Advances in Bioscience and Biotechnology
Vol.4 No.4A(2013), Article ID:30626,4 pages DOI:10.4236/abb.2013.44A009
Combination of specific monoclonal antibodies allow identification of soluble aggregates of Aβ1-42 by sandwich ELISA
![]()
1Department of Applied Molecular Chemistry, Graduate School of Industrial Technology, Nihon University, Chiba, Japan
2Department of Applied Molecular Chemistry, College of Industrial Technology, Nihon University, Chiba, Japan
3Department of Sustainable Engineering, College of Industrial Technology, Nihon University, Chiba, Japan
4Immuno Probe Co., Ltd., Ranzan, Japan
5Department of Social Medicine, School of Public Health, Zhejiang University, Hangzhou, China
Email: *kouno.hideki@nihon-u.ac.jp
Copyright © 2013 Takenori Shimizu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 16th, 2013; revised March 11th, 2013; accepted April 13th, 2013
Keywords: Aβ1-42; Monoclonal Antibody; Soluble Aggregates; ELISA
ABSTRACT
Aggregate amyloid beta protein1-42 (Aβ1-42) can typically be found in the early stage of Alzheimer’s disease (AD). Aβ1-42 self-assembles and is highly toxic to neurons. Thus, recognizing aggregated Aβ1-42 is very important for elucidation of Aβ1-42 structure and for the diagnosis of AD. In this study, the specificity of the 79-3 monoclonal antibody against soluble aggregate Aβ1-42 was measured by sandwich Enzyme-Linked Immuno Sorbent Assay (ELISA). Eight monoclonal antibodies against both soluble aggregates and amorphous aggregates were used as primary antibodies. Soluble aggregates and amorphous aggregates were used as antigen. As secondary antibody, HRP was labeled with the 79-3 monoclonal antibody. The reactivity of the 79-3 monoclonal antibody against soluble aggregates was confirmed in all combinations, but little reactivity against amorphous aggregates was found. Furthermore, we performed the above sandwich ELISA using the 37-11 antibody, which is reactive against large oval aggregates (LOA) that occur in micro aggregates, instead of the 79-3 antibody. The 77-3 antibody is 1 of the 8 monoclonal antibodies against soluble aggregates; amorphous aggregates also reacted with the 37-11 antibody. These results indicated that soluble aggregates are specifically recognized by a combination of different antibodies. The combined use of these antibodies can be applied to the diagnosis of AD and to defining the structure of the Aβ1-42.
1. INTRODUCTION
Amyloid beta protein is 1 of the proteins that cause Alzheimer’s disease. Aβ is produced in the human brain and is a major constituent of senile plaques [1,2]. There are mainly 2 types of Aβ, viz. Aβ1-40 and Aβ1-42. Aβ1-42 shows greater toxicity against neuronal cells than do Aβ1-40 [3]. Aβ1-42 represents the monomeric form of the protein; it can, however, also form oligomers from these monomers [4].
In solution, Aβ1-42 is aggregated by self-assembly, and various sizes of Aβ1-42 aggregate have been reported. For example, Aβ-derived diffusible ligands (ADDLs) occur as 17 kDa and 42 kDa aggregates [5]. Low molecular weight (LMW) aggregates are oligomers of Aβ1-42 of approximately 14 kDa [6]. The globulomer in which methionine at the N-terminus of Aβ1-42 is displaced, is approximately 64 kDa, while non-displaced globulomers are 38 - 48 kDa [7,8]. Medium-sized aggregates of Aβ1-42, ASPDs, are 158 - 669 kDa, and aggregated Aβ1-42 of 80 - 500 kDa have also been reported [9,10]. In the class of large Aβ1-42 aggregates, there are large oval aggregates (LOA) that contain an added Aβ16-20 (KLVFF) peptide, as well as aggregates of Aβ1-42 that contain an added Fc-KLVFF [11,12]. The Aβ16-20 (KLVFF) peptide binds to the Aβ1-42 β-sheet and inhibits Aβ1-42 fibril formation. Recently, soluble Aβ has been reported to occur during the process of Aβ aggregation, which is more toxic than the fibril and monomer forms [13,14].
Immunoassays are an effective way to capture the causative agent of a disease, and can be used to recognize the structure of Aβ1-42 by capturing the aggregates of different sizes. It is important that the size of the aggregated Aβ1-42 is identified in this way [15]. Antibodies against various Aβ1-42 aggregates have been produced to date. Among the small Aβ1-42 aggregates, the antibody 6E10 has been used to capture ADDLs and N-Met-Aβ1-42 globulomers, which form comparatively small oligomers. In the context of medium-sized Aβ1-42 aggregates, some antibodies have been reported, but it is difficult to produce a specific antibody against aggregates in this sizerange.
