Journal of Environmental Protection
Vol.5 No.2(2014), Article ID:43353,8 pages DOI:10.4236/jep.2014.52019
Impacts of Wood Poaching on Vegetation Structure and Composition in Mukuvisi Woodland, Zimbabwe
1Department of Wildlife and Safari Management, Chinhoyi University of Technology, Chinhoyi, Zimbabwe; 2Tropical Resource Ecology Programme, Department of Biological Sciences, University of Zimbabwe, Harare, Zimbabwe.
Email: *nmbok@yahoo.co.uk
Copyright © 2014 Never Muboko et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Never Muboko et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received December 16th, 2013; revised January 17th, 2014; accepted February 15th, 2014
Keywords:Alternative Energy; Composition; Miombo Woodlands; Mukuvisi Woodland; Structure; Wood Poaching
ABSTRACT
Our study focused on the effects of wood poaching on the vegetation structure and composition in Mukuvisi Woodland, Zimbabwe. Mukuvisi Woodland, located within the precincts of Harare urban area, Zimbabwe, suffers from high illegal wood utilization pressure stemming from the need to fulfill alternative energy demands created by persistent electricity shortages and an unstable economic environment, particularly between 2000 and 2008. This results in a continuous flux of vegetation and a disturbed animal habitat driven mainly by anthropogenic activities. Due to the heterogeneity in vegetation utilisation trends, we used the stratified systematic random sampling technique, where the site was divided into two strata, central and boundary. Twelve 30 × 20 m permanent plots were established in which species name, species diversity, height, basal area, plant status, fire evidence, number of stems and saplings were recorded and assessed in April and May 2012. A total of 968 woody plants were assessed representing 47 woody plant species. All woody vegetation variables recorded and assessed showed no significant difference (P > 0.05) between the two strata, i.e., central and boundary, in Mukuvisi Woodland, except sapling density (P = 0.022). Principal Component Analysis indicated evidence of fire impacts on vegetation structure. The study concludes that illegal wood harvesting in Mukuvisi Woodland has not yet reached alarming proportions and can be contained. The study recommends collaborative arrangements with key stakeholders, promotion of the use of alternative energy sources and increased environmental education and awareness campaigns.
1. Introduction
At a national scale, Comiskey and Sunderland [1] state that Zimbabwe lost about 29.5% of its forest cover or around 6,540,000 ha with the highest coverage in protected areas between 1990 and 2010. This amid growing concerns that wood poaching is negatively impacting on woodlands dynamics in African savanna [2]. Woody vegetation is important as it provides products and services such as fuel, timber, fodder, especially in developing countries [3,4], habitats for avifauna, canopy environments for wildlife and nutrient rich pools within a harsh landscape [5-7]. Wood harvesting and strong selectivity for both woody species and size class are rarely factored into fuel wood harvesting models or impacts on woody vegetation structure and composition. Large scale woody resource utilisation may cause changes in woody species composition and structural distribution patterns [8,9]. Anthropogenic disturbances, such as energy needs and encroachments into protected areas, have increased dramatically in recent decades, influencing woodland dynamics at different spatial levels across the African savanna [1,8,10,11]. Mukuvisi Woodland’s location close to an urban area, City of Harare, Zimbabwe, is perceived by most conservationists to be a threat to its long-term integrity. This threat is directly linked to wood poaching [12] since the woodland is surrounded by communities that sometimes rely on fuel wood for domestic purposes due to the electricity woes in Harare, among other factors.
Wood poaching is regarded as a threat to protected area woodland in close proximity to human habitations [13]. Zimbabwe faced economic challenges between 2000 and 2008 which led to increased power cuts, among other shortages, leading to local people in urban areas to heavily rely on wood fuel and gas among other energy sources. Excessive woody plant species extraction raises concerns of ecological implications on biodiversity and ecosystem processes. Effective biodiversity conservation is only possible when accurate, timely information is available regarding disturbance impact on seemingly intact woody vegetation nodes and the interactive dynamics between biophysical factors and socioeconomic factors [14]. Despite its status and recognition as a protected wildlife and woodland area, inadequate documentary evidence exists on the impacts of wood poaching on vegetation structure and composition of the Mukuvisi Woodland. The objectives of this study were twofold: 1) to determine the preferred tree species harvested by wood poachers in Mukuvisi Woodland based on cut trees on the ground; and 2) to assess the impacts of wood poaching on vegetation structure and composition in Mukuvisi Woodland.
2. Materials and Methods
2.1. Study Area
Mukuvisi Woodland, located at latitude 17˚50'35S and longitude 31˚5'42E, covers approximately 274 hectors [15] and is situated in the highveld of Zimbabwe with a distance of approximately 5 km south east of Harare city centre (Figure 1). The land is owned by Harare City Council, but leased to a private operator, Mukuvisi Woodland Association [15]. Surrounding Mukuvisi Woodland is low to medium density suburbs and light industrial areas. Mukuvisi Woodland is found between the Mukuvisi and Chiraura rivers [16]. The 274 hectares are zoned into segments which comprise approximately 106 ha of game area, 156 ha of the public walking area and 12 ha of the interpretive and administration area. Mukuvisi Woodland has a diverse vertebrate fauna that includes mammals, birds, reptiles and fish. Mammals include herbivores such as zebra (Equus quagga), giraffe (Giraffa camelopardalis), wildebeest (Connocaetus taurinus), impala (Aepyceros melampus) and eland (Taurotragus oryx). The presence of mammal carnivores is not documented, although existence of small carnivorous animals cannot be ruled out. Mammal community is dominated by impala which approximately constitutes 70% of the total biomass [16]. There is a prolific bird life which includes the spotted creeper (Salpornis spilonotus), Violet backed sunbird (Anthrepes longuemarei), miombo blue eared starling (Lamprotornis elisabeth), and white eared barbet (Stactolaena leucotis). The dominant tree species include Julbernadia globiflora, Brachystegia speciformis, Brachystegia boehmii and Parinari curatellifolia, while Heteropogon contortus, Hyparrehenia filipendula, Sporobolus pyramidalis and Hyparrehenia dissoluta represent dominant grass species [16,17].
