Journal of Environmental Protection
Vol.4 No.4(2013), Article ID:30728,9 pages DOI:10.4236/jep.2013.44044
Size-Resolved Water-Soluble Ionic Composition of Ambient Particles in an Urban Area in Southern Poland
![]()
1Institute of Environmental Engineering, Polish Academy of Sciences, Zabrze, Poland; 2Ecologistics Division, Institute of Environmental Protection Engineering, Wroclaw University of Technology, Wroclaw, Poland.
Email: wioletta@ipis.zabrze.pl
Copyright © 2013 Wioletta Rogula-Kozłowska et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 10th, 2013; revised March 12th, 2013; accepted April 10th, 2013
Keywords: Ambient Aerosol; DEKATI; Mass Size Distribution; SIA; Ammonium Sulfate; Ammonium Nitrate; Neutralization Ratio; Upper Silesia
ABSTRACT
The ambient concentrations of PM-related anions (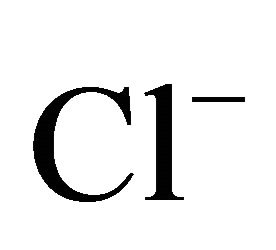 ,
, ,
,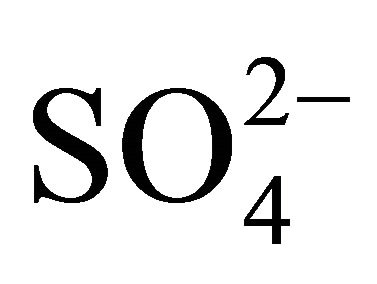 ) and cations (Na+,
) and cations (Na+, 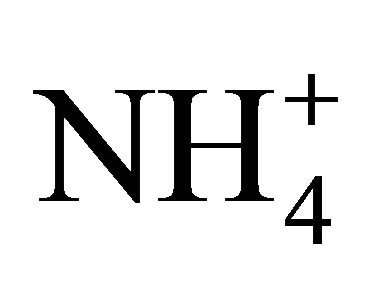 , K+, Ca2+, Mg2+), total and contained in the PM fractions, were investigated in a typical urban area within the Silesian Agglomeration. A DEKATI low pressure impactor (DLPI) was used to sample PM and separate it into 13 fractions. The PM concentrations were determined gravimetrically, the ion content of the PM water extracts—by means of ion chromatography (Herisau Metrohm AG ion chromatograph). In general, sulfate, nitrate, and ammonia had the greatest ambient concentrations. PM1 contained over 60% of the PM-related sulfate and nitrate mass and 90% of the ammonia mass. Also the majority of Na+ and Cl− were bound onto fine particles. Instead, more of the PM-related K+, Ca2+ and Mg2+ mass were in PM2.5-10 than in PM2.5. In the fine particles (sub-fractions of PM1.6) sulfate, nitrate and ammonia occur mainly as (NH4)2SO4 and NH4NO3. In the sub-fractions of PM1.6-10 sulfate and nitrate might also occur as K2SO4, CaSO4, Ca(NO3)2 or NaNO3.
, K+, Ca2+, Mg2+), total and contained in the PM fractions, were investigated in a typical urban area within the Silesian Agglomeration. A DEKATI low pressure impactor (DLPI) was used to sample PM and separate it into 13 fractions. The PM concentrations were determined gravimetrically, the ion content of the PM water extracts—by means of ion chromatography (Herisau Metrohm AG ion chromatograph). In general, sulfate, nitrate, and ammonia had the greatest ambient concentrations. PM1 contained over 60% of the PM-related sulfate and nitrate mass and 90% of the ammonia mass. Also the majority of Na+ and Cl− were bound onto fine particles. Instead, more of the PM-related K+, Ca2+ and Mg2+ mass were in PM2.5-10 than in PM2.5. In the fine particles (sub-fractions of PM1.6) sulfate, nitrate and ammonia occur mainly as (NH4)2SO4 and NH4NO3. In the sub-fractions of PM1.6-10 sulfate and nitrate might also occur as K2SO4, CaSO4, Ca(NO3)2 or NaNO3.
1. Introduction
To assess the impact of atmospheric aerosol on the environment, including air quality, ecosystems, human health and climate change, it is necessary to know its concentration, chemical composition and mass size distribution of PM (ambient particulate matter) components [1-8]. Knowledge of the mass size distribution of PM components is helpful in determining mechanisms of aerosol formation, as well as physical and chemical changes, it is subjected to on a given area [9-13].
Besides the obvious and relatively well-recognized relation between the content of various toxic compounds in ambient dust and human health [14-17], another example of a dust chemical composition impact on the environment, is the effect of some water-soluble inorganic compounds on the acidity and conductivity of aerosols. Under certain conditions, the water-soluble sulfur and nitrogen compounds contained in the dust, contribute to acidification of precipitation and/or deposition, whereas the deposition of particles rich in the water-soluble calcium, magnesium, potassium or sodium compounds, increases the alkalinity of the environment [18-21].
Water-soluble ions, next to elemental carbon and organic matter, dominate the mass of PM. In urban areas, mass of sulfates 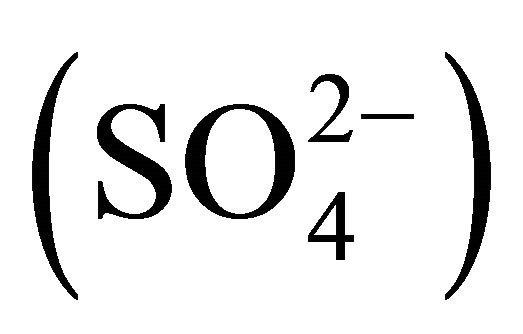 and nitrates
and nitrates 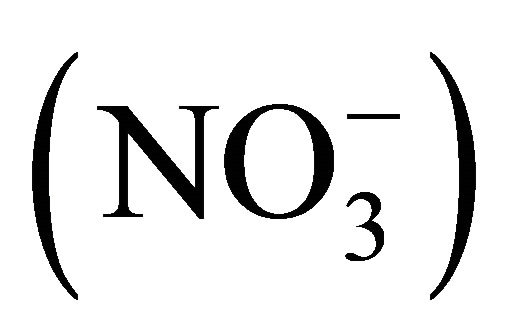 associated with particulate matter is even ~80% of all water extracted ions (Table 1, [22]) and ~15% - 50% of the total mass of PM2.5 (fine particles, with aerodynamic diameters not exceeding 2.5 µm) [23-26].
associated with particulate matter is even ~80% of all water extracted ions (Table 1, [22]) and ~15% - 50% of the total mass of PM2.5 (fine particles, with aerodynamic diameters not exceeding 2.5 µm) [23-26].