Here, we prepared soluble aggregates and amorphous aggregates of Aβ1-42. Soluble aggregates smaller than LOA were recognized by a combination of antibodies against soluble aggregates, those against soluble aggregates and amorphous aggregates, and those against LOA, by sandwich ELISA. This approach allows structural analysis of Aβ1-42 aggregates and facilitates diagnosis of AD.
2. MATERIALS AND METHODS
2.1. Preparation of Aggregated Aβ1-42
Aβ1-42 was purchased from AnyGen Co. Ltd., Korea. Amorphous aggregates were prepared as follows. A suspension of 0.44 mM Aβ1-42 was prepared in Milli Q water and incubated for 30 min at 4˚C. The solution was prepared in Dulbecco’s PBS without Ca2+ or Mg2+ (Wako, Osaka, Japan) 0.22 mM. To this solution, 2.2 mM of Aβ16-20 (AnyGen Co. Ltd.) was added and the mixture stirred at 7 rpm for 16 h, at 37˚C [11].
For soluble aggregates, Aβ1-42 (100 μg) was dissolved in 200 μl of 1,1,1,3,3,3-hexafluoro-2-propanol. This solution was incubated for 16 h at 4˚C and then incubated for 3 h at 37˚C [16]. Thereafter, the solution was lyophilized; these steps were repeated twice. After the final lyophilization, a solution of Aβ1-42 was prepared at a concentration of 1 mg/ml in Milli Q water.
2.2. Reactivity of Monoclonal Antibodies during the Preparation of Aggregated and Fibrillar Aβ1-42
The 79-3 monoclonal antibody against soluble aggregates and the 78-6 monoclonal antibody against soluble aggregate and amorphous aggregate were used. Reactivity of these monoclonal antibodies were assessed during the preparation of aggregated and fibrillar Aβ1-42. The measurements were carried out every 4 h, up to 24 h.
2.3. ELISA
As primary antibodies, 8 monoclonal antibodies against soluble aggregates and amorphous aggregates were used. These were used to coat 96-well plates (F96 MAXISORP NUNC-IMMUNO PLATE; Thermo Fisher SCIENTIFIC Inc. Rochester NY) (100 μl/well, at a concentration of 2 μg/ml). The plates were incubated for 60 min at 37˚C and washed with PBS containing 0.05% Tween. Blocking buffer (Immunoblock; DS Pharma Biomedical) were used to coat the 96 well plates (200 μl/well) and incubated for 60 min at 37˚C. The plates were washed with PBS containing 0.05% Tween 5 times. Soluble aggregates and aggregates were then used to coated these prepared 96-well plates (100 μl/well at a concentration of 1 μg/ml). The plates were then incubated for 60 min at 37˚C, and washed with PBS containing 0.05% Tween 5 times.
As secondary antibodies, the 37-11 and 79-3 antibodies labeled with horse-radish peroxidase (HRP) were used to coat the 96-well plates (100 μl/well at a concentration of 0.144 μg/ml). The plates were incubated for 60 min at 37˚C and were washed with PBS containing 0.05% Tween 5 times. Citrate buffer solution (25 ml), containing 10 mg O-phenylalanine dichloride (SigmaAldrich, St. Louis, MO) and 30% hydrogen peroxide, was then used to coat the wells (100 µl/well) and the plates were incubated for 20 min at room temperature. Absorbance was then measured using a microplate reader at 492 nm.
3. RESULTS AND DISCUSSION
3.1. Reactivity of the 79-3 and 78-6 Monoclonal Antibodies during Preparation of Aggregated Aβ1-42
The size of the amorphous aggregates of Aβ1-42 in the presence of Aβ16-20-containing LOA is 50 - 400 nm. The size of the soluble aggregates is nearly all approximately 50 nm, although some are 300 nm. The reactivity of the 79-3 and 78-6 monoclonal antibodies decreased during preparation of the amorphous aggregate of Aβ1-42 in the presence of Aβ16-20-containing LOA (Figure 1). After 16 h, the reactivity of the 79-3 monoclonal antibody was lower than that of the 78-6 antibody. This result indicated that the 79-3 monoclonal antibody is more specific for the soluble aggregate than the 78-6 monoclonal antibody.