Annual rainfall ranges between 650 - 850 mm recorded between November and March and summer temperatures range between 16˚C and 30˚C [16]. Frost in May and June is common on the vlei and along the Chiraura and Mukuvisi river banks. The soils of the Mukuvisi protected area are nutrient poor, a characteristic of most of the miombo soils [18]. Coarse-grained granite [19] underlay Mukuvisi Woodland and gives rise to coarsetextured sandy soils.
2.2. Sampling Design and Data Collection
The study area was stratified into two study strata, namely the central area and the park boundary. Sample plots were randomly placed based on Mukuvisi Woodland topographical map using grid intercept method across the defined two study strata. A total of 12 sample plots were sampled between April and May 2012. Four plots were 100 m from the boundary and the remaining 8 were located 5 km from the boundary and were in the central area of Mukuvisi Woodland. Sampling plots measuring 30 × 20 m were delineated on each of the strata. Woody species occurring within a sample plot were identified using field identification guides [20]. Further, the following variables were assessed in each plot following methods outlined by Gandiwa and Kativu [21]: tree height, basal circumference, plant status (dead or alive), tree stumps as a result of poaching, fire evidence, number of stems and saplings.
2.3. Data Analysis
Data were tested for normality using KolmogorovSmirnov test in STATISTICA version 6 for Windows [22] and were found to be normal. Differences across the study strata were determined using t-tests for independent sample groups. Species diversity across the strata was determined using the Shannon-Weiner diversity index [23]. A principal component analysis (PCA) was used to analyze woody vegetation composition and structure across the strata, following Davies and Fearn [24]. In addition, hierarchical cluster analysis (HCA), using the Ward’s method, was used to analyze woody species
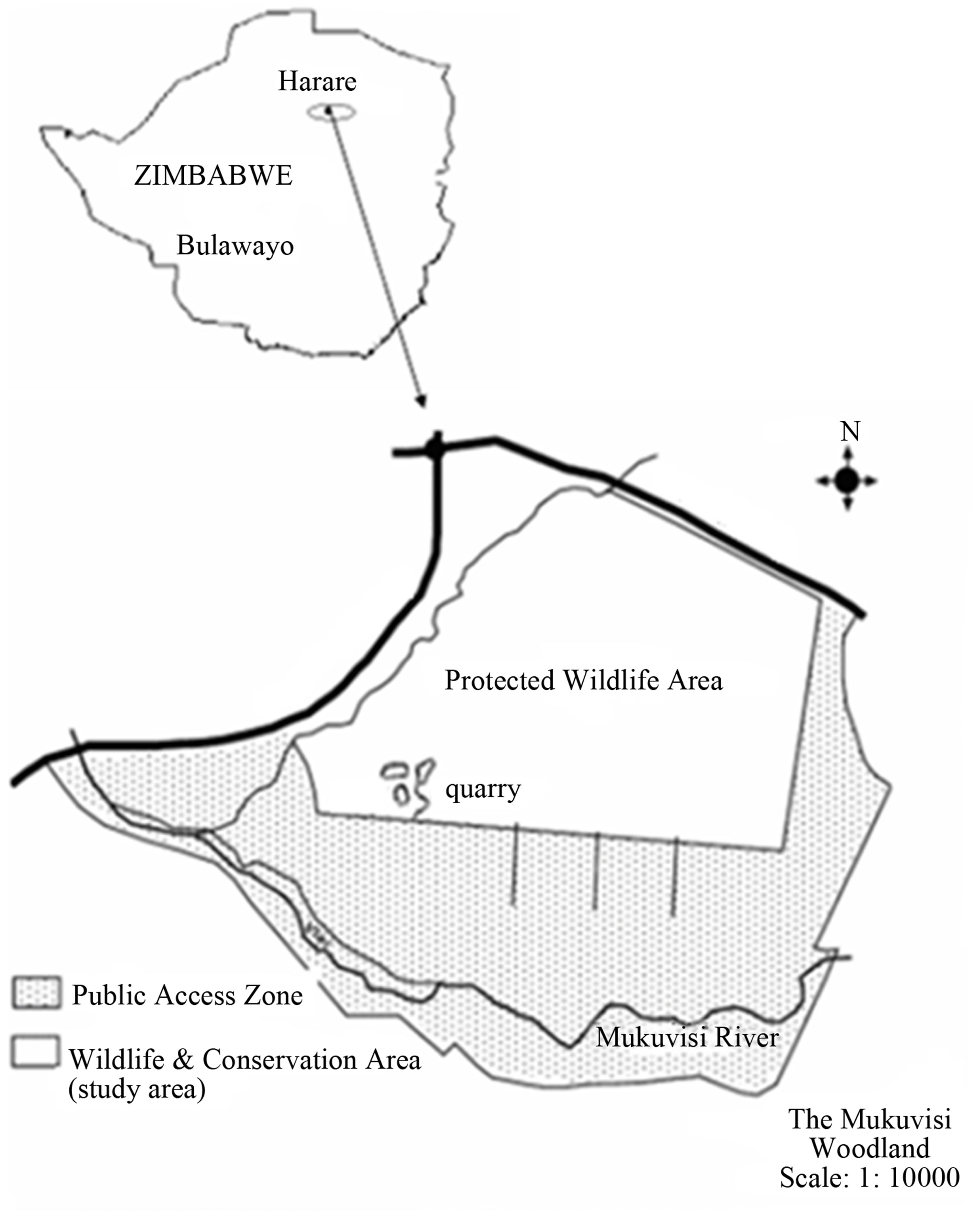
Figure 1. Mukuvisi Woodland, Harare, Zimbabwe. Source: modified from Chiseva [17].
