Applied Mathematics
Vol.07 No.10(2016), Article ID:67581,16 pages
10.4236/am.2016.710097
Review Study of Detection of Diabetes Models through Delay Differential Equations
Dimplekumar Chalishajar, David H. Geary, Geoffrey Cox
Department of Applied Mathematics, Virginia Military Institute, Lexington, VA, USA

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 28 October 2015; accepted 19 June 2016; published 22 June 2016
ABSTRACT
Mathematical models based on advanced differential equations are utilized to analyze the glucose-insulin regulatory system, and how it affects the detection of Type I and Type II diabetes. In this paper, we have incorporated several models of prominent mathematicians in this field of work. Three of these models are single time delays, where either there is a time delay of how long it takes insulin produced by the pancreas to take effect, or a delay in the glucose production after the insulin has taken effect on the body. Three other models are two-time delay models, and based on the specific models, the time delay takes place in some sort of insulin production delay or glucose production delay. The intent of this paper is to use these multiple delays to analyze the glucose-insulin regulatory system, and how if it is not properly working at any point, the high risk of diabetes becomes a reality.
Keywords:
Delay Differential Equations, MATLAB, Diabetes, Insulin-Glucose Model

1. Introduction
The human body needs to maintain a level of glucose concentration that is not too high in order for it to function properly. In order for glucose to be produced and utilized, insulin production and utilization must occur. If insulin is not produced, or not enough of it is produced, then proper glucose levels cannot be maintained, which leads to the disease of diabetes. Diabetes mellitus is a condition in which there is too much glucose in our blood. The pancreatic endocrine hormones insulin and glucagon are responsible for regulating and maintaining your body’s glucose concentration level. Insulin is produced in the pancreas, and is released from 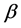 cells. These
cells. These 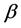 cells stimulate the cells to absorb enough glucose from the blood for the fuel or energy that they need. Insulin also stimulates the liver to absorb and store any left-over glucose. Glucagon is released when the glucose concentration level is too low. This stimulates the liver cells to release the left-over glucose into the blood, so cells will have enough energy to carry out their functions. If a person’s blood glucose concentration level is constantly above the normal range for humans, they suffer from the chronic condition, known as diabetes.
cells stimulate the cells to absorb enough glucose from the blood for the fuel or energy that they need. Insulin also stimulates the liver to absorb and store any left-over glucose. Glucagon is released when the glucose concentration level is too low. This stimulates the liver cells to release the left-over glucose into the blood, so cells will have enough energy to carry out their functions. If a person’s blood glucose concentration level is constantly above the normal range for humans, they suffer from the chronic condition, known as diabetes.
There are two type of diabetes. Type I diabetes, or insulin-dependent diabetes, is an autoimmune disease that occurs when the insulin-producing 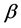 cells in the pancreas are attacked and destroyed by other cells in the body, causing the pancreas to produce little or no insulin at all. The cure for Type I diabetes is to have insulin replacement treatment for the rest of their life. This type of diabetes is known as juvenile diabetes, because it normally develops before the age of 40, often times before then. It is the least common of the two (10%).
cells in the pancreas are attacked and destroyed by other cells in the body, causing the pancreas to produce little or no insulin at all. The cure for Type I diabetes is to have insulin replacement treatment for the rest of their life. This type of diabetes is known as juvenile diabetes, because it normally develops before the age of 40, often times before then. It is the least common of the two (10%).
Type II, or non-insulin dependent diabetes, is a metabolic disorder which develops when the body can still produce insulin, but not enough, or when the insulin that is produced does not work properly, or better known as insulin resistance. Type II diabetes, is more likely to occur if there is a history of it in your family, and is often associated with obesity. Symptoms for Type II diabetes develop slowly over time, if at all, and occur mostly in people over the age of 40. Life expectancy for the Type I diabetes is reduced by about 15 years, and 10 years for Type II diabetes. If diabetes is left untreated or improperly managed, it can lead to heart disease, blindness, kidney disease, and non-traumatic limb amputations.
Due to these factors and harmful effects of diabetes, many researchers have been motivated to study the glucose-insulin endocrine metabolic regulatory system with hopes to better understand the mechanistic functions and causes of the dysfunction of the metabolic system. Many types of models have been made to better understand the role of the insulin-glucose regulatory system, including ordinary differential equations, and delay differential equation, both of which are included in this paper.