Sulfates, nitrates and ammonia are used to determine the share of secondary inorganic aerosol (SIA) in the mass of ambient dust. Oxidation of SO2 in the air, then a binary nucleation of H2SO4-H2O and ternary H2SO4- H2O-NH3, results in the formation of dust particles, mostly smaller than 1 µm [19,21,27,28]. These particles, together with nitrate (V) ammonium emerging in the analogous reaction of nitric acid (V) with ammonia, form
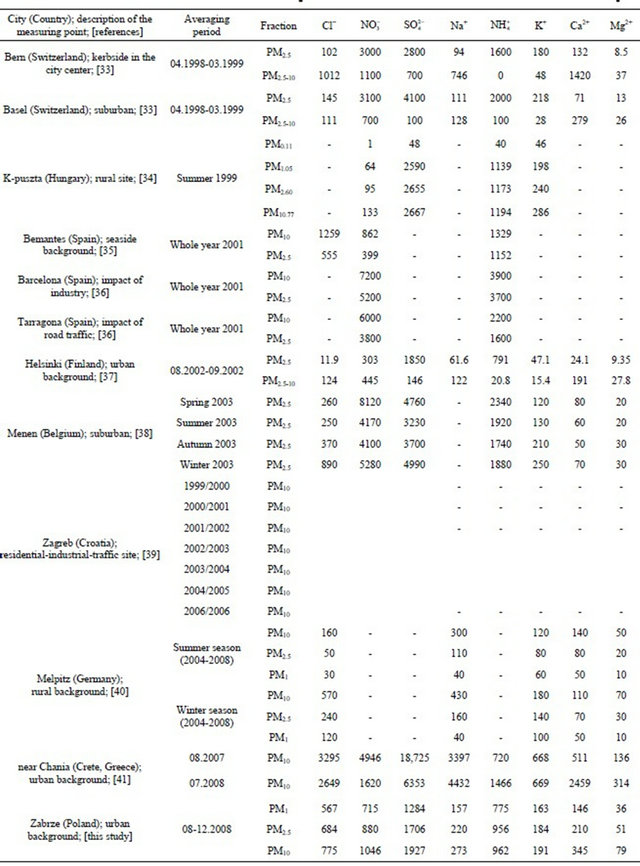
Table 1. Ambient concentrations of water-soluble ions (ng·m−3) related to various PM fractions at various sites in Europe.
the SIA. In the air poor in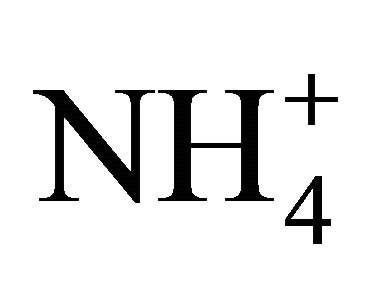 , sulfuric acid H2SO4 can react with mineral dust or sea salt components, generally creating coarse particles of CaSO4 or (Na)2SO4.
, sulfuric acid H2SO4 can react with mineral dust or sea salt components, generally creating coarse particles of CaSO4 or (Na)2SO4.
The goal of the work was to determine concentration and mass size distribution of eight water-soluble ions (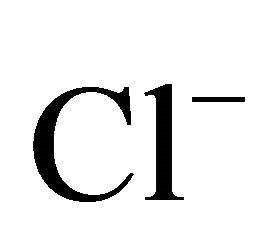 ,
,  ,
, 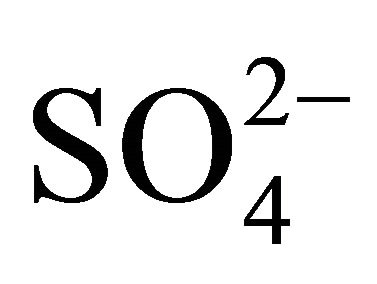 , Na+,
, Na+, 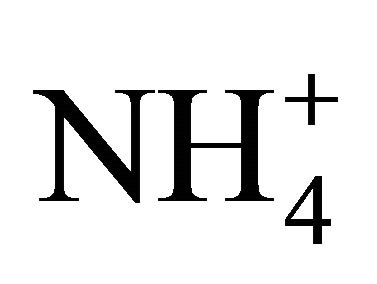 , K+, Ca2+, Mg2+) related to thirteen PM fractions in a typical urban area of southern Poland. Possible chemical composition of secondary inorganic aerosol in 13 dust fractions was also estimated.
, K+, Ca2+, Mg2+) related to thirteen PM fractions in a typical urban area of southern Poland. Possible chemical composition of secondary inorganic aerosol in 13 dust fractions was also estimated.
2. Material and Methods
The site of experiment (Zabrze, Poland, Figure 1) is located in area representative of the air pollution conditions for the central part of Upper Silesia and it meets the criteria of urban background site (Directive 2008/50/EC). Conditions at this point, characterize well dust concentration in residential areas exposed to municipal and industrial emissions in the Upper Silesia [29].
Samples have been collected from August to December 2008. Fourteen measurements were carried out and each lasted about a week. Dust was collected using a thirteen stage DEKATI low pressure impactor (DLPI) [13].
Masses of dust collected on aluminum substrates, were determined by weighing substrates before and after exposure, on a Mettler Toledo microbalance (accuracy 2 µg). Before weighing the substrates were kept in weighing room for 48 hours (temperature 20˚C ± 2˚C, relative air humidity 48% ± 5%). Concentrations of PM fractions were calculated by dividing each fraction’s mass by the volume of air, from which it was collected. Dust samples were kept in a refrigerator in tight and lightproof containers until the analysis.
Thirteen samples were fixed for chromatography analysis - for each fraction, a collective sample from 14 weeks was prepared. Samples were placed in ROTH extraction containers. For the extraction, 50 cm3 of deionized water was added to each container and the containers were tightly capped to prevent leaking during the extraction. Extracts were then placed in an ultrasonic
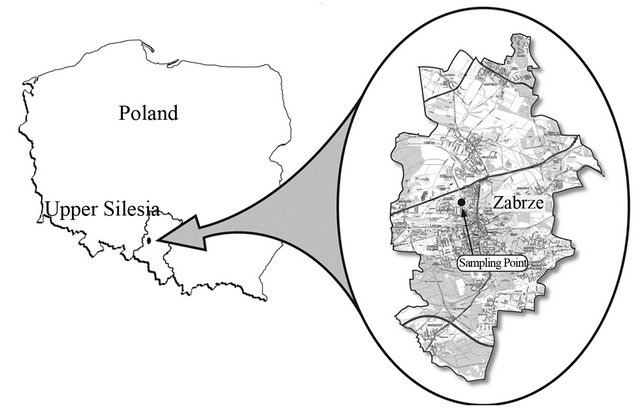
Figure 1. Location of the sampling point.
bath (60 min), at a temperature not exceeding 15˚C. Then, the extraction containers were placed in a vortex mixer and shaken overnight at about 18˚C and 60 cycles per minute. Extracts were then filtered through a CRONUS microporous filter with a PES membrane with a porosity of 0.2 microns.
The ion content in the extracts was determined using Metrohm ion chromatograph (Metrohm Herisau AG, Switzerland), equipped with 818 IC Pump, 819 IC Detector, 837 IC Eluent Degasser, 830 IC Interface, 820 IC Separation Center, Metrodata 2.3 programme). The method was previously validated on the basis of certified reference material (CRM Fluka products nos. 89316 and 89886, the standard recovery ranged in 92% - 109%). Detection limits were at the level of: 0.02 mg·l−1 for NH4+, 0.05 mg·l−1 for Cl–, 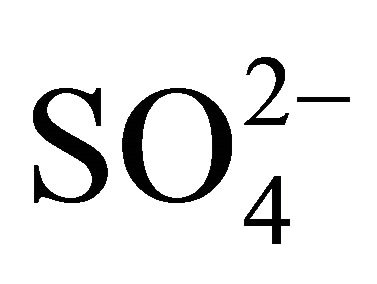 and K+, 0.07 mg·l−1 for
and K+, 0.07 mg·l−1 for  and Na+, 0.12 mg·l−1 for Ca2+ and Mg2+.
and Na+, 0.12 mg·l−1 for Ca2+ and Mg2+.