The 78-6 antibody reacts not only with soluble aggregates, but also with amorphous aggregates.
3.2. Measurement of Soluble Aggregates by Sandwich ELISA
To confirm the specificity of the 79-3 monoclonal antibody for soluble aggregates of Aβ1-42, we performed an evaluation of the specificity of the 79-3 monoclonal an-
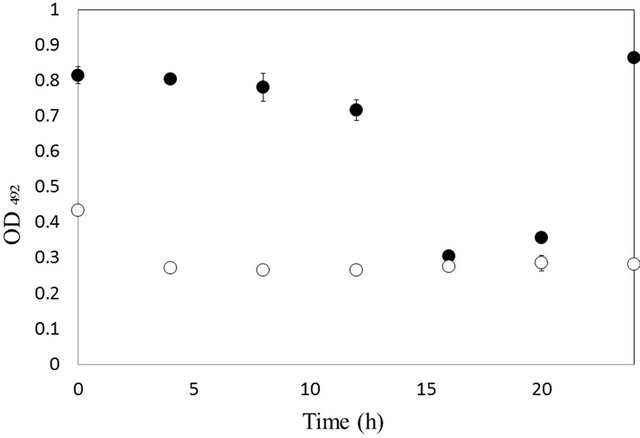
Figure 1. Reactivity of the antibodies 79-3 (○), against soluble aggregates, and 78-6 (●), against soluble aggregates and amorphous aggregates, in the presence of 2.2 mM Aβ16-20.
tibody labeled with HRP against soluble aggregates of Aβ1-42 with that of the 78-6 monoclonal antibody, using sandwich ELISA. As antigen, soluble aggregates and amorphous aggregates were used. As primary antibodies, 72-10, 73-1, 76-3, 80-4, 75-2, 77-3, and 83-3, which react against both soluble aggregates and amorphous aggregates, as does the 78-6 antibody, were used. The reactivity of the 79-3 monoclonal antibody against soluble aggregates was high (Figure 2); on the other hand, the reactivity of the 79-3 monoclonal antibody against amorphous aggregates was low. By combining the different monoclonal antibodies, it was possible to detect a combination of soluble Aβ1-42 aggregates. These results indicated that these 2 types of antibodies have different epitope sites.
The structure and size of soluble aggregates are different from those of amorphous aggregates. 4G8 and 82E1 are specific monoclonal antibodies against the N-terminus of Aβ [17]. 11A1 is a specific monoclonal antibody directed against positions 22 and 23 of Aβ1-42 oligomers [18]. Thus, the 8 monoclonal antibodies used as primary antibodies reacted with Aβ1-42 of various dimensions. The 79-3 monoclonal antibody recognized only soluble aggregates. Therefore, the 79-3 monoclonal antibody recognizes an epitope on the surface of Aβ1-42.
Furthermore, we have attempted to construct a similar sandwich ELISA using the monoclonal antibodies that reacts against LOA other than 79-3, such as 37-11.
HRP was used to label the 37-11 monoclonal antibody, as for 79-3, and the reactivity of the 37-11 antibody was evaluated against those of the 9 monoclonal antibodies that react against soluble aggregates and amorphous aggregates, using sandwich ELISA. These results are shown in Figure 3. The results indicated that a combination of the 37-11 and 77-3 antibodies yielded the highest reactivity, whereas those of the other were low. The 37-11 monoclonal antibody is specific for LOA. How-
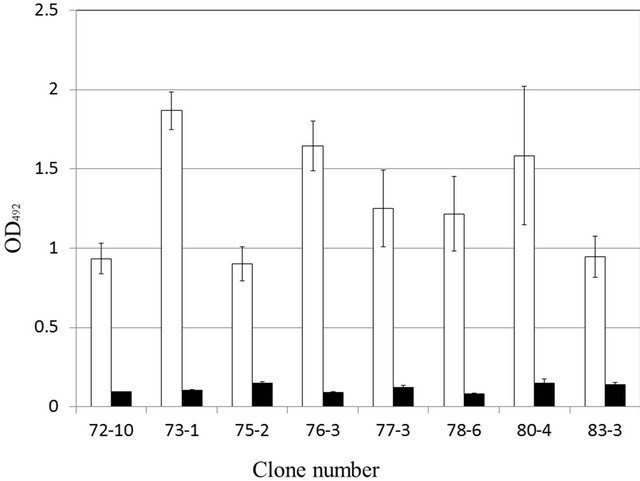
Figure 2. Reactivity of the 79-3 monoclonal antibody against soluble aggregates (□) and amorphous aggregates (■) by sandwich ELISA.