In the glucose-insulin endocrine metabolic regulatory system, the two pancreatic endocrine hormones, insulin and glucagon, are the primary regulatory factors. Numerous in-vivo and in-vitro experiments indicate that insulin secretion consists of two oscillations occurring with different time scales. One is rapid oscillations (5 - 15 min) and the second is ultradian oscillations (50 - 150 min). The mechanisms underlying both types of oscillations are still not fully understood. The rapid oscillations may arise from an intra-pancreatic pacemaker mechanism resulting in periodic secretary bursting of insulin from pancreatic 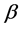 cells. This rapid oscillation is usually super imposed on the slow ultradian oscillation. The ultradian oscillations of insulin secretion are assumed to result from instability in the glucose-insulin endocrine hormones of the metabolic regulatory system.
cells. This rapid oscillation is usually super imposed on the slow ultradian oscillation. The ultradian oscillations of insulin secretion are assumed to result from instability in the glucose-insulin endocrine hormones of the metabolic regulatory system.
In this paper, we propose a number of delay differential equation (DDE) models by introducing two and three explicit time delays in order to model the glucose-insulin levels in the metabolic regulatory system. The two and three time delay models not only confirm many experimental observations, but also demonstrate robustness, and produce simulation profiles in better agreement with physiological data. As a result, we suspect that one of the possible causes of ultradian insulin secretion oscillations is the time delay of the insulin secretion simulated by the elevated glucose concentration. Our analysis shows that three time delay model is more accurate than two time delay.
In this paper we use several proposed Delay Differential Equation models (DDE) to show the delays of the metabolic system. We use single delay models in addition of multiple delay models to form a new proposed model combining the theories of the previous models used.
2. Delay Differential Equations
A delay differential equation (DDE) is an equation where the evolution of the system at a certain time, solution t, depends on the state of the system at an earlier time; say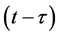 . This is distinct from the ordinary differential equation (ODE) where the derivatives depend only on the current value of the independent variable. The solution of the ODEs therefore requires knowledge of not only the current state, but also of the state of a certain time previously.
. This is distinct from the ordinary differential equation (ODE) where the derivatives depend only on the current value of the independent variable. The solution of the ODEs therefore requires knowledge of not only the current state, but also of the state of a certain time previously.
2.1. Differential Delay Equation Methods
Many approaches of DDE solutions start from the problem in the formulations
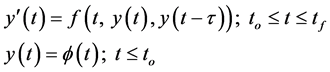 (2.1)
(2.1)
The function 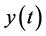 represents the same physical quantity that evolves overtime. The derivative
represents the same physical quantity that evolves overtime. The derivative  depends on past values of
depends on past values of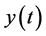 ,
,  represents the initial function, and
represents the initial function, and  is the delay term.
is the delay term.
The theory of DDEs has become an important area of investigation stimulated by their numerous applications to problem in mechanics, electric, and electronic engineering, medicines, biology, ecology, etc. Since many systems arising from realistic models heavily depend on history (which is characterized by the effect of finite (or infinite) delay on all equations), so there is a need to study partial functional delay differential system with the delay argument; and many evolution process characterized the fact that at certain moments of time they experience a change of state abruptly, this process is subject to short time perturbation whose duration is negligible in comparison with the duration of the process.
There are several approaches to finding numerical solutions to (2.1). These include the direct application of linear multiple step method and Runge-Kutta (4th order mainly) method for ODEs. If the delay is non-constant, the methods are combined with interpolation.
The first approaches to the numerical solution of DDEs of the above form were characterized by the direct application of formulas for ODEs, called linear multi-step methods. For example, Euler’s forward method, which is
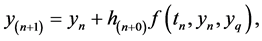 (2.2)
(2.2)
for the same integer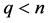 .
.
Beuman’s method ( [1] , Beuman et al., 2003) avoids the need for interpolation in solving the numerical solution of (2.1). To simplify first we consider the case of the constant delay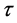 . Then the discontinuity points are
. Then the discontinuity points are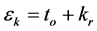 . In the first interval
. In the first interval
In the second interval 

Hence in general, over the interval 

where,
passing from k to k + 1 means shifting the interval of integration from 








2.2. An Example of a Delay Differential Equation
Since 


Therefore,
Using
Therefore,
In the next subinterval, 

The differential equation becomes
Since
Therefore,
2.3. Solving Differential Delay Equations in MATLAB
Using the routine dde23 ( [2] , Thompson et al., 2001) in MATLAB, it is easier to solve a large class of delay differential equations (DDE). The problem is restricted to solving systems of equations of the form
for constant delays 











The first formula used in the triple is an approximation used to advance the integration, written in terms of the increment function

The second formula is used only for selection the step size

The third formula is often described as a continuous extension of the first formula, and has the form

