Advances in Bioscience and Biotechnology
Vol.1 No.2(2010), Article ID:2093,5 pages DOI:10.4236/abb.2010.12018
Development and validation of HPTLC method for niacin and simvastatin in binary combination
![]()
Department of Chemistry, Ramnarian Ruia College, Mumbai, India.
Email: pravishkumar1981@yahoo.com
Received 21 February 2010; revised 11 March 2010; accepted 28 March 2010.
Keywords: HPTLC; Methanol; Niacin; Simvastatin
ABSTRACT
A simple, sensitive and validated HPTLC method has been developed to determine Niacin and simvastatin simultaneously in synthetic mixture form. Chromatographic separation was achieved on a RP18 plate using a mixture of Methanol: Water: Acetic acid (80:20:0.1) at a wavelength of 236 nm. Linearity of the method was found to be in the concentration range of 12.5-37.5 μg/spot for niacin and 0.25-0.75 μg/spot for simvastatin with correlation coefficient greater than 0.999. The method can be used for simultaneous determination of Niacin and Simvastatin.
1. INTRODUCTION
Niacin (Figure 1) chemically designated as Pyridine 3 carboxylic acid reduce triglyceride, levels, is also effective for increasing serum HDL levels [1]. It has also been demonstrated that this drug lowers the incidence of coronary heart disease in humans [1]. A number of analytical methods have been developed for its determination in pharmaceutical formulations or in biofluids either alone or in combination with other drugs [2-8]. These include determination of niacin by liquid chromatography–mass spectrometry [9], HPLC [9-12], flow injection and spectrofluorimetric analysis.
Simvastatin (Figure 2), a hypolipidemic drug belong-
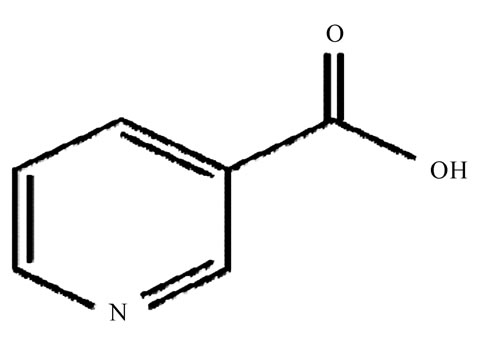
Figure 1. Niacin.
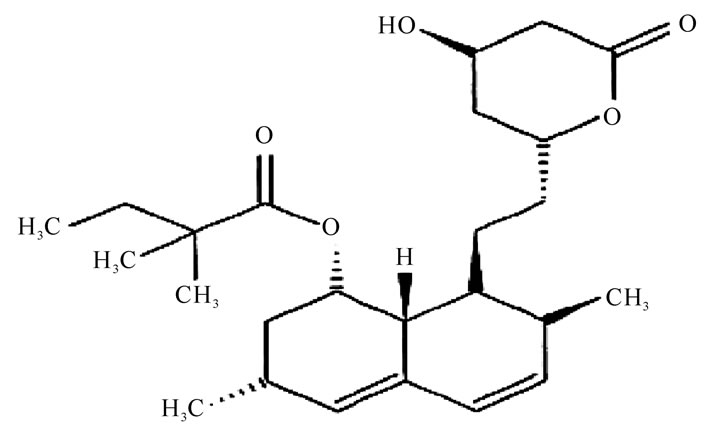
Figure 2. simvastatin.
ing to the class of pharmaceuticals called statins is chemically designated as [(1S,3R,7R,8S,8aR)-8-[2[(2R,4R)-4-hydroxy-6-oxo-oxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8ahexahydronaphthalen-1-yl] 2,2-dimethylbutanoate. It is used for the treatment of hypercholesterolemia [13]. An HMG-CoA reduces ase inhibitor, acts by decreasing cholesterol synthesis and by increasing low density lipoprotein (LDL) catabolism via increased LDL receptor activity [14]. Different analytical methods have been reported for the determination of simvastatin, which include HPLC [15-17], HPLC-MS/MS [18], spectrophotometer [19].