cluster composition across the strata [25].
3. Results
3.1. Preferred Woody Vegetation Species
A total of 117 woody stumps were recorded, mostly close to the boundary, translating to about 12.1% of poached woody trees across the strata. Figure 2 shows a tree stump left after cutting. One would notice that a type of a tree-cutting saw, probably a chainsaw basing on the tree trunk diameter and the precision displayed, was used as opposed to the common hand-held axe. This may explain mechanised techniques used by wood poachers to evade detection.
As indicated by the prevalence of recorded stumps, J. globiflora (69%) and B. speciformis (23%) had high tree stumps, showing that they were the most preferred and harvested while species like Vangueria infausta, Protea roupelliae roupelliae, Combretum molle and Newtonia hildebrandtii, were not only less common, but also least preferred with each accounting for less than two percent of stumps recorded.

Figure 2. One of the many tree stumps in Mukuvisi Woodland. Photo credit: T. Chigumira.
3.2. Woody Vegetation Structure and Composition
A total of 968 woody plants, representing 47 woody species were recorded in the 12 sample plots. The dominant woody species recorded include J. globiflora, B. speciformis, B. boehmii and Parinari curatellifolia. All the measured or recorded woody vegetation variables indicated no significance difference (P > 0.05) between the strata (Table 1).
The Principal Component Analysis of the woody vegetation variables showed a variance of 34% (eigenvalue = 3.4) on Factor 1 and 23% (eigenvalue = 2.3) on Factor 2 in the woody species composition and structure (Figure 3). Sample plots C2, C3, C5, and C6 were drawn from the central area of Mukuvisi Woodland. These have high tree density, species diversity, shrub height and dead plant density. Those near the boundary, sample plots B1, B2, B3 and B4 show high shrub density, sapling density and a larger basal area compared to the plots in the central area. Fire damage was high in plot C4 and tree height is higher in plot C7 as shown by its outlying characteristic. Positively correlated were tree height and basal area, similarly to tree density and woody species diversity. Tree height and shrub density were negatively correlated to fire damage, whereas, sapling density was also negatively correlated to dead plants.
The Hierarchical cluster analysis (HCA) using the Ward’s method with Euclidian distances showed that there are 47 species in abundance data in the study sites. We noted two sub clusters A and B. Sub cluster A, sample plot B4 and C8 show a slight variability in species
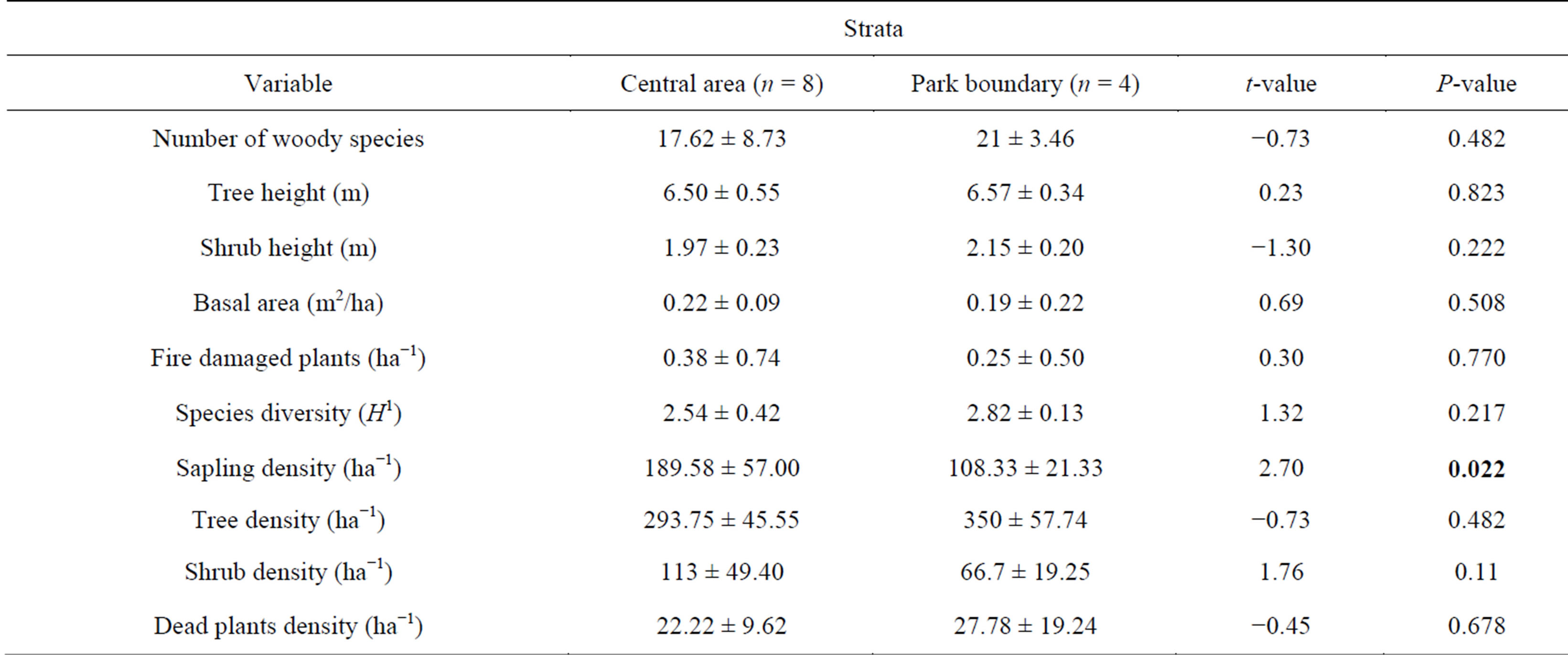
Table 1. Summary of measured/recorded woody vegetation variables (Mean ± Standard Deviation) across Mukuvisi Woodland study strata, Zimbabwe.