The coefficients, 






When the delay term is greater than the step size and we have an available approximation 
for all


stage and the formulas are explicit, the function 





When the step size is greater than the smallest delay, the “history” term 






DDE 23 predicts 

A typical introduction of DDE 23 has the form
sol = DDE23(ddefile, lags, history, tspan);
where ddefile is the name of the function for evaluation the DDE, lags is the constant delays, supplied as an array. history can be specified as the name of a function that evaluates the solution and returns it as a column vector, and tspan is the interval of integration. See ( [4] , Wang et al., 2007) chapter Numerical Results for some examples of using DDE 23 to solve some simple delay differential equation models.
3. Mathematical Models for Diabetes
3.1. Modeling through Ordinary Differential Equations
The insulin-glucose model that is used in this paper was originally developed by Sturis. The purpose of the model was to show the slow oscillations that take place inside of the pancreas and liver, and could give some insight on how they can lead to diabetes if not functioning properly ( [5] , Tolic et al., 2000).
The secretion of insulin in the glucose-insulin endocrine metabolic system occurs in an oscillatory manner over a range of 50 - 150 minutes. Two time delays exist in the time. One is due to the electric action inside of the 
The two time delay model of the glucose-insulin regulatory system can be seen in Figure 1.
There are three feedback loops in this model: glucose stimulates pancreatic insulin secretion, insulin stimulates glucose uptake and inhibits hepatic glucose production, and glucose enhances its own uptake. The system also includes two significant time delays: the first is the fact that the physiological action of insulin after the utilization of glucose is correlated with the concentration of insulin. The second is associated with the time lag between the appearance of insulin in the plasma and its inhibitory effect on the hepatic glucose production ( [5] , Tolic et al., 2000).
The model has three main variables: the amount of glucose in the plasma and intercellular space, G, the amount of insulin in the plasma, 


The equations describing the dynamics of the model are



Figure 1. Two time delay glucose-insulin regulatory system model. The dash-dot-dot lines indicate that insulin inhibits hepatic glucose production with a time delay; the dash-dot lines indicate insulin secretion from the β cells simulated by elevated glucose concentration levels and the short dashed line indicates the insulin induced glucose utilization in cells with time delay; the dashed lines indicate low glucose concentration levels triggering α cells in the pancreas to release glucagon.



Insulin production: Insulin can only be produced by 




Insulin degradation and clearance: The liver and the kidney are the primary sites of insulin degradation and clearance. Insulin that is not cleared by the liver or the kidney is eventually removed from other tissues (i.e. muscles). Insulin degradation is process of the kidney and liver that controls insulin action by inactivating and removing the excess of the hormone. Insulin degradation is directly proportional to the insulin concentration. Insulin clearance is the process of regulating the cellular response to the hormone by decreasing insulin availability.
Glucose production: The formation of glucose in the body is caused by two sources. The first is the consumption of carbohydrates, or compounds of carbon, oxygen and hydrogen, that form sugars, starches and fibers. These carbohydrates are absorbed into the bloodstream through hydrolysis. The consumption of carbohydrates takes place through meal ingestion, oral intake (coffee with sugar), etc. The second source of glucose production is the liver. When level of glucose concentration in the bloodstream drops, the 

Glucose Utilization: Glucose utilization, like glucose production consists of two parts, insulin-independent utilization, and insulin-dependent utilization. Insulin-independent glucose consumers are the brain and nerve cells. This glucose intake of the brain and nerve cells is given as

This function represents the dependency on the glucose concentration alone ( [6] , Li et al., 2006). Glucose utilization for muscles and fat tissues depend not only on glucose concentrations levels, but also insulin concentration levels. The 

The insulin dependent term is given as

Production of glucose controlled by insulin concentration (I) is stated as hepatic glucose production. In healthy people the pancreas has the ability to constantly measure levels of glucose in the bloodstream. The pancreas then responds to the glucose levels and releases a certain amount of insulin to counteract the glucose intake. The formula to show the above reaction is

3.2. Analysis of Insulin Glucose Feedback Model
In order to fully understand certain feature of the model, the insulin-glucose feedback model has been simplified. The simplified model has two important properties: it is analytically tractable, and it shows the same main characteristics as the original model (i.e. self-sustained oscillations and values of the state variables in the same range as the original model) ( [5] , Tolic et al., 2000).
The function 




The function 



Table 1. Parameters of the functions in Equations (7)-(11). Values (data) are obtained experimentally in laboratory.
Table 2. Commonly accepted experimental values used in these models.
standard model to for the detection of diabetes without delay is studied and analyzed. It is well accepted, reasonable and reliable model, refer [7] .
The simplified model is