It was found that Simvastatin plus niacin provides marked clinical and angiographic ally measurable benefits in patients with coronary disease and low HDL levels [20]. The US Food and Drug Administration (FDA) has approved a fixed-dose combination of niacin and simvastatin for use in patients with complex lipid abnormalities where treatment with niacin or simvastatin alone is not sufficient [21] The FDA has approved maximum dose of niacin 1000mg and 20 mg of simvastatin per tab. The combine dosage form of Niacin and simvastatin are available in market.
According to the information collected from literature there is no reported method for simultaneous determination of Niacin and simvastatin. In the present work we have focused on deciding the optimum chromatographic conditions for the simultaneous determination of Niacin and simvastatin in a pharmaceutical preparation.
We describe in this paper a simple, sensitive and validated HPTLC method for the simultaneous determination of Niacin and Simvastatin .The developed method can be applied successfully for quality control and for other analytical purposes.
2. MATERIAL AND METHODS
2.1. Chemicals and Reagents
Niacin and simvastatin reference substances with claimed purity of 99.70% and 99.65% respectively were taken from Precise Pharma (Turbhe, mumbai) Methanol (HPLC grade), Triethyl amine and acetic acid (analytical reagent grade) were purchased from Merck (Mumbai). All excepients used were of pharmaceutical grade. Mobile phase was filtered using 0.45 μm cellulose acetate filters made by Millipore (USA) whereas; Whatmann filter papers No. 41 (purchased from the local market) were used in the preparation of sample solution.
2.2. Chromatographic Conditions
The chromatographic estimation were performed using stationary phase precoated C18 aluminum sheets (10 × 10 cm, prewashed with methanol and dried in oven at 50℃ for 5 min) mobile phase, Water: Methanol: Acetic acid (20:80:0.1), chamber and plate saturation time of 30 min, migration distance allowed was 74 mm, wavelength scanning was done at 236 nm, keeping the slit dimension at 5 × 0.45 mm.
2.3. Preparation of Standard Solution
A Stock solution of niacin and simvastatin was prepared at about 10000 μg/ml and 200 μg/ml respectively in Diluent. Make standard in duplicate and spot 2.5 μl of each solution.
2.4. Determination of Simvastatin and Niacin in There Combined Dosage Forms
The content of twenty tablets where taken and weighed .powder equivalent to Niacin 1000 mg and 20 mg simvastatin in 100 ml volumetric flask add 50 ml of diluent and flask was sonicated for 5 min .The flask was sonicated and the volume was diluted to the mark with diluent .The above solution was filtered using what man filter paper No. 1.
2.5. Linearity
Linearity of the proposed method was checked by analyzing seven solutions in the range of 5000-15000 μg/ml for niacin (5000, 7500, 10000, 12500, 15000 μg/ml) and 100-300 μg/ml for simvastatin (100,150,200,250,300 μg/ ml). Each level was made in triplicate and spot 2.5 μl of each solution.
2.6. Accuracy
Method accuracy was performed by adding known amounts of niacin and simvastatin to the pre analyzed sample and then comparing the added concentration with the found concentration. Three levels of solutions were made which correspond to 50, 100 and 150% of the nominal analytical concentration. Each level was made in triplicate and spot 2.5 μl of each solution.
2.7. Specificity
Commonly used exceipients (starch, microcrystalline cellulose and magnesium stearate, lactose,) were spiked in to a pre weighed quantity of drugs .The chromatogram was taken by appropriate dilution and the quantities of drug were determined.
2.8. Ruggedness
Ruggedness of the method was performed by two different parameters i.e. days and analyst .The results of estimation by proposed method are very much similar under variety of condition.
2.9. Precision
For evaluating the within-day precision, results of six replicate analyses of samples were calculated on a single day. The between-day precision was calculated from the same samples analyzed on different days.
2.10. LOD and LOQ
For calculating the LOD and LOQ values, solutions with known decreased concentrations of analytes were injected into the HPTLC system. The limit of detection (LOD) and quantification (LOQ) were then measured by calculating the minimum level at which the analytes can be readily detected (signal to noise ratio of 3:1) and quantified (signal to noise ratio of 10:1) with accuracy, respectively.