3. Results and Discussion
PM-related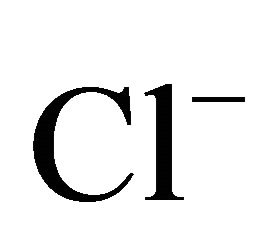 ,
,  ,
, 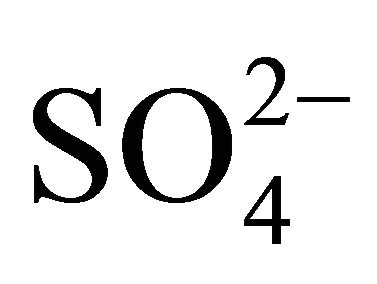 , Na+,
, Na+, 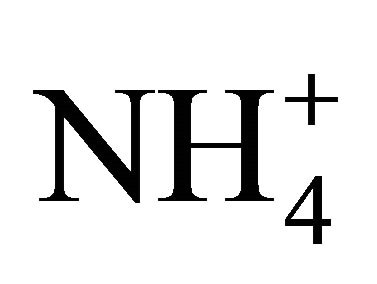 , K+, Ca2+, Mg2+ concentrations from Zabrze, were compared with concentrations of these ions from various sites in Europe (Table 1). The concentration of the PM1-related ion was calculated by summing its concentrations in following fractions: 0.03 - 0.06 µm, 0.06 - 0.108 µm, 0.108 - 0.17 µm, 0.17 - 0.26 µm, 0.26 - 0.40 µm, 0.40 - 0.65 µm and 0.65 - 1.0 µm. In the case of ions associated with PM2.5, additionally concentrations from fractions: 1.0 - 1.6 µm and 1.6 - 2.5 µm were included and in case of PM10, besides previously mentioned, ion concentrations of 2.5 - 4.4 µm; 4.4 - 6.8 µm and 6.8 - 10.0 µm range were summed.
, K+, Ca2+, Mg2+ concentrations from Zabrze, were compared with concentrations of these ions from various sites in Europe (Table 1). The concentration of the PM1-related ion was calculated by summing its concentrations in following fractions: 0.03 - 0.06 µm, 0.06 - 0.108 µm, 0.108 - 0.17 µm, 0.17 - 0.26 µm, 0.26 - 0.40 µm, 0.40 - 0.65 µm and 0.65 - 1.0 µm. In the case of ions associated with PM2.5, additionally concentrations from fractions: 1.0 - 1.6 µm and 1.6 - 2.5 µm were included and in case of PM10, besides previously mentioned, ion concentrations of 2.5 - 4.4 µm; 4.4 - 6.8 µm and 6.8 - 10.0 µm range were summed.
Most of ions’ concentrations in Zabrze were comparable to concentrations noted between 1998-2008 in Europe. For example, concentration of sulfates in particulate matter in Zabrze, was comparable to the concentration recorded at two sites in Switzerland, suburban station in Menen (Belgium) and urban background station in Helsinki (Finland). Generally, higher concentrations than in Zabrze are listed in Asian countries [13,30, 31]. Concentration of Cl− associated with fine dust in Zabrze was extraordinarily high comparing to values recorded in other parts of Europe and similar to concentrations of chlorine in Menen and Melpitz, recorded in these cities during the winter season (Table 1).
Sulfates, nitrates and ammonia associated with PM1, PM2.5 and PM10, had the highest concentration of the eight analyzed ions in Zabrze (Tables 1 and 2). Average mass shares of 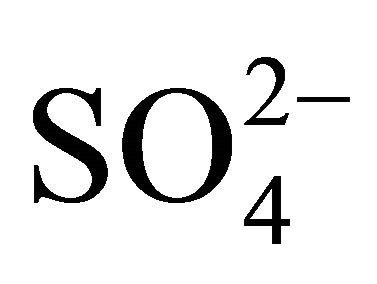 and
and  in the PM2.5, are about 80% of the total mass (the sum of the masses in all 13 fractions) of sulfates and nitrates, and the average mass share of
in the PM2.5, are about 80% of the total mass (the sum of the masses in all 13 fractions) of sulfates and nitrates, and the average mass share of 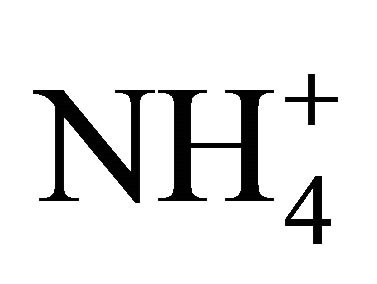 is even up to 98% of the total mass of ammonia. More than 60% of sulfates and nitrates mass were related to particles with an aerodynamic diameter
is even up to 98% of the total mass of ammonia. More than 60% of sulfates and nitrates mass were related to particles with an aerodynamic diameter
Table 2. Ambient concentrations of PM (µg·m−3) and PM-related ions (ng·m−3) from 13 original DLPI fractions of PM at the urban background site.
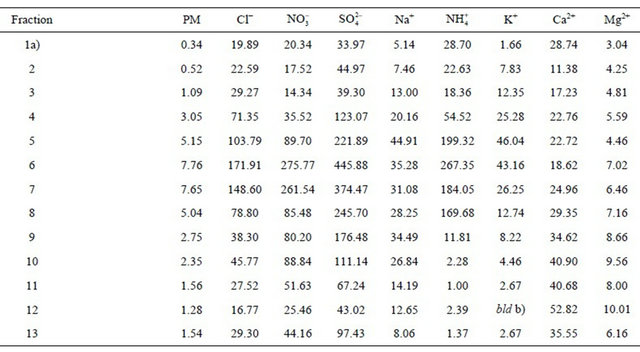
a. 1—0.03 - 0.06 µm; 2—0.06 - 0.108 µm; 3—0.108 - 0.17 µm; 4—0.17 - 0.26 µm; 5—0.26 - 0.40 µm; 6—0.40 - 0.65 µm; 7—0.65 - 1.0 µm; 8—1.0 - 1.6 µm; 9—1.6 - 2.5 µm; 10—2.5 - 4.4 µm; 11—4.4 - 6.8 µm; 12—6.8 - 10.0 µm; 13—>10.0 µm; b. below limit of detection.
≤1 μm. As to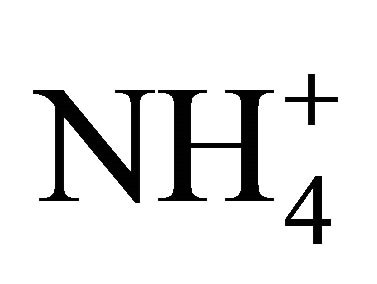 , it was close to 90%.