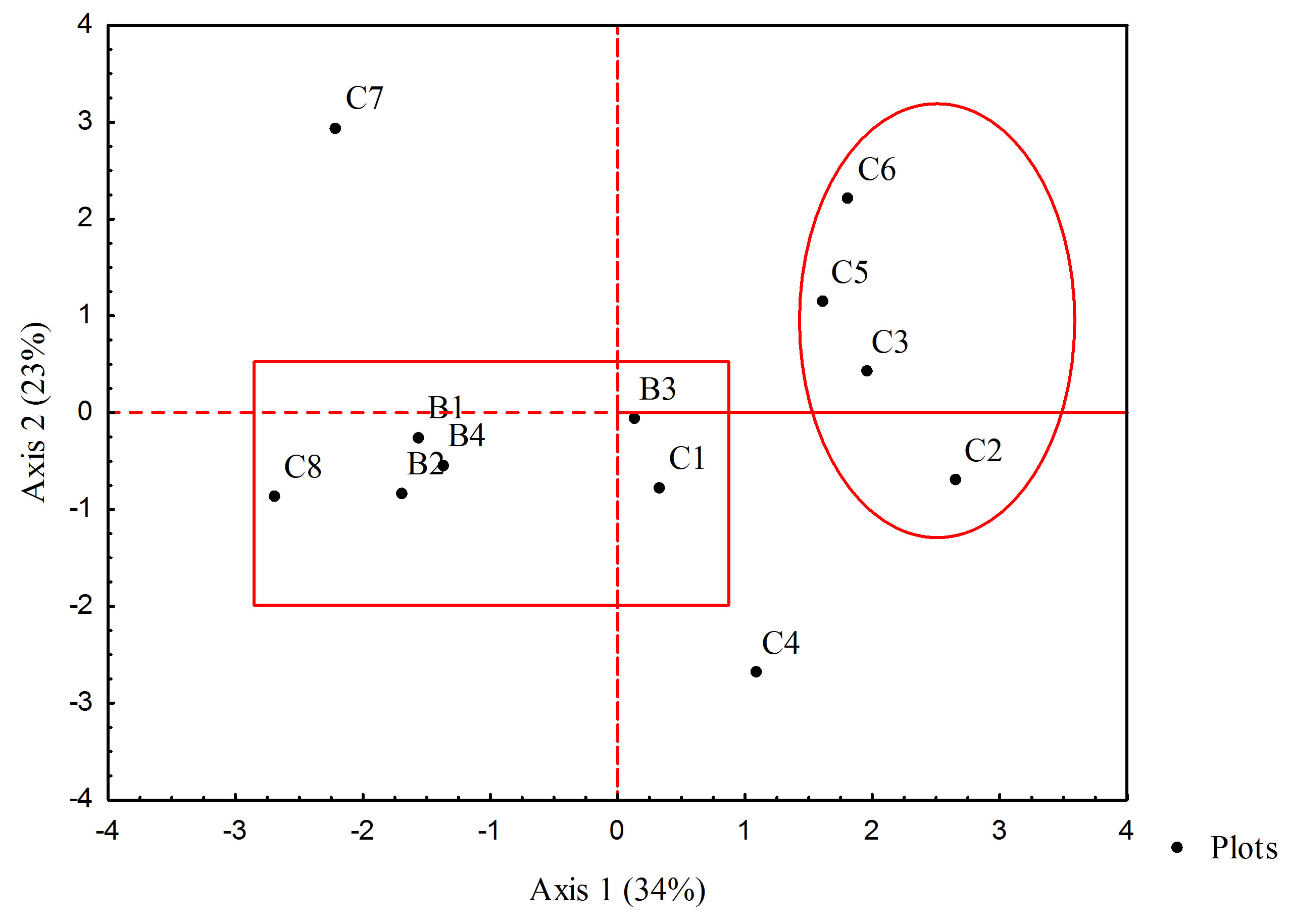
Figure 3. The Principal Component Analysis bipot showing relationships of the woody vegetation variables as observed in Mukuvisi Woodland, Zimbabwe. Notes: B represents boundary plots and C represents plots located in the central area of Mukuvisi Woodland.
composition compared to the sample plots B1, B2 and B3. In sub cluster B, sample plots C1, C2, C3, C5 and C7 show little variability in terms of species composition (Figure 4).