3. RESULTS
In the present work conditions were optimized for the development and validation of a simple and accurate HPTLC method for the simultaneous determination of niacin and simvastatin in synthetic mixture form. Method development was started with water and methanol in the ratio of 50:50 (v/v). At this composition although both components were separated large resolution but peak shape was not good. The methanol contents of the mobile phase were then increased to decrease resolution and R.F and 0.1% acetic acid was added to reduce the tailing. At the composition of 20:80:0.1(Water: Methanol: Acetic acid) both components were separated with a good resolution and good peak shape. The most appropriate mobile phase composition was thus found to be Water: Methanol; Acetic acid in the ratio of 20:80:0.1.
Under the Described experimental conditions, sharp peaks that belong to niacin and simvastatin were obtained at retention factor of 0.100 and 0.65 minutes respectively as shown in Figure 3.
The developed chromatographic method was validated using ICH guidelines [22]. Validation parameters performed include linearity, limit of detection and quantitation, selectivity, ruggedness, accuracy and repeatability the calibration curve was linear over the concentration range of 5000-15000 μg/ml for niacin and 100-300 μg/ml for simvastatin. The correlation coefficient in both cases was found to be greater than 0.999 which manifests a linear relationship between concentration and the peak area. The linear regression equation for niacin was found to be Y = 3.2342 X + 149.27 with correlation coefficient equal to 0.999. The linear regression equation for simvastatin was found to be Y = 11.499 X + 4.8 with value of correlation coefficient equal to 0.99997. In this study, the LOD was found to be 1200 μg/ml and 24 μg/ml for niacin and simvastatin respectively. The LOQ was found to be 4000 μg/ml and 80 μg/ml for niacin and simvastatin respectively. The recovery and the relative standard deviation for each of the analytes are given in Table 1.
The results of within-day and between-day precision are presented in Table 2.
Chromatogram of niacin and simvastatin in sample in given in figure 4 showing selectivity of the proposed method.
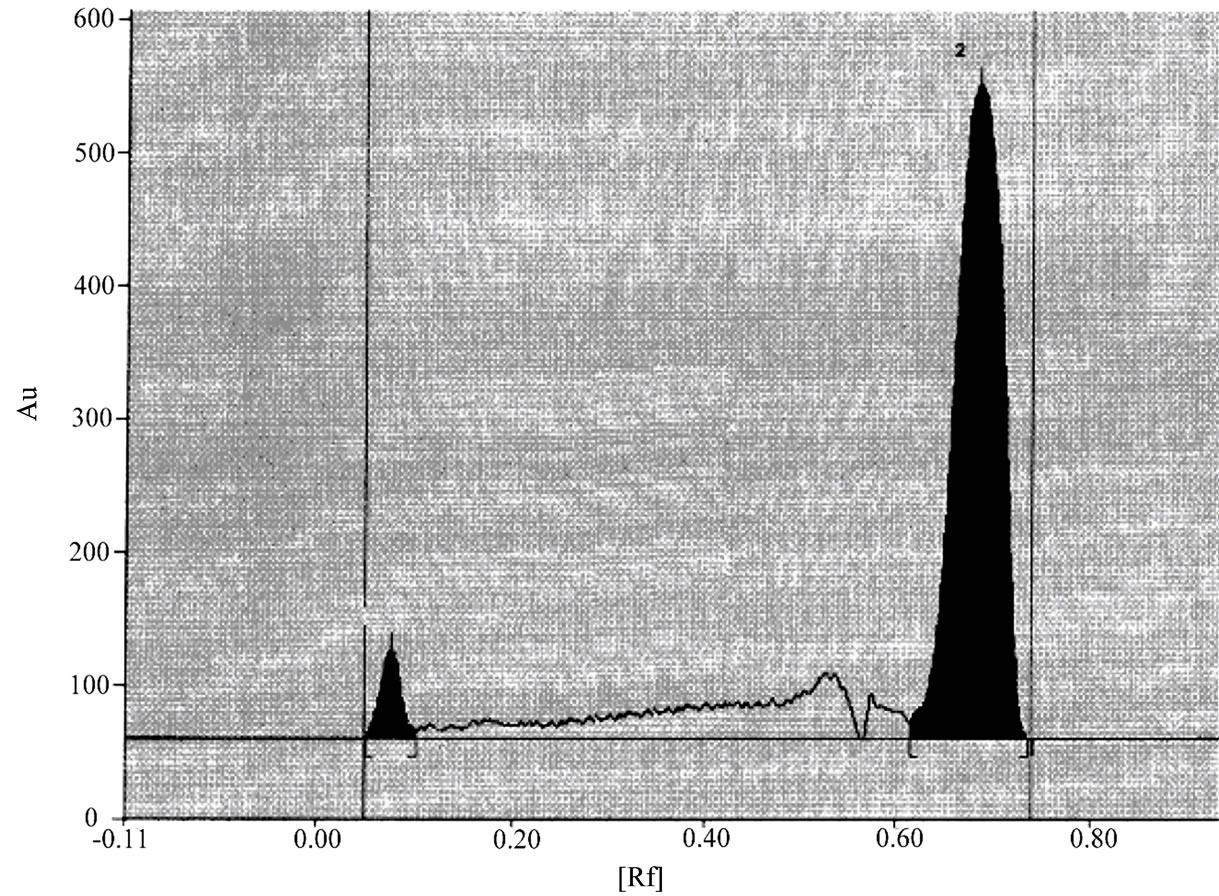
Figure 3. Chromatograms of Niacin and simvastatin reference substances.
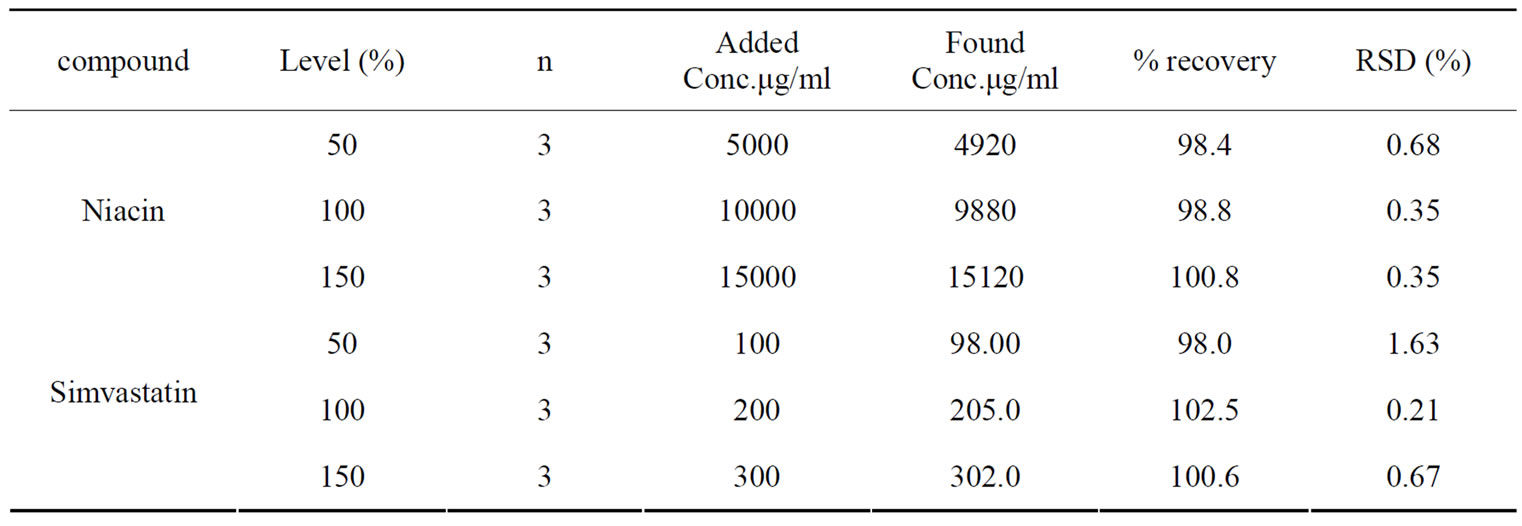
Table 1. Accuracy of the proposed HPTLC method.
