at High Temperatures,” Indian Journal of Pure and Applied Physics, Vol. 50, No. 6, 2012, pp. 387- 397.
- S. K. Srivatsava and P. Sinha, “Analysis of Thermal Expansion of NaCl and KCl Crystals,” Indian Journal of Physics, Vol. 85, No. 8, 2011, pp. 1257-1265.
- S. K. Srivatsava, P. Sinha and M. Panwar, “Thermal Expansivity and Isothermal Bulk Modulus of Ionic Materials at High Temperatures,” Indian Journal of Pure and Applied Physics, Vol. 47, 2009, pp. 175-179.
- J. E. Rapp and H. D. Merchant, “Thermal Expansion of Alkali Halides from 70 to 570 K,” Journal of Applied Physics, Vol. 44, No. 9, 1973, pp. 3919-3923.
- P. D. Pathak, J. M. Trivedi and N. G. Vasavada, “Thermal Expansion of NaF and RbBr and Temperature Variation of the Frequency Spectrum of NaF,” Acta Crystallographica Section A, Vol. 29, No. A29, 1973, pp. 477-479.