4. Discussion
4.1. Woody Vegetation Species Preference, Poaching and Fire
Our results showed that woody vegetation composition of the conserved Mukuvisi Woodland area was dominated by J. globiflora, B. spiciformis and B. boehmii, specifically resembling the Miombo woodlands of southern African savanna [26-29]. Regardless of the high representation of J. globiflora and B. speciformis and many other endemic woody plants at local or regional scale, these Miombo woody species are threatened by anthropogenic activities especially wood poaching as recorded in this study and this could be attributed to their hardwood type preferred for fuel wood, poles, construction and carpentry [5]. Our results are consistent with those of others like Pote et al. [5] and Furukawa et al. [30] who observed that these species besides being dominant in miombo woodlands are also preferred because of their basal circumference, as evidenced in this case by the stump size. Mukuvisi Woodland, being almost surrounded by urban residential areas is subjected to anthropogenic pressure where human livelihoods like energy needs are heavily dependent on wood derived from the study area. High wood poaching impacts were close to the boundary compared to the central parts as wood poachers want easy and quick exit from the park to reduce chances of being caught [31] and increasing distance from human settlements has been observed to correlate positively to wood biomass [32]. However, wood poaching may allow regrowth on under-represented species and woody biomass may accumulate rapidly after disturbance [31] as evidenced by the presence of saplings in the study area.
Our study results showed that fire damage was negatively correlated to tree size (tree height and basal area) and sapling density, whereas, fire damage was positively correlated to dead plant density (Figure 3). Anthropogenic fires in Africa form the ancient disturbance regimes that have probably shaped the miombo vegetation [33,34]. Effects of repeated burning include topkill of woody plants [35,21]. Repeated topkill of small trees and saplings makes them susceptible to “fire trap”, which suppress recruitment to large tree size [36]. It has been recorded that savanna woodlands respond variably to long term burning [37]. Our study results suggests that fire had little effect on tree density and woody species diversity as recorded in earlier studies elsewhere in the African savanna [34].
4.2. Woody Vegetation Structure and Composition
There were no significant differences among all assessed variables across the strata except sapling density. This indicates that wood poaching between the strata are not yet pronounced. Significant difference in sapling density
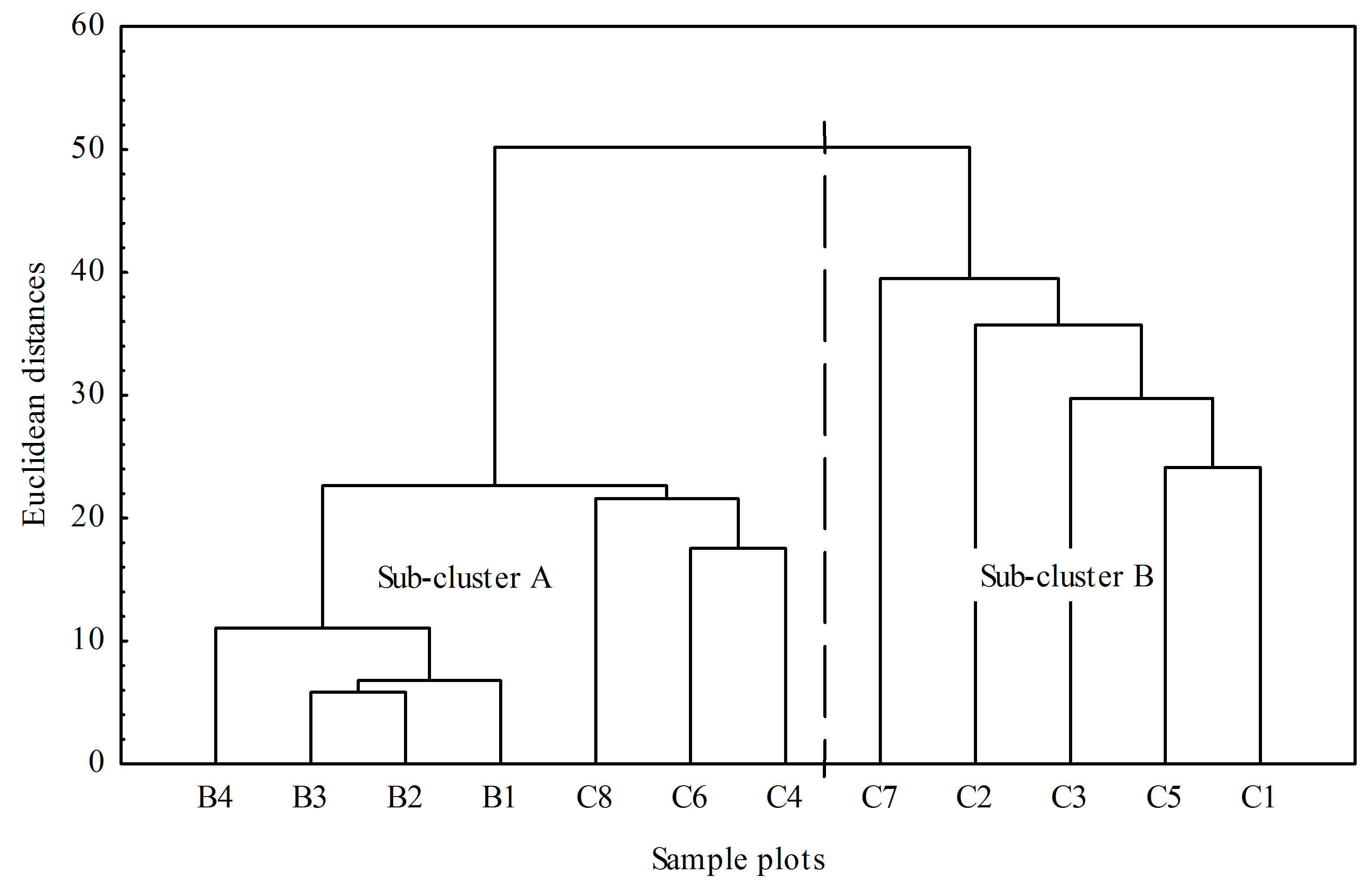
Figure 4. Classification dendrogram of woody species cluster composition between the strata in Mukuvisi Woodland. Notes: B represents boundary plots and C represents central area plots.