, it was close to 90%.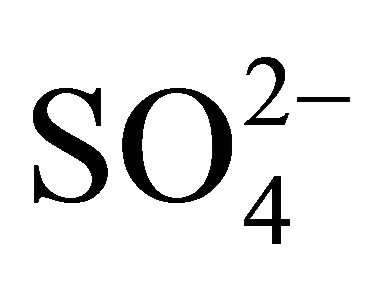 ,
,  and
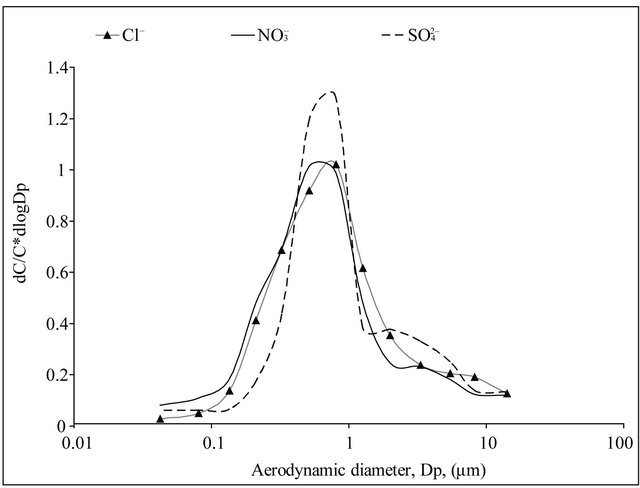
and 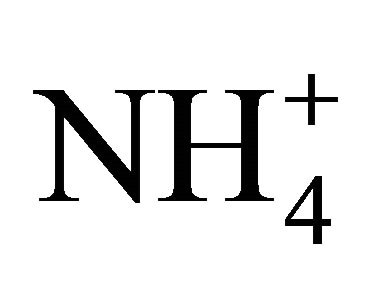 concentrations were highest in the range of 0.26 - 1 µm. Very similar, bimodal mass size distribution of
concentrations were highest in the range of 0.26 - 1 µm. Very similar, bimodal mass size distribution of 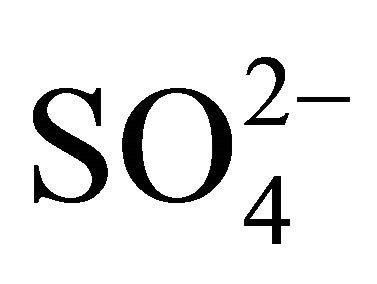 and
and , with a maximum occurring between 0.4 - 1 μm (Figure 2(a)), means that these ions are parts of the same compounds in the dust. The main mechanism of their formation are presumably the transformation processes of PM gaseous precursors occurring in the atmosphere. PM-related
, with a maximum occurring between 0.4 - 1 μm (Figure 2(a)), means that these ions are parts of the same compounds in the dust. The main mechanism of their formation are presumably the transformation processes of PM gaseous precursors occurring in the atmosphere. PM-related 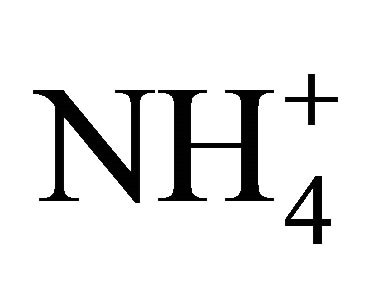 had multimodal mass size distribution, with a one maximum occurring in the range of 0.4 - 1 μm and two maxima between 1.6 - 10 μm (Figure 2(b)).
had multimodal mass size distribution, with a one maximum occurring in the range of 0.4 - 1 μm and two maxima between 1.6 - 10 μm (Figure 2(b)).
On the areas where sea spray or sea water evaporation (marine aerosols) and road salt are main sources of sodium and chloride, ambient concentrations of Na+ and Cl− related to PM2.5-10 (coarse dust, ambient particles with aerodynamic diameters exceeding 2.5 and not greater than 10 µm) are generally higher than the concentrations of PM1- and PM2.5-related Na+ and Cl− (Table 1). It is clear that in Zabrze, Na+ and Cl− are related mostly with fine dust particles [26,32]. PM2.5-related Na+ and 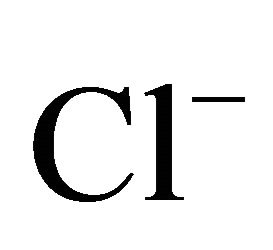 were respectively 80 and 85% of their total concentration in the air of Zabrze. The highest concentrations of PMrelated Na+ and
were respectively 80 and 85% of their total concentration in the air of Zabrze. The highest concentrations of PMrelated Na+ and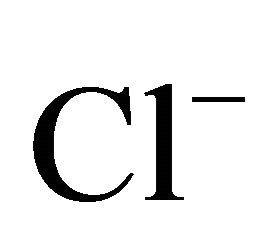 , occurred in similar particle sizes range, as in the case of highest
, occurred in similar particle sizes range, as in the case of highest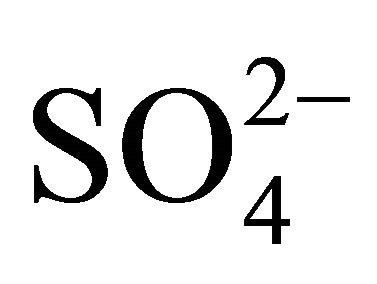 ,
,  and
and 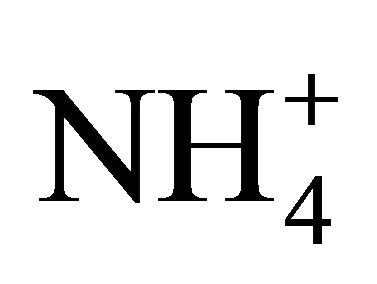 concentrations (Table 2). Both, Na+ and Cl−, were characterized by unimodal mass size distribution and its maximum occurred in the range of 0.4 - 1 μm (Figures 2(a) and (b)). This indicates the anthropogenic origin of these ions (combustion processes). It is most likely that Na+ and
concentrations (Table 2). Both, Na+ and Cl−, were characterized by unimodal mass size distribution and its maximum occurred in the range of 0.4 - 1 μm (Figures 2(a) and (b)). This indicates the anthropogenic origin of these ions (combustion processes). It is most likely that Na+ and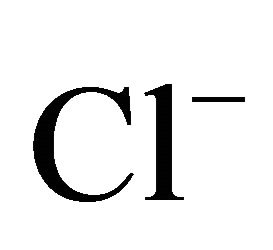 , occur in the dust mainly as a sodium chloride.
, occur in the dust mainly as a sodium chloride.
The concentration of K+, Mg2+ and Ca2+ associated with each of 13 fractions, did not exceed 53 ng·m−3 (Table 1). Masses of these cations were distributed differently among PM fractions. More than 95% of the total mass of K+ was concentrated in the PM2.5, over 25% of which were PM0.26-0.4 and PM0.4-0.65. Distribution of Ca2+ and Mg2+ masses among 13 fractions was more variable, although the share of PM2.5-10-related ions’ mass, was much bigger than their contribution in the fine dust particles amount, and was more than 50% of total mass of these ions in the Zabrze air.
Potassium and calcium were characterized by unimodal mass distribution with a maximum—as in the case of 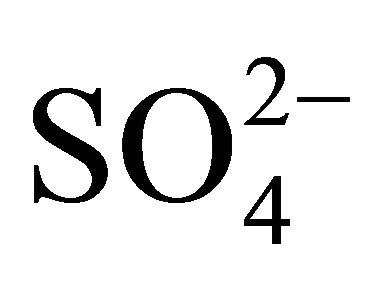
 , Na+ and Cl−—in the range of 0.26 - 0.65 μm (Figure 1(b)), whereas magnesium was determined with multimodal size mass distribution, without clearly dominant maximum. Highest potassium concentrations occurred for particles in the range of 0.17 - 1 μm (Table 2). However, higher Mg2+ and Ca2+ concentrations occurred for particles with an aerodynamic diameter larger than 2.5 μm. Therefore, it seems that K+ and Ca2+ may be present in the compounds with
, Na+ and Cl−—in the range of 0.26 - 0.65 μm (Figure 1(b)), whereas magnesium was determined with multimodal size mass distribution, without clearly dominant maximum. Highest potassium concentrations occurred for particles in the range of 0.17 - 1 μm (Table 2). However, higher Mg2+ and Ca2+ concentrations occurred for particles with an aerodynamic diameter larger than 2.5 μm. Therefore, it seems that K+ and Ca2+ may be present in the compounds with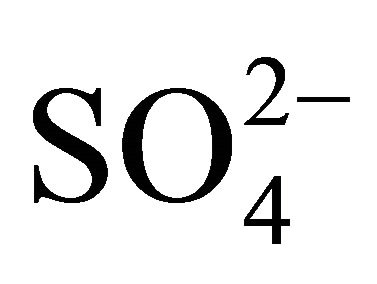 ,
,  , Na+ and Cl− ions, and their most probable source in Zabrze air are combustion processes. Relatively high proportion of Mg2+ in the coarse fraction of particulate matter, proves that mechanical processes, including re-suspension of the soil and road dust could have had an influence on these ions concentration levels.
, Na+ and Cl− ions, and their most probable source in Zabrze air are combustion processes. Relatively high proportion of Mg2+ in the coarse fraction of particulate matter, proves that mechanical processes, including re-suspension of the soil and road dust could have had an influence on these ions concentration levels.