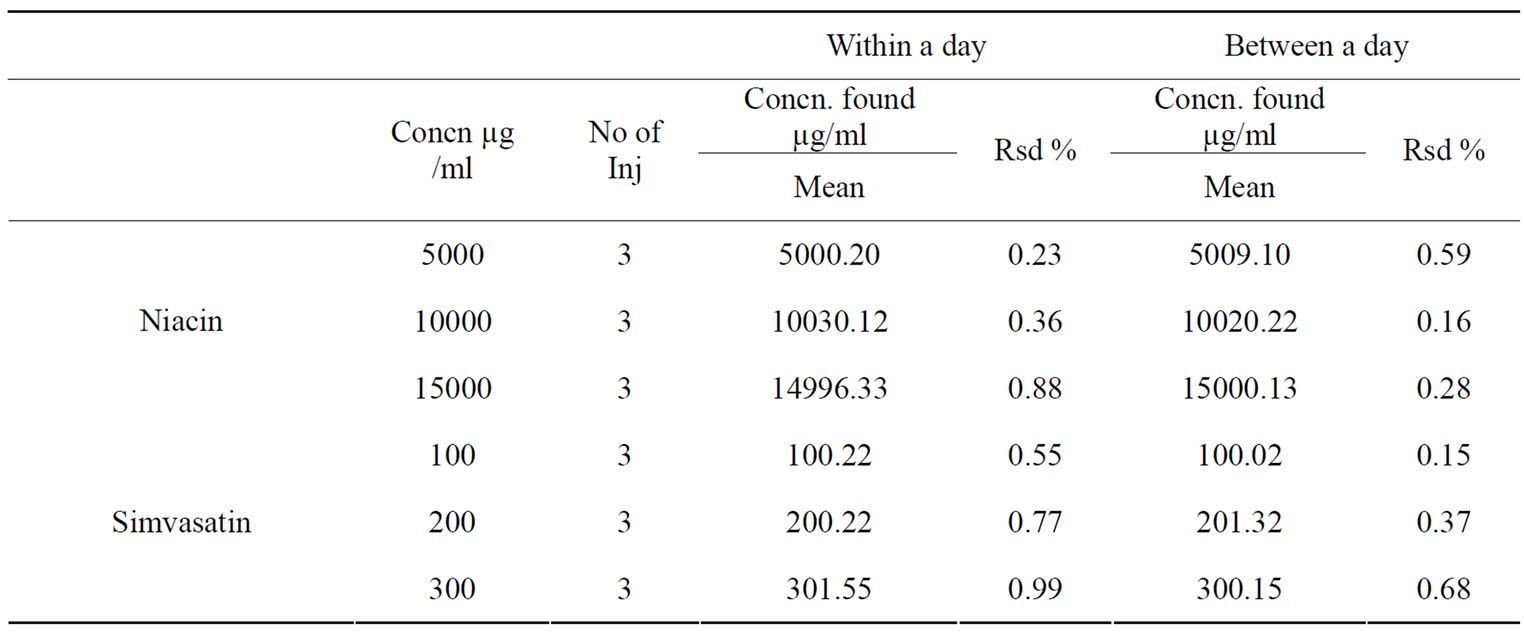
Table 2. Precision of the proposed HPTLC method.