between the central and boundary strata may explain the higher impact of fire damage rather than wood poaching in the central area. The most observed trend is that human disturbed vegetation is characterised by high sapling density.
For woody species of slow growth rate, recruitment is interpreted as viable when woody species have a larger proportion of saplings than larger trees, and recruitment is considered poor when there are larger proportions of trees than saplings [38-40]. On the basis of this indicator, our study results of high proportion of tree density as compared to saplings could suggest less vibrant recruitment of woody species in Mukuvisi Woodland. There was evidence of large tree extraction on Mukuvisi Woodland as represented by relatively low tree densities, high number of stumps and high dead plant density. Wood poaching is known to affect micro-sites for plant establishment and the resultant woody plant diversity and density [41,42], this could be a contributing factor to relatively low woody species diversity and high dead wood density in Mukuvisi Woodland. Consistent with observations by Fadiman [43], species diversity was recorded to be decreasing in the central area as preferred species are getting scarce along the park boundary hence harvesting is now taking place in the interior. That herbivory is an ongoing activity in the studied woodland, together with fire, could have confounded the results of our study and, together with the effect of time, masked wood poaching impacts on the regeneration potential of miombo woodlands. Mukuvisi Woodland dominated by the miombo tree species, like the Brachystegia species of the Arabuko-Sokoke Forest, Kenya, exhibit a low diversity of canopy tree species [44]. This scenario, if left unchecked, is disastrous to biodiversity conservation and therefore, shows the importance of policy and law enforcement, particularly in the conservation and protection of micro-sites conserving remnant indigenous vegetation in areas where these are threatened by expanding urban settlements. Although we noted that there are some law enforcement activities in the study area and a perimeter game fence to try and ensure protection, there is need to increase surveillance and monitoring to cope with increasing pressure for wood fuel and forest products.
5. Conclusion
The dominant tree species in Mukuvisi Woodland, J. globiflora and B. spiciformis are highly targeted by wood poachers. We conclude that there is moderate to high levels of woody poaching as indicated by the cut trees and also sapling density that was relatively high. Moreover, woody poaching was more evident close to the boundary of the Mukuvisi Woodland than the inner areas. However, our results suggest that there is slight modification of the woody vegetation in Mukuvisi Woodland, especially the structural component. Therefore, we recommend increased law enforcement and conservation awareness education to reduce woody species poaching. Consistent with other authors like Du Plessis [3], we recommend proactive approaches to deal with this menace including calling for responsible authorities to improve energy provision and looking for alternative power sources to augment hydro-electricity deficit. The paradox is that we live in a part of the world endowed with abundant wind energy, solar radiation and in some parts, water and high biogas potential. Further, it is also our assumption that with increased wood poaching, it is likely that these wood poachers also kill wildlife. On that basis, we recommend further investigations into human-wildlife dynamics focusing on the wood poachers or neighbouring communities and effectiveness of current law enforcement efforts in the area to get a holistic picture.
Acknowledgements
The authors extend their appreciation to Mukuvisi Woodland management for allowing this study to be carried out in their property. We also acknowledge staff in the Department of Wildlife and Safari Management at Chinhoyi University of Technology for their support and constructive criticism.
REFERENCES
- J. A. Comiskey and T. C. H. Sunderland, “Takamanda: The Biodiversity of an African Rainforest,” Smithsonian Institution, SI/MAB Series, No. 8, 2003.
- I. Backens, B. Petersen, L. Stromquist and C. Ruffo, “Tree Communities and Structural Dynamics in Miombo (Brachystegia-Julbernadia) Woodland, Tanzania,” Forest Ecology and Management, Vol. 230, 2006, pp. 171-178. http://dx.doi.org/10.1016/j.foreco.2006.04.033
- M. A. Du Plessis, “The Effects of Fuel Wood Removal on the Diversity of Some Cavity-Using Birds and Mammals in South Africa,” Biological Conservation, Vol. 74, No. 2, 1995, pp. 77-82. http://dx.doi.org/10.1016/0006-3207(95)00016-W
- S. I. Higgins, C. M. Shackleton and E. R. Robinson, “Changes in Woody Community Structure and Composition under Contrasting Landuse Systems in a Semi-Arid Savanna, South Africa,” Journal of Biogeography, Vol. 26, No. 3, 1999, pp. 619-627. http://dx.doi.org/10.1046/j.1365-2699.1999.t01-1-00317.x
- J. Pote, C. Shackleton, M. Cocks and R. Lubke, “Fuelwood Harvesting and Selection in Valley Thicket, South Africa,” Journal of Arid Environments, Vol. 67, No. 2, 2006, pp. 270-287. http://dx.doi.org/10.1016/j.jaridenv.2006.02.011
- E. Gandiwa, “Importance of Dry Savanna Woodlands in Rural Livelihoods and Wildlife Conservation in SouthEastern Zimbabwe,” Nature & Faune, Vol. 26, 2011, pp. 60-66.