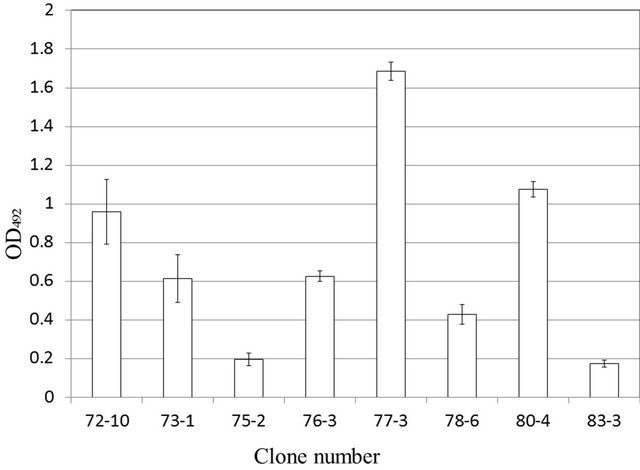
Figure 3. Reactivity of the 37-11 monoclonal antibody against soluble aggregates by sandwich ELISA.
ever it is possible that the 37-11 antibody could recognize soluble aggregates different from LOA by changing the measurement system of the sandwich ELISA. These results suggested that the epitopes of the 37-11 and 77-3 monoclonal antibodies differ.
In this study, we were able to recognize soluble aggregates by sandwich ELISA. Further refinement of the size of aggregated Aβ1-42 would be possible and may facilitate the analysis of the structure of Aβ1-42 and the diagnosis of AD.
4. ACKNOWLEDGEMENTS
We thank the president of Immuno Probe Co. Ltd., Mr. Hiroshi Nomura, for technical assistance in producing the monoclonal antibodies.
REFERENCES
- Hardy, J.A. and Higgins, G.A. (1992) Alzheimer’s disease: The amyloid cascade hypothesis. Science, 256, 184- 185. doi:10.1126/science.1566067
- Hardy, J. and Selkoe, D.J. (2002) The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science, 297, 353-356. doi:10.1126/science.1072994
- Jhoo, H., Kim, H.C., Nabeshima, T., Yamada, K., Shin, E.J., Jhoo, W.K., Kim, W., Kang, K.S., Jo, S.A. and Woo, J.I. (2004) Beta-amyloid (1-42)-induced learning and memory deficits in mice: Involvement of oxidative burdens in the hippocampus and cerebral cortex. Behavioural Brain Research, 155, 185-196. doi:10.1016/j.bbr.2004.04.012
- Takahashi, T. and Mihara, H. (2008) Peptide and protein mimetics inhibiting amyloid-peptide aggregation. Accounts of Chemical Research, 41, 1309-1318. doi:10.1021/ar8000475
- LeVine 3rd, H. (1993) Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: Detection of amyloid aggregation in solution. Protein Science, 2, 404-410. doi:10.1002/pro.5560020312
- Gong, Y., Chang, L., Viola, K.L., Lacor, P.N., Lambert, M.P., Finch, C.E., Krafft, G.A. and Klein, W.L. (2003) Alzheimer’s disease-affected brain: Presence of oligomeric Abeta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proceedings of the National Academy of Sciences USA, 100, 10417-10422. doi:10.1073/pnas.1834302100
- Barghorn, S., Nimmrich, V., Striebinger A., Krantz, C., Keller, P., Janson, B., Bahr, M., Schmidt, M., Bitner, R.S., Harlan, J., Barlow, E., Ebert, U. and Hillen, H. (2005) Globular amyloid beta-peptide oligomer—A homogenous and stable neuropathological protein in Alzheimer’s disease. Journal of Neurochemistry, 95, 834-847. doi:10.1111/j.1471-4159.2005.03407.x
- Matsumura, S., Shinoda, K., Yamada, M., Yokojima, S., Inoue, M., Ohnishi, T., Shimada, T., Kikuchi, K., Masui, D., Hashimoto, S., Sato, M., Ito, A., Akioka, M., Takagi, S., Nakamura, Y., Nemoto, K., Hasegawa, Y., Takamoto, H., Inoue, H., Nakamura, S., Nabeshima, Y., Teplow, D.B., Kinjo, M. and Hoshi, M. (2011) Two distinct amyloid beta-protein (Abeta) assembly pathways leading to oligomers and fibrils identified by combined fluorescence correlation spectroscopy, morphology, and toxicity analyses. Journal of Biological Chemistry, 286, 11555-11562. doi:10.1074/jbc.M110.181313