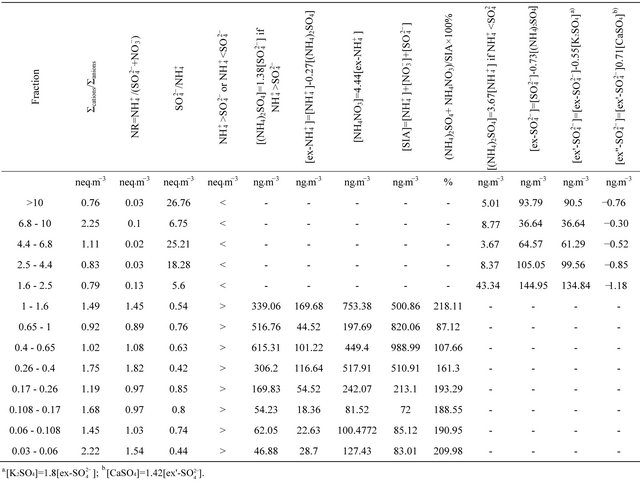
To assess the neutralizing capacity of occurring simultaneously in the air sulfates and nitrates by ammonium ion, neutralizing ratio (NR) was calculated for each fraction of particulate matter. NR is the ratio of 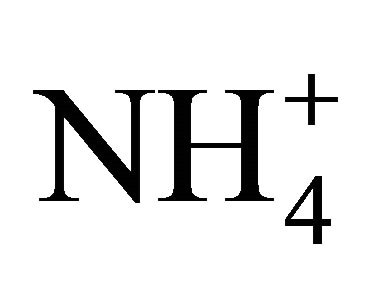 concentration (in normal equivalent, neq·m−3) and the sum of
concentration (in normal equivalent, neq·m−3) and the sum of  and
and 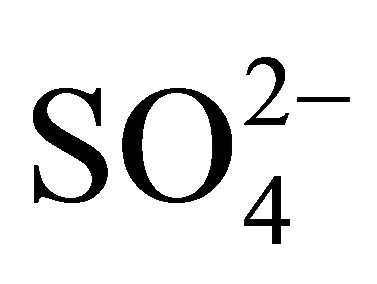 concentrations (in neq·m−3)— Table 3.
concentrations (in neq·m−3)— Table 3.
For particles not greater than 1.6 µm, NR values ranged from ≈ 1 (PM0.65-1, PM 0.17-0.26, PM0.108-0.17 and PM0.06-0.108) to 1.82 (PM0.26-0.4). It means that the amount of 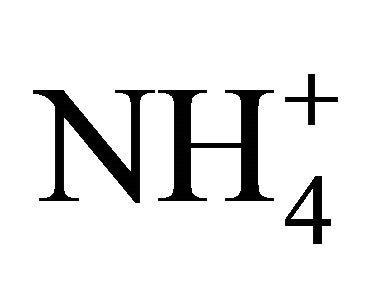 related to these dust fractions, was sufficient to neutralize sulfuric and nitric acid completely. This result also proves that ambient fine dust (PM1.6) in Zabrze is alkaline (NR ≥ 1).
related to these dust fractions, was sufficient to neutralize sulfuric and nitric acid completely. This result also proves that ambient fine dust (PM1.6) in Zabrze is alkaline (NR ≥ 1).
Stoichiometric ratio for (NH4)2SO4 of 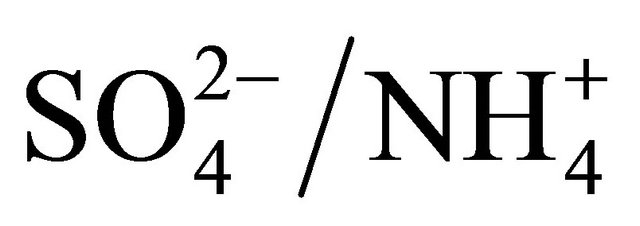 is 2.67. In all fractions of particles ≤ 1.6 µm, the ratio of
is 2.67. In all fractions of particles ≤ 1.6 µm, the ratio of 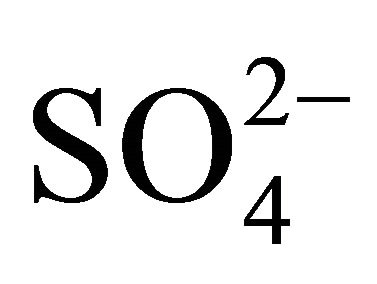 and
and 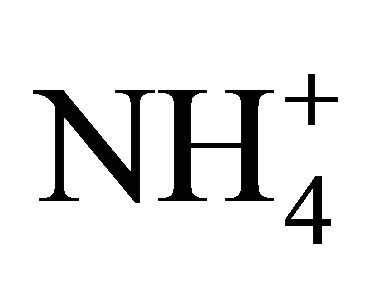 (in neq·m−3) is much lower than 2.67. It confirms the previous deduction, that PM1.6-related
(in neq·m−3) is much lower than 2.67. It confirms the previous deduction, that PM1.6-related
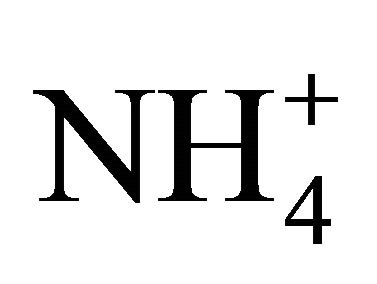 in Zabrze occurred in a greater amount than needed to react with the PM1.6-related
in Zabrze occurred in a greater amount than needed to react with the PM1.6-related 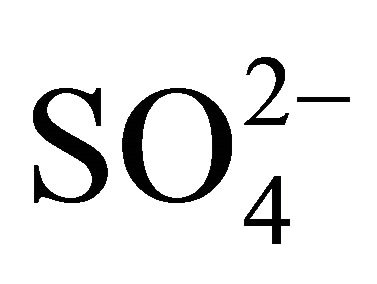 completely. Also the condition
completely. Also the condition 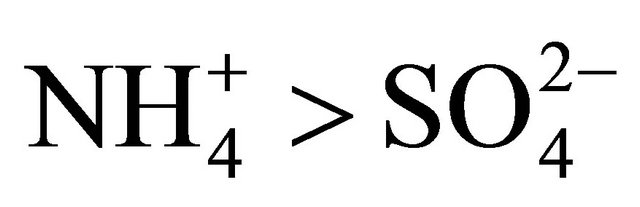 (in neq·m−3) is satisfied. Therefore, the concentration of (NH4)2SO4 may be estimated from the formula:
(in neq·m−3) is satisfied. Therefore, the concentration of (NH4)2SO4 may be estimated from the formula:
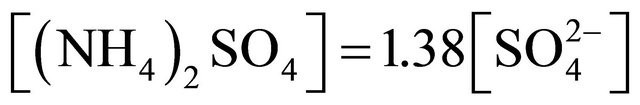 (1)
(1)
The concentration of (NH4)2SO4 associated with particles ≤ 1.6 µm, fit within the limits of 615.31 ng·m−3 (PM0.4-0.65) to 46.88 ng·m−3 for PM0.03-0.06. The amount (concentration) of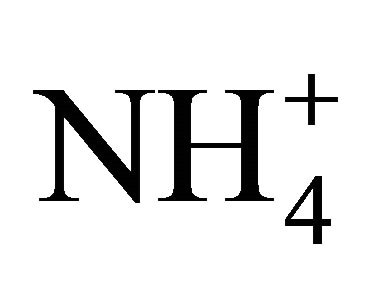 , remaining after reaction with
, remaining after reaction with 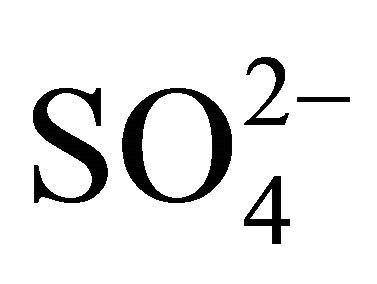 (ammonium ion excess
(ammonium ion excess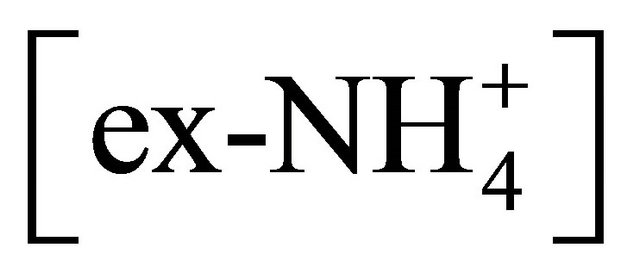 ) and ammonium nitrate concentration associated with each fractions of particles ≤ 1.6 µm, was calculated from the following formulas:
) and ammonium nitrate concentration associated with each fractions of particles ≤ 1.6 µm, was calculated from the following formulas:
 (a)
(a)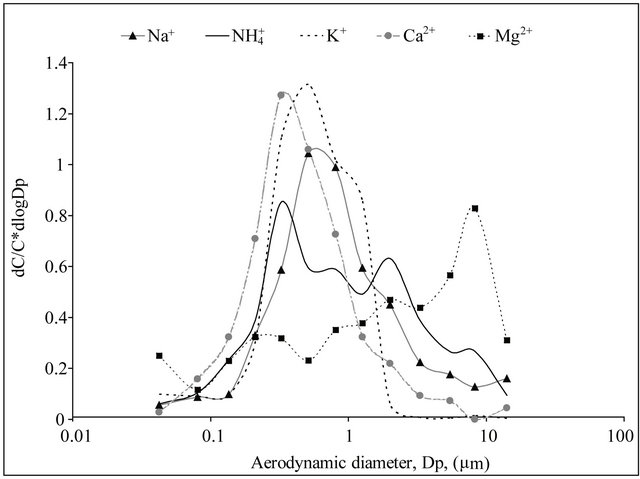 (b)
(b)
Figure 2. Mass size distribution of PM-related ions in Zabrze, Poland.