The time evolution of plasma glucose and insulin concentration that result from the simplified model does not differ significantly from the results of the original model.
In the simulation of an exogenous insulin infusion, equation, 

where m = 21 mU/min. A was set to zero for the constant infusion and to 0.3 for the oscillatory infusion. The period T = 120 min. Since the glucose infusion rate in the experiments equals 420 mg/min, using Table 1 and Table 2 (experimental data). The parameter p in Equation (3.14) was set to 285 mg/min to compensate for the difference between the rate of the glucose infusion of 420 and 216 mg/min ( [5] , Tolic et al., 2000).
From an exact solution of the two first order differential equations (3.13) and (3.18), the numerical solutions to (3.15)-(3.17) were computed. For large t, the solutions are periodic functions with period T and may be expressed in the form

The only equation in the simplified model with non-linear terms is Equation (3.14). It can be expressed in the form

where

and

Equation (20) has the general solution

where


and

3.3. Modeling through Delay Differential Equations
In section 3.1 we have discussed the dynamics of the insulin-glucose feedback system through Equations (1)-(6). The hepatic glucose production time delay is stimulated by introducing three auxiliary variables, 

Then
When
Using Taylor’s Series Expansion for the function of one variable, we get
I: Explicit Single Time Delay Differential Equation Model
In [8] , Engleborghs et al. (2001) replaced the auxiliary variables
where the functions











This model consists of two negative feedback loops, describing the effects of insulin on glucose utilization and glucose production, respectively. Both loops include the stimulatory effect of glucose on insulin secretion. The model mimics the delayed insulin-dependent glucose uptake by splitting the insulin into two separate compartments, plasma and interstitial space. The hepatic glucose production time delay is simulated by introducing three variables 
Unfortunately, this model overlooked the time delay from glucose stimulated insulin release to the delayed insulin-dependent glucose uptake. Due to the complex chemical reactions of the 
II. Single Time Delay Proposed by Drozdov and Khanina (1995) [10]
The rate of insulin secretion 


The numerator in the right-hand side of the numerator in 
The function 
The rate of glucose production 





The delay in this proposed model is a delay in the 




The model is as follows
where 


III: Explicit Two Time Delay Differential Equation Model
This is a single delay DDE model used for insulin therapies for both Type I and Type II diabetes mellitus and insulin degregation rate assumes Michaelis-Menten kinetics. The model equations proposed by Wang, Li, and Kuang (2009) [11] , is as follows
The major analytical results of this model were to obtain a sufficient condition for global asymptotical stability induced by a Liapunov function with the hepatic glucose production time delay satisfying the cases when 





For Type I diabetes, b = 0, (means no insulin is secreted from the pancreas). For Type IIdiabetes,



IV: Alternative Explicit Two Time Delay Differential Equation Model (Insulin Therapies Related Model)
The idea of insulin therapy is to mimic the normal reaction of the beta cells in the pancreas when they are stimulated by an increase in glucose concentration of the blood, for example after meal ingestion. Numerous experiences have demonstrated that insulin is released from beta cells in two oscillatory models: pulsatile oscillations and ultradin oscillations. Insulin therapies must mimic the insulin secretion at these two time scales and are normally introduced based on clinical experiences, although mathematical models have been proposed for some specific situations ( [4] , Wang et al., 2007).
In the paper by Wang, the following generic model is put forward to simulate the pancreatic insulin secretion with insulin infusion after blood glucose concentration increases for Type I diabetes. We model the effect of time delay t2 in glucose utilization by 

The notations in models (III) and (IV) are the same as the models discussed previously. Data are used from Table 1 and Table 2 to set up the MATLAB codes.
V: Alternative Explicit Two Time Delay Differential Equation Model (Two-Time Delay Model proposed by Li, Kuang, Mason (2006)) [6] .
Li, Kuang, and Mason consider two time delays: 




The second time delay, 

This model suggests that the time delay from insulin secretion stimulated by glucose to the insulin becoming “remote insulin” is not negligible; especially the delay of insulin secretion triggered by elevated glucose. Therefore, we suspect that one of the possible causes of ultradian insulin secretion oscillations is the time delay of the insulin secretion stimulated by the elevated glucose concentration.
In this two time delay model, 




Based on our numerical simulations and analysis, we suspect that time total time delay, 
VI: Another Alternative Two Time Delay Differential Equation Model, (Palumbo, Panunzi, DeGaetano [2004]) [12]
To our knowledge, there are no mathematical models considering 

where 

There are two possibilities of delay of insulin release by the pancreas 


This model describes the dynamics of the glucose-insulin feedback system which involves an equation for the number of 