- S. Syampungani, P. W. Chirwa, F. K. Akinnifesi, G. Sileshi and O. C. Ajayi, “The Miombo Woodlands at the Cross Roads: Potential Threats, Sustainable Livelihoods, Policy Gaps and Challenges,” Natural Resources Forum, Vol. 33, No. 2, 2009, pp. 150-159. http://dx.doi.org/10.1111/j.1477-8947.2009.01218.x
- C. Mligo, “Anthropogenic Disturbance on the Vegetation in Mukurunge Woodland, Bagamoyo District, Tanzania,” Tanzania Journal of Science, Vol. 37, 2011, pp. 94-108.
- R. Matsika, B. F. Erasmus, and W. C Twine, “A Tale of Two Villages: Assessing the Dynamics of Fuelwood Supply in Communal Landscapes in South Africa,” Environmental Conservation, Vol. 40, No. 1, 2012, pp. 71- 83. http://dx.doi.org/10.1017/S0376892912000264
- E. M. Shumba, “Integration of Biodiversity in National Forestry Planning Programme,” Paper prepared for an international workshop “Integration of Biodiversity in National Forestry Planning Programme”, CIFOR Headquarters, Bogor, Indonesia on 13-16 August 2001.
- P. Dewees, B. Campbell, Y. Katerere, A. Sithole, B. A. Cunningham, A. Angelesen and S. Winder, “Managing Miombo Woodlands of Southern Africa; Policies, Incentives and Options for Rural Poor,” Program on forest (PROFOR), Washington DC USA, 2011.
- E. Distefano, “Human-Wildlife Conflict Worldwide: A collection of case studies, analysis of management strategies and good practices,” SARD Initiative Report, FAO, Rome, 2005.
- C. A. Mackenzie, C. A. Chapman and R. Sengupta, “Spatial patterns of illegal resource extraction in Kibale National Park, Uganda”, Environmental Conservation, Vol. 39, No. 1, 2011, pp. 38-50. http://dx.doi.org/10.1017/S0376892911000282
- R. J. Hobbs, S. Arico, J. Aronson, J. S. Baron, P. Bridgewater, V. A. Cramer, P. R. Epstein, J. J. Ewel, C. A. Klink, A. E. Lugo, D. Norton, D. Ojima, D. M. Richardson, E. W. Sanderson, F. Valladares, M. Vila, R. Zamora and M. Zobel, “Novel Ecosystems: Theoretical and Management Aspects of the New Ecological World Order,” Global Ecology and Biogeography, Vol. 15, 2006, pp. 1-7. http://dx.doi.org/10.1111/j.1466-822X.2006.00212.x
- M. A. Hyde, B. T. Wursten and P. Ballings, “Flora of Zimbabwe: Location Details: Mukuvisi Woodland,” Harare, 2013.
- R. Bulton, “The Makabusi Historical Background, Mukuvisi Woodland Association,” Quick Print Publishers, Harare, 1995.
- S. Chiseva, “Influence of Brachystegia Spiciformis and Parinari Curatellifolia on Herbaceous Flora and Soils in Mukuvisi Woodland, Harare,” MSc Thesis, University of Zimbabwe, Harare, 2012.
- B. Campbell, “The Miombo in Transition: Woodlands and Welfare in Africa,” Center for International Forest Research (CIFOR), Bogor, 1996.
- J. Baldock, M.T. Styles, S. Kalbskopf and E. Muchemwa, “The Geology of the Area around Harare Greenstone Belt and Surrounding Granitic Terrain,” Bulletin 94, Zimbabwe Geological Survey, Harare, 1991.
- K. Coates-Palgrave, “Trees of Southern Africa,” 2nd Edition, Kyodo Printing Company Private Limited, Singapore, 1997.
- E. Gandiwa and S. Kativu, “Influence of Fire Frequency on Colophospermum mopane and Combretum apiculatum Woodland Structure and Composition in Northern Gonarezhou National Park, Zimbabwe,” Koedoe, Vol. 51, No. 1, 2009, p. 13.
- StatSoft Inc, “STATISTICA for Windows, Version 6, StatSoft Inc. 2300,” Tulsa, 2001.
- J. A. Ludwig and J. F. Reynolds, “Statistical Ecology, A Primer on Methods and Computing,” John Wiley and Sons, New York, 1988.
- A. M. C. Davies and T. Fearn, “Back to Basics: The Principles of Principal Component Analysis,” Near infrared (NIR) Publications, Chichester, 1992.
- P. E. Green, F. J. Carmone and S. M. Smith, “Multidimensional Scaling: Concepts and Applications,” Allyn and Bacon, London, 1989.
- P. R. Guy, “The Influence of Elephants and Fire on Brachsytegia-Julbernardia Woodland in Zimbabwe,” Journal of Tropical Ecology, Vol. 15, No. 1, 1989, pp. 215- 226. http://dx.doi.org/10.1111/j.1466-822X.2006.00212.x
- I. M. Grundy, “Wood Biomass Estimation in Dry Miombo Woodland in Zimbabwe,” Forest Ecology Management, Vol. 72, No. 2-3, 1995, pp. 109-117. http://dx.doi.org/10.1016/0378-1127(94)03467-B
- E. N. Chidumayo, “Development of Brachsytegia-Julbernardia Woodland after Clear Felling in Central Zambia: Evidence from High Resilience,” Applied Vegetation Science, Vol. 7, 2004, pp. 237-242.