Table 3. Proportions of the ionic equivalent concentrations and probable composition of secondary inorganic aerosol in 13 original DLPI fractions of PM at the urban background site in Zabrze, Poland.
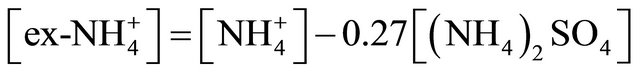 (2)
(2)
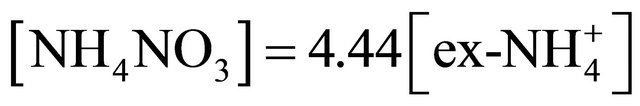 (3)
(3)
NH4NO3 concentration ranged from 753.38 ng·m−3 (for PM1-1.6) to 81.52 ng·m−3 (for PM0.108-0.17).
(NH4)2SO4 and NH4NO3 concentrations sum share, in a total SIA concentration 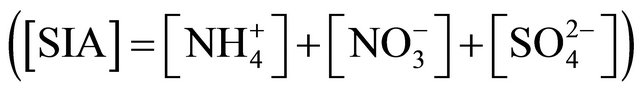 for fractions of particles ≤ 1.6 µm, is shown in Table 3. For the fraction with ((NH4)2SO4+NH4NO3)/SIA value exceeding 1, the share is overestimated. Still, stoichiometric calculations that have been carried out, show that these two compounds constitute the entirety of SIA in ambient particles not greater than 1.6 µm. The most probable distribution of (NH4)2SO4 and NH4NO3 concentrations between the sum of these compounds concentrations were obtained for PM0.65-1 and PM0.4-0.65, where the share of ((NH4)2SO4 + NH4NO3) in the SIA did not exceed 100%. There are also these two fractions, in which the predominant part in the SIA takes ammonium sulfate, while the concentrations of these two dust fractions in the air are the highest of all 13 (Table 2).
for fractions of particles ≤ 1.6 µm, is shown in Table 3. For the fraction with ((NH4)2SO4+NH4NO3)/SIA value exceeding 1, the share is overestimated. Still, stoichiometric calculations that have been carried out, show that these two compounds constitute the entirety of SIA in ambient particles not greater than 1.6 µm. The most probable distribution of (NH4)2SO4 and NH4NO3 concentrations between the sum of these compounds concentrations were obtained for PM0.65-1 and PM0.4-0.65, where the share of ((NH4)2SO4 + NH4NO3) in the SIA did not exceed 100%. There are also these two fractions, in which the predominant part in the SIA takes ammonium sulfate, while the concentrations of these two dust fractions in the air are the highest of all 13 (Table 2).
NR for particles greater than 1.6 µm, was much smaller than 1 (Table 3). However, it doesn’t mean that ions associated with these particles are not fully neutralized. The concentration sum ratio of anions to cations (Σcations/Σanions, in neq·m−3) is in the range of 1, for all fractions.
In all fractions of particles greater than 1.6 µm, the concentration ratios of 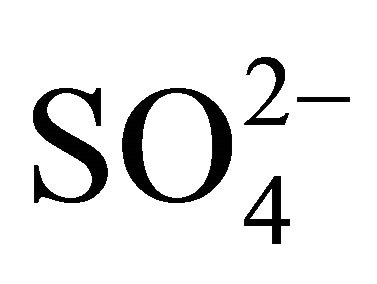 and
and 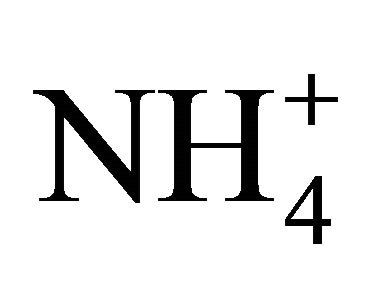 (in neq·m−3) is considerably higher than 2.67. Also the relation
(in neq·m−3) is considerably higher than 2.67. Also the relation 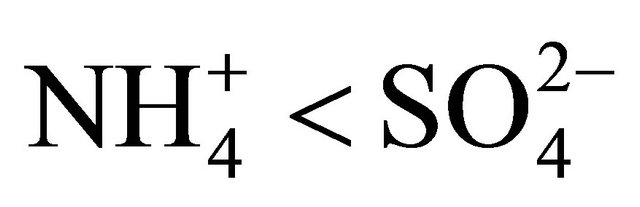 is satisfied (concentrations in neq·m−3). It means that in these PM fractions,
is satisfied (concentrations in neq·m−3). It means that in these PM fractions, 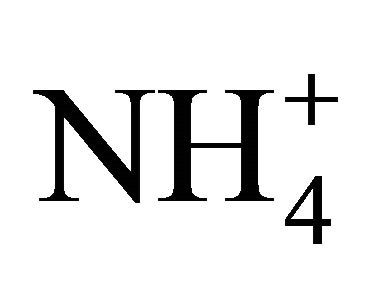 could neutralize some part of
could neutralize some part of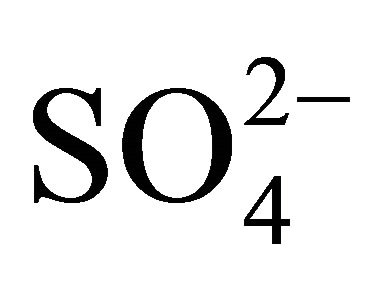 , forming (NH4)2SO4 but there was not enough of
, forming (NH4)2SO4 but there was not enough of 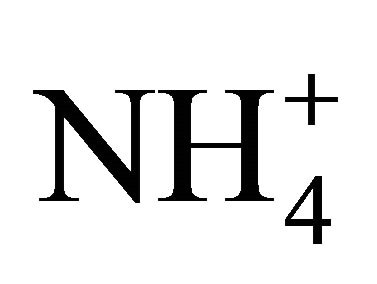 to react the whole
to react the whole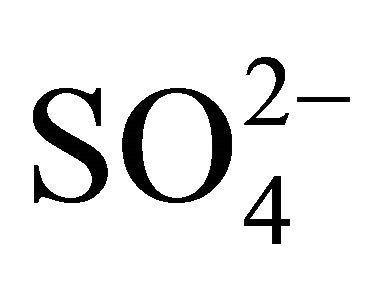 . Thus, there was not enough of
. Thus, there was not enough of 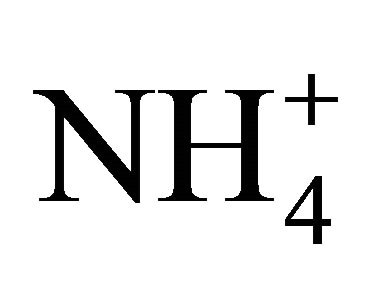 to form ammonium nitrate. Therefore, the (NH4)2SO4 concentration for particles greater than 1.6 µm, can be calculated from the for mula:
to form ammonium nitrate. Therefore, the (NH4)2SO4 concentration for particles greater than 1.6 µm, can be calculated from the for mula:
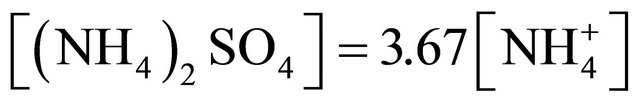 (4)
(4)
The concentration of (NH4)2SO4 associated with particles greater than 1.6 µm, ranged from 3.67 ng·m−3 (PM4.4-6.8) to 43.34 ng·m−3 for PM1.6-2.5.