VII: Multiple Time Delay Model
The final model, which has not been considered, is a multiple delay differential equation, made from combinations of the previous four models given. The model is as follows:
This model is made up from all of the previous sections, taking common delays from each and making one model out of the previous five, and adding one additional delay in








This function represents all the delays we have presented so far in this paper. The time delays in the 







The delay in the 



Remark: The parameter values assumed in Figures 1-3 are experimental values. Such values may have significant effects on the results studied in this paper.
Summary: In this paper, we studied delay differential equation (DDE) models by introducing two and three explicit time delays and model the glucose-insulin endocrine metabolic regulatory system. The three-time delay model not only confirms many exciting experimental observations, but also demonstrates robustness, and produces simulation profiles in better agreement with observed data. As a result, we suspect that one of the possible causes of ultradian insulin secretion oscillations is the time delay of the insulin secretion simulated by the elevated glucose concentration. Like the two-time delay models, three-time delay models can also be generalized to get more accuracy in the detection of diabetes.
Figure 2. Glucose profiles [models (IV)-(VII)].
Figure 3. Insulin profiles [models (IV)-(VII)].
Acknowledgements
We are thankful to Dr. Roger Thelwell (James Madison University, VA) for working with us throughout the process to set up the MATLAB codes.
Cite this paper
Dimplekumar Chalishajar,David H. Geary,Geoffrey Cox, (2016) Review Study of Detection of Diabetes Models through Delay Differential Equations. Applied Mathematics,07,1087-1102. doi: 10.4236/am.2016.710097
References
- 1. Beuman, A. and Zennaro, M. (2003) Numerical Methods for Delay Differential Equations. Oxford University Press, NY, USA.
- 2. Thompson, S. and Shampine, L.F. Solving Delay Differential Equations with dde23.
http://www.radford.edu/thompson/webddes/tutorial - 3. Thompson, S. and Shampine, L.F. (2001) Solving Delay Differential Equations in MATLAB. Applied Numerical Mathematics, 37, 441-458.
- 4. Wang, H.Y., Li, J.X. and Kuang, Y. (2007) Mathematical Modeling & Qualitative Analysis of Insulin Therapies. Mathematical Biosciences, 210, 17-33.
http://dx.doi.org/10.1016/j.mbs.2007.05.008 - 5. Tolic, I.M., Mosekilde, E. and Sturis, J. (2000) Modeling the Insulin-Glucose Feeback System: The Significance of Pulsatile Insulin Secretion. Journal of Theoretical Biology, 207, 361-375.
http://dx.doi.org/10.1006/jtbi.2000.2180 - 6. Li, J.X., Kuang, Y. and Mason, C.C. (2006) Modeling the Glucose-insulin Regulatory System and Ultradin Insulin Secretory Oscillations with Two Explicit Time Delays. Journal of Theoretical Biology, 242, 722-735.
http://dx.doi.org/10.1016/j.jtbi.2006.04.002 - 7. Chalishajar, D. and Stanford, A. (2014) Mathematical Analysis of Insulin-Glucose Feedback System of Diabetes. International Journal of Engineering and Applied Sciences, 5, 36-58.
- 8. Englrborgh, K., Lemaire, V., Belair, J. and Roose, D. (2001) Numerical Bifurcation Analysis of Delay Differential Equations Arising from Physiological Modeling. Journal of Mathematical Biology, 42, 361-385.
http://dx.doi.org/10.1007/s002850000072 - 9. Sturis, J., Scheen, A.J., Leproult, R., Polonsky, J.S. and Van Cauter, E. (1995) 24-Hours Glucose Profilesduring Continuous or Oscillatory Insulin Infusion. Journal of Clinical Investigation, 95, 1464-1471.
http://dx.doi.org/10.1172/JCI117817 - 10. Drozdov, A. and Khanina, H. (1995) A Model for Ultradin Oscillations of Insulin and Glucose. Mathematical and Computer Modelling, 22, 23-38.
http://dx.doi.org/10.1016/0895-7177(95)00108-E - 11. Wang, H.Y., Li, J.X. and Kuang, Y. (2009) Enhanced Modeling of the Glucose-Insulin System and Its Applications in Insulin Therapies. Journal of Biological Dynamics, 3, 22-38.
http://dx.doi.org/10.1080/17513750802101927 - 12. Palumbo, P., Panunzi, S. and De Gaetano, A. (2004) Qualitative Behavior of a Family of Delay-Differential Models of the Glucose-Insulin System. Istituto Di Analisi Dei Sistemi Ed Informatica, 620, 3-28.








































