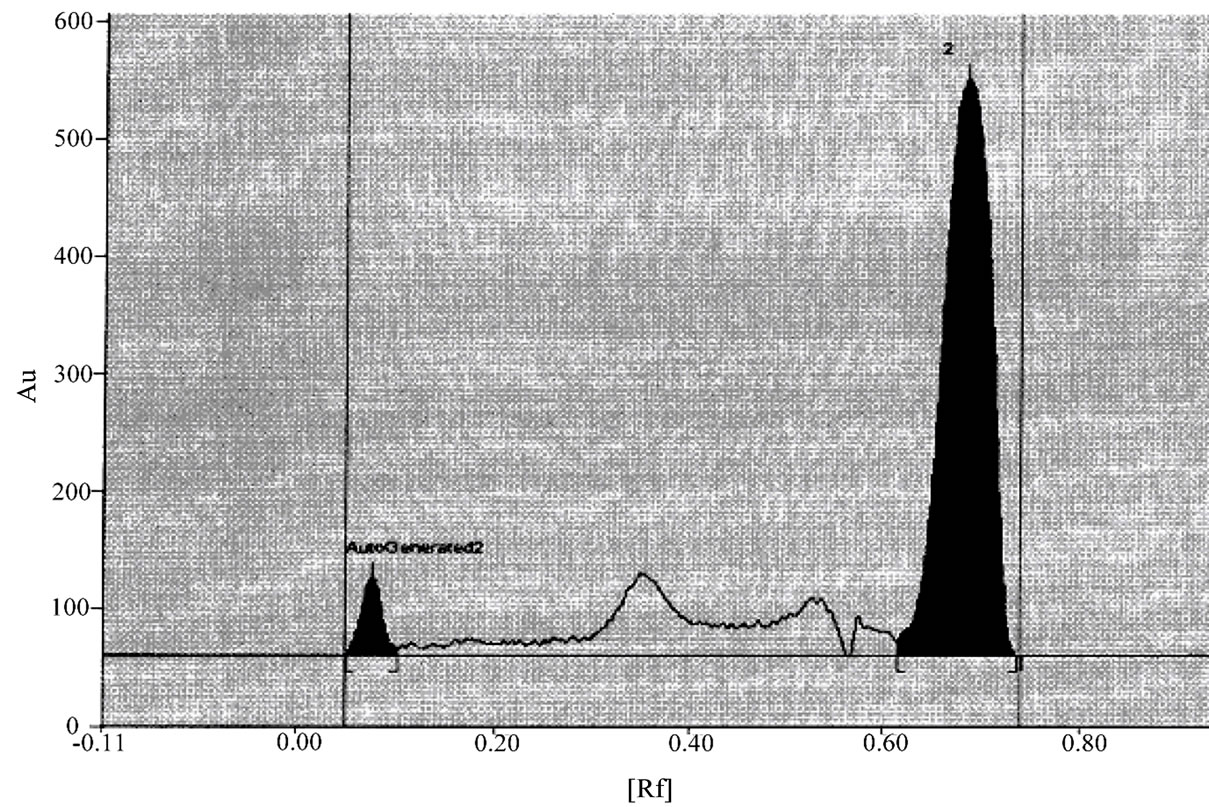
Figure 4. Chromatograms of Niacin and simvastatin in sample solution.
4. CONCLUSIONS
A simple and accurate reverse phase HPTLC method has been developed for the simultaneous determination of niacin and simvastatin. The method was validated by testing its linearity, accuracy, precision, limits of detection and quantization, selectivity and robustness.
5. ACKNOWLEDGEMENTS
The authors are great full to precise pharma for providing gift sample of simvastain and niacin.
REFERENCES
- Susman (1995) Niacin reduces triglycerides, increases good cholesterol in diabetics. Doctors Guide Publishing Limited.
- Kumar, V. and Shah, R.P., (2008) LC and LC–MS methods for the investigation of polypills for the treatment of cardiovascular diseases: Part 1. Separation of active components and classification of their interaction/degradation products. Saranjit Singh Journal of Pharmaceutical and Biomedical Analysis, 47(3), 508-515.
- Chaudhari, B.G., Patel, N.M., Shah, P.B. and Modi, K.P. (2006) Development and validation of a HPTLC method for the simultaneous estimation of atorvastatin calcium and ezetimibe. Indian Journal of pharamaceutical Science, 68(6), 793-796.
- Jain, N. and Raghuwanshi, R. (2008) Development and validation of RP-HPTLC method for simultaneous estimation of atorvastatin calcium and fenofibrate in tablet dosage forms. Deepti Jain Indian Journal of pharmaceutical Science, 70(2), 263-265.