It is impossible to determine precisely concentrations of all compounds constituting the secondary inorganic aerosol in Zabrze, still, estimating on the basis of stoichiometric relations. However, it can be shown that the amount of 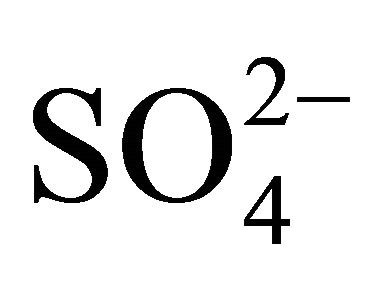 in the particles greater than 1.6 µm is enough to react the whole
in the particles greater than 1.6 µm is enough to react the whole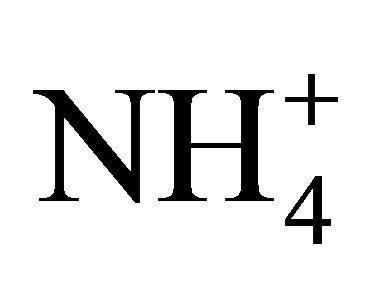 .
.
The rest of the 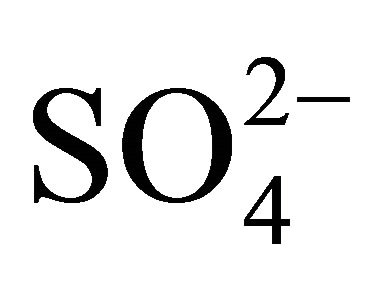 (sulfate ion excess
(sulfate ion excess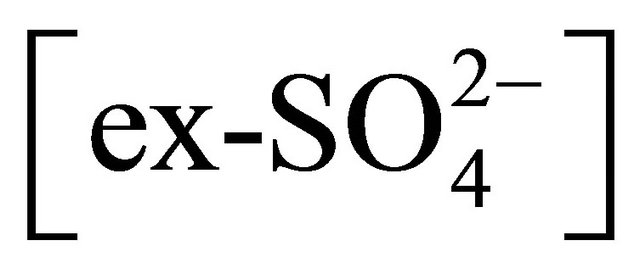 ) could react i.a. with potassium and calcium ions, forming K2SO4 and CaSO4. This would prove specific, similar to
) could react i.a. with potassium and calcium ions, forming K2SO4 and CaSO4. This would prove specific, similar to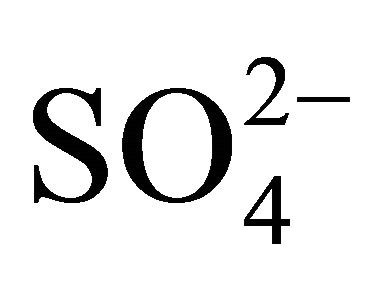 , mass size distributions of K+, Ca2+ (Figures 2(a) and (b)). The concentration of the rest of
, mass size distributions of K+, Ca2+ (Figures 2(a) and (b)). The concentration of the rest of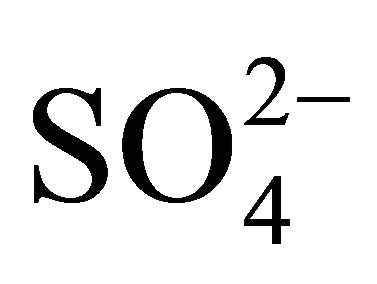 , that remained after:
, that remained after:
• reaction with NH4+ forming (NH4)2SO4; 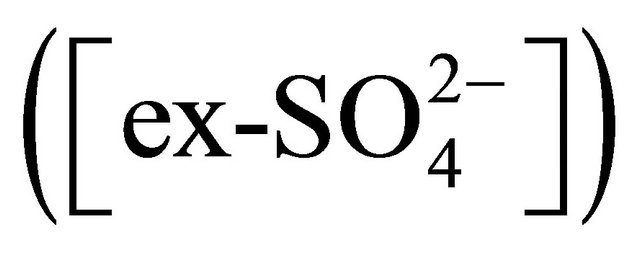 • reaction with
• reaction with 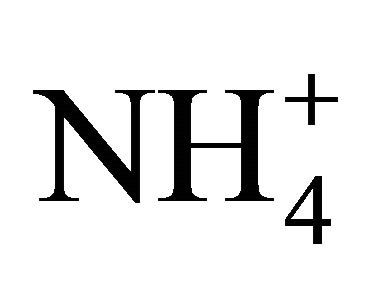 forming (NH4)2SO4 and K+ forming K2SO4;
forming (NH4)2SO4 and K+ forming K2SO4;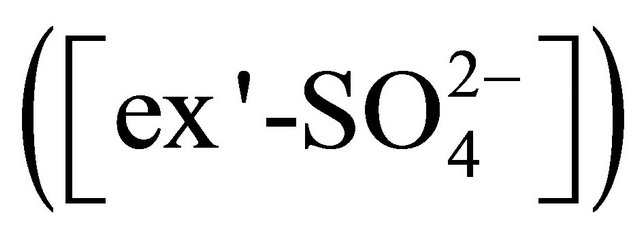 • reaction with
• reaction with 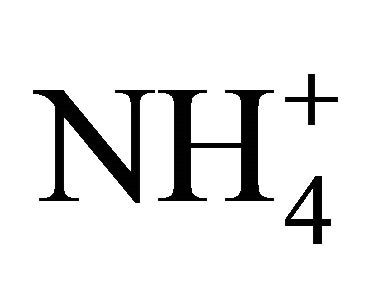 forming (NH4)2SO4, K+ forming K2SO4 and Ca2+ forming CaSO4;
forming (NH4)2SO4, K+ forming K2SO4 and Ca2+ forming CaSO4; 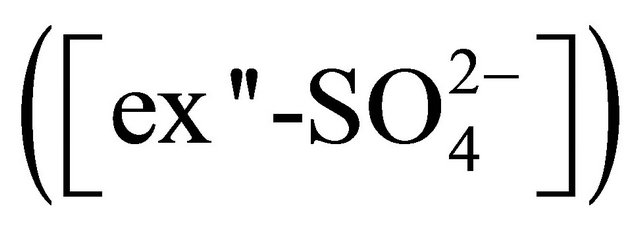
can be calculated (in PM1.6-2.5, PM2.5-4.4, PM4.4-6.8, PM6.8-10, PM>10) from the following formula:
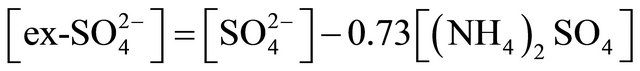 (5)
(5)
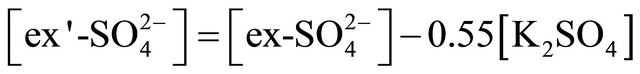 (6)
(6)
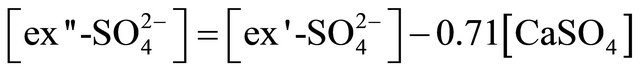 (7)
(7)
Using values listed in Table 3, it can be concluded that for PM>1.6, there was not enough sulfate ion to complete reaction of calcium ions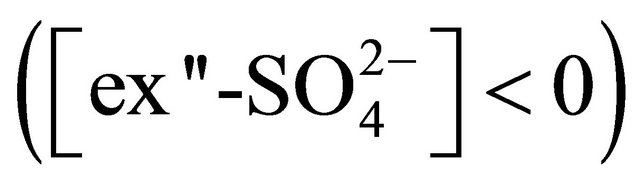 .