- S. Dondeyne, A. Wijffels, L. B. Emmanuel, J. Deckers, and M. Hermy, “Soils and Vegetation of Angai Forest: Ecological Insights from a Participatory Survey in South Eastern Tanzania,” African Journal of Ecology, Vol. 42, No. 3, pp. 198-207. http://dx.doi.org/10.1111/j.1365-2028.2004.00513.x
- T. Furukawa, K. Fujiwara, S. K. Kiboi and P. B. C. Mutiso, “Can Stumps Tell What People Want: Pattern and Preference of Informal Wood Extraction in an Urban Forest of Nairobi, Kenya,” Biological Conservation, Vol. 144, No. 12, 2011, pp. 3047-3054.
- C. A. McKenzie, C. A. Chapman and R. Sengupta, “Spatial Patterns of Illegal Resource Extraction in Kibale National Park,” Uganda, Foundation for Environmental Conservation, Vol. 39, No. 1, 2011, pp. 38-50. http://dx.doi.org/10.1017/S0376892911000282
- E. N. Chidumayo, “Changes in Miombo Woodland Structure under Different Land Tenure and Use Systems in Central Zambia,” Journal of Biogeography, Vol. 29, No. 12, 2002, pp. 1619-1626. http://dx.doi.org/10.1046/j.1365-2699.2002.00794.x
- A. Sheuyange, G. Oba and R. B. Weladji, “Effects of Anthropogenic Fire History on Savanna Vegetation in Northeastern Namibia,” Journal of Environmental Management, Vol. 75, No. 3, 2005, pp. 189-198. http://dx.doi.org/10.1016/j.jenvman.2004.11.004
- E. Gandiwa, “Effects of Repeated Burning on Woody Vegetation Structure and Composition in a Semi-Arid Southern Africa Savanna,” International Journal of Environmental Science, Vol. 2, No. 2, 2011, pp. 0976-4402.
- B. W. Enslin, A. L. F. Potgieter, H. C. Biggs and R. Biggs, “Long Term Effects of Fire Frequency and Season on the Woody Vegetation Dynamics of the Sclerocarya birrea/Acacia nigrescens Savanna of the Kruger National Park,” Koedoe, Vol. 43, No. 1, 2000, pp. 27-37. http://dx.doi.org/10.4102/koedoe.v43i1.206
- C. M. Ryan and M. Williams, “How Does Fire Intensity and Frequency Affect Miombo Woodland Tree Populations and Biomass?” Ecological Applications, Vol. 21, No. 1, 2011, pp. 48-60. http://dx.doi.org/10.1890/09-1489.1
- S. I. Higgins, W. J. Bond, E. C. February, A. Bronn, D. I. W. Euston-Brown, B. Enslin, N. Govender, L. Rademan, S. O’regan, A. L. F. Potgieter, S. Scheiter, R. Sowry, L. Trollope and W. S. W. Trollope, “Effects of Four Decades of Fire Manipulation on Woody Vegetation Structure in Savanna,” Ecology, Vol. 88, No. 5, 2007, pp. 1119- 1125. http://dx.doi.org/10.1890/06-1664
- R. Condit, R. Sukumar, S. P. Hubbell and R. Foster, “Predicting Population Trends from Size Distributions: A Direct Test in a Tropical Tree Community,” The American Naturalist, Vol. 152, No. 4, 1998, pp. 495-509. http://dx.doi.org/10.1086/286186
- S. J. Wright, H. E. Muller-Landau, R. Condit and S. P. Hubbell, “Gap-Dependent Recruitment, Realized Vital Rate, and Size Distribution of Tropical Trees,” Ecology, Vol. 84, 2003, pp. 3174-3185. http://dx.doi.org/10.1890/02-0038
- A. M. Lykke, “Assessment of Species Composition Change in Savanna Vegetation by Means of Woody Plants’ Size Class Distributions and Local Information,” Biodiversity and Conservation, Vol. 7, No. 10, 1998, pp. 1261-1275. http://dx.doi.org/10.1023/A:1008877819286
- R. J. Hobbs, “Disturbance, Diversity and Invasion: Implications for Conservation,” Conservation Biology, Vol. 6, No. 3, 1992, pp. 325-337. http://dx.doi.org/10.1046/j.1523-1739.1992.06030324.x
- A. Alelign, Y. Yemshaw, D. Teketay and S. Edwards, “Socio-Economic Factors Affecting Sustainable Utilization of Woody Species in Zegie Peninsula, North-Western Ethiopia,” Tropical Ecology, Vol. 52, 2011, pp. 13-24.
- M. Fadiman, “Starvation Taught Me Art: Tree Poaching, Gender and Cultural Shifts in Wood Curio Carving in Zimbabwe,” Ethnobotany Research and Applications, Vol. 6, 2008, pp. 335-346.
- J. O. Oyugi, J. S Brown and J. C. Whelan, “Effects of Human Disturbance on Composition and Structure of Brachystegia Woodland in Arabuko-Sokoke Forest,” Kenya, African Journal of Ecology, Vol. 46, No. 3, 2007, pp. 374-383. http://dx.doi.org/10.1111/j.1365-2028.2007.00850.x
NOTES
*Corresponding author.