- Bieschke, J., Herbst, M., Wiglenda, T., Friedrich, R.P., Boeddrich, A., Schiele, F., Kleckers, D., Lopez Del Amo, J.M., Grüning, B.A., Wang, Q., Schmidt, M.R., Lurz, R., Anwyl, R., Schnoegl, S., Fändrich, M., Frank, R.F., Reif, B., Günther, S., Walsh, D.M. and Wanker, E.E. (2011) Small-molecule conversion of toxic oligomers to nontoxic β-sheet-rich amyloid fibrils. Nature Chemical Biology, 20, 93-101. doi:10.1038/nchembio.719
- Yu, L., Edalji, R., Harlan, J.E., Holzman, T.F., Lopez, A.P., Labkovsky, B., Hillen, H., Barghorn, S., Ebert, U., Richardson, P.L., Miesbauer, L., Solomon, L., Bartley, D., Walter, K., Johnson, R.W., Hajduk, P.J. and Olejniczak, E.T. (2009) Structural characterization of a soluble amyloid beta-peptide oligomer. Biochemistry, 48, 1870-1877. doi:10.1021/bi802046n
- Shimizu, T., Yoshimune, K., Komoriya, T., Akiyama, T., Ye, H. and Kohno, H. (2013) Monoclonal antibodies against large oval aggregates of Aβ1-42. Journal of Bioscience and Bioengineering, 115, 216-220. doi:10.1016/j.jbiosc.2012.09.007
- Wei, C.W., Peng, Y., Zhang, L., Huang, Q., Cheng, M., Liu, Y.N. and Li, J. (2011) Synthesis and evaluation of ferrocenoyl pentapeptide (Fc-KLVFF) as an inhibitor of Alzheimer’s Aβ1-42 fibril formation in vitro. Bioorganic and Medical Chemistry Letters, 21, 5818-5821. doi:10.1016/j.bmcl.2011.07.111
- Klaver, A.C., Patrias, L.M., Finke, J.M. and Loeffler, D.A. (2011) Specificity and sensitivity of the Abeta oligomer ELISA. Journal of Neuroscience Methods, 195, 249-254. doi:10.1016/j.jneumeth.2010.12.001
- Zhao, L.N., Long, H., Mu, Y. and Chew, L.Y. (2012) The toxicity of amyloid β oligomers. International Journal of Molecular Science, 13, 7303-7327. doi:10.3390/ijms13067303
- Klaver, A.C., Patrias, L.M., Coffey, M.P., Finke, J.M. and Loeffler, D.A. (2010) Measurement of anti-Abeta1-42 antibodies in intravenous immunoglobulin with indirect ELISA: The problem of nonspecific binding. Journal of Neuroscience Methods, 187, 263-269. doi:10.1016/j.jneumeth.2010.01.018
- Noguchi, A., Matsumura, S., Dezawa, M., Tada, M., Yanazawa, M., Ito, A., Akioka, M., Kikuchi, S., Sato, M., Ideno, S., Noda, M., Fukunari, A., Muramatsu, S., Itokazu, Y., Sato, K., Takahashi, H., Teplow, D.B., Nabeshima, Y., Kakita, A., Imahori, K. and Hoshi, M. (2009) Isolation and characterization of patient-derived, toxic, high mass amyloid beta-protein (Abeta) assembly from Alzheimer disease brains. Journal of Biological Chemistry, 284, 32895-32905. doi:10.1074/jbc.M109.000208
- Horikoshi, Y., Sakaguchi, G., Becker, A.G., Gray, A.J., Duff, K., Aisen, P.S., Yamaguchi, H., Maeda, M., Kinoshita, N. and Matsuoka, Y. (2004) Development of Abeta terminal end-specific antibodies and sensitive ELISA for Abeta variant. Biochemical and Biophysical Research Communications, 319, 733-737. doi:10.1016/j.bbrc.2004.05.051
- Murakami, K., Horikoshi-Sakuraba, Y., Murata, N., Noda, Y., Masuda, Y., Kinoshita, N., Hatsuta, H., Murayama, S., Shirasawa, T., Shimizu, T. and Irie, K. (2010) Monoclonal antibody against the turn of the 42-residue amyloid β-protein at positions 22 and 23. ACS Chemical Neuroscience, 17, 747-756. doi:10.5692/clinicalneurol.51.890
NOTES
*Corresponding author.