- Kova´, L.N. and Atı´nsky´, D.S., (2008) HPLC methods for the determination of simvastatin and atorvastatin. PetrSolich Trac Trends in Analytical Chemistry, 27(4), 352-367.
- Jemal, M., Ouyang, Z. and Powell, M.L.(2000) A strategy for a post-method-validation use of incurred biological samples for establishing the acceptability of a liquid chromatography/tandem mass-spectrometric method for quantitation of drugs in biological samples. Journal Pharm Biomed Anal, 16(16), 1538-1547.
- Zhang, N., Yang, A., Rogers, J.D. and Zhao, J.J. (2004) Journal Pharm Biomed Anal, 34, 175-187.
- Nirogi, R., Mudigonda, K. and Kandikere, V. (2004) Chromatography–mass spectrometry methods for the quantitation of statins in biological samples. Journal of pharmaceutical and Biomedical Analysis. 44, 379-387.
- Research & Development report, may 2000, Australian Government Analytical Laboratories.
- Khor, S.-C. and Tee, E.-S. (1996) Development of a HPLC method for the simultaneous determination of several b-vitamins and ascorbic acid. Malaysian Journal of Nutrition, 2(1), 49-65.
- Euro Fir guidelines for assessment of Methods of Analysis KellieWindahi, V Craige Trenerry and Caroline Ward.
- Pfuhl, P., Karcher, U., Haring, N., Baumeister, A., Tawab, M.A. and Schubert-Zsilaveez, M. (2005) Simultaneous Determination of Niacin, Niacinamide and Nicotinuric Acid in Human Plasma. Journal of Pharmaceutical and Biomedical Analysis, 36(5), 1045-1052.
- Todd, P.A. and Goa, K.L., (1990) Simvastatin: A review of its pharmacological properties and therapeutic potential in hyperlipidaemia. Drugs, 40(4), 583-607.
- Pasternak, R.C., Smith, S.C.J., Merz, C.N.B., Grundy, S.M., Cleeman, J.I. and Lenfant, C. (2002) ACC/ AHA/ NHLBI clinical advisory on the use and safety of statins. Journal Of The American Heart Association, 40, 567-572.
- Dave, T. and Diab, E. Analysis of Simvastatin Tablets by High Speed LC. Application Notes 405, Thermo Fisher Scientific, San Jose, 1-6.
- Ashfaq, M., Khan, I.U., Quatab, S.S. and Razz, S.N. (2007) Hplc determination of ezetimibe and simvastatin in pharmaceutical formulations. Journal of the Chilean Chemical Society, 52(3), 1220-1224.
- Ashfaq, M., Khan, I.U. and Asghar, M.N. (2008) Development and Validation of Liquid Chromatographic Method for Gemfibrozil and Simvastatin in Binary Combination. Journal of the Chilean Chemical Society, 53(3), 1617-1619.
- Yang, H., Feng, Y. and Luana, Y. (2003) Determination of Simvastatin in human plasma by liquid chromatography–mass spectrometry. Journal of Chromatography B, 785(2) 369-375.
- Basavaiah, K. and Tharpa, K. (2008) The development and validation of visible spectrophotometric methods for simvastatin determination in pure and the tablet dosage forms. Chemical Industry and Chemical Engineering Quarterly, 14(3), 205-210.
- Brown, G.B., Zhao, X.-Q., Chait, A., Fisher, L.D., Cheung, M.C., Morese, J.S., Dowdy, A.A., Marino, E.K., Bolson, E.L., Alaupovic, P., Frohlich, J. and Albers, J.J. (2001) Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. The New England Journal of Medicine, 345(22), 1583-1592.
- Riordan, M.O. and Waknine, Y. (2008) FDA approves combination niacin and simvastatin. Medical News, Medscape.
- I.C.H (Q2A) (1994) Note for guidance on validation of analytical methods, definition and terminology. International Conference on Harmonization, Human Medicines Evaluation Unit, the European Agency for the Evaluation of Medicinal Products.