.
Therefore, it can be concluded, that the secondary inorganic aerosol in Zabrze, in the case of compounds occuring in particles greater than 1.6 µm, is mainly composed of ammonium sulfate, potassium sulfate and calcium sulfate. Certainly, there are also nitrates in these particles, however, in contrast to particles not greater than 1.6 µm, there is no ammonium nitrate but probably NaNO3 and/or Ca(NO3)2.
4. Conclusions
Most of ions’ concentrations in Zabrze were comparable to concentrations presented in the literature. Generally, higher concentrations than in Zabrze are listed in Asian countries, this concerns particularly to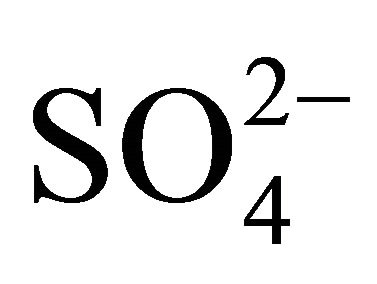 ,
, 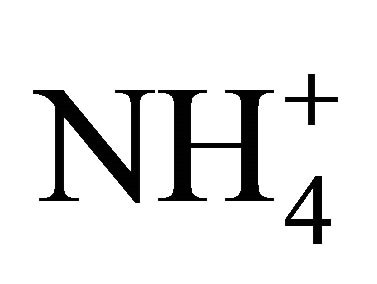 , K+, Mg2+ and Ca2+ . Concentration of
, K+, Mg2+ and Ca2+ . Concentration of 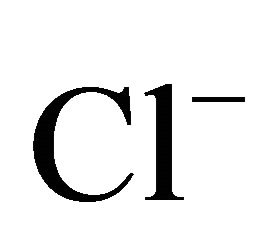 associated with fine dust in Zabrze was extraordinarily high, comparing to values recorded in other parts of the world.
associated with fine dust in Zabrze was extraordinarily high, comparing to values recorded in other parts of the world.
Sulfates, nitrates and ammonium had the highest concentration of the eight analyzed ions in Zabrze. More than 60% of 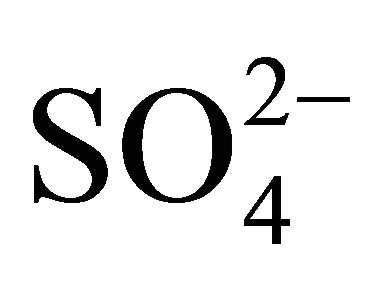 and
and  and 90% of
and 90% of 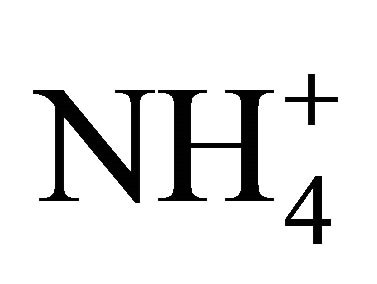 mass, was concentrated in particles with an aerodynamic diameter ≤ 1 micron. Na+ and Cl− were also mostly associated with fine dust particles. Ions mentioned above, as well as K+ and Ca2+, had similar mass size distributions, and generally, maxima of these distributions were in the same particle size ranges. This indicates the anthropogenic origin of seven of eight analyzed ions (combustion processes), associated with dust in Zabrze.
mass, was concentrated in particles with an aerodynamic diameter ≤ 1 micron. Na+ and Cl− were also mostly associated with fine dust particles. Ions mentioned above, as well as K+ and Ca2+, had similar mass size distributions, and generally, maxima of these distributions were in the same particle size ranges. This indicates the anthropogenic origin of seven of eight analyzed ions (combustion processes), associated with dust in Zabrze.
Relatively high proportion of Mg2+ in the coarse fraction of particulate matter, proves that mechanical processes, including re-suspension of the soil and road dust could have had an influence on Mg2+ concentration in the air.
In particles not greater than 1.6 µm, the amount of ammonium ion is sufficient to neutralize sulfuric and nitric acid, therefore, in dust precursors gas conversions, ammonium sulfate and nitrate are formed. In fractions of particles greater than 1.6 μm, the amount of ammonium ion is not sufficient to neutralize the nitric acid. Therefore, in these fractions, inorganic aerosol is composed of ammonium sulfate and other compounds, including K2SO4 and CaSO4, and also NaNO3 and/or Ca(NO3)2.
5. Acknowledgements
The work was partially supported by grant No. N N523 564038 from the Polish Ministry of Science and Higher Education.
REFERENCES
- J. Schwartz, “Air Pollution and Daily Mortality: A Review and Meta-Analysis,” Environmental Research, Vol. 64, No. 1, 1994, pp. 36-52. doi:10.1006/enrs.1994.1005
- K. R. Spurny, “Chemical Mixtures in Atmospheric Aerosols and Their Correlation to Lung Diseases and Lung Cancer Occurrence in the General Population,” Toxicology Letters, Vol. 88, No. 1-3, 1996, pp. 271-277. doi:10.1016/0378-4274(96)03749-6
- C. A. Pope and D. W. Dockery, “Health Effects of Fine Particulate Air Pollution: Lines that Connect,” Journal of the Air & Waste Management Association, Vol. 56, No. 6, 2006, pp. 709-742. doi:10.1080/10473289.2006.10464485
- G. Majewski and W. Przewoźniczuk, “Study of Particulate Matter Pollution in Warsaw Area,” Polish Journal of Environmental Studies, Vol. 18, No 2, 2009, pp. 293-300.
- P. Huszar, K. Juda-Rezler, T. Halenka, H. Chervenkov, D. Syrakov, B. C. Krueger, P. Zanis, D. Melas, E. Katragkou, M. Reizer, W. Trapp and M. Belda, “Effects of Climate Change on Ozone and Particulate Matter over Central and Eastern Europe,” Climate Research, Vol. 50, No. 1, 2011. pp. 51-68. doi:10.3354/cr01036
- M. Kowalska, M. Skrzypek, F. Danso and J. KaszniaKocot, “Relative Risk of Total and Cardiovascular Mortality in the Eldery as Related to Short-Term Increases of PM2. 5 Concentrations in Ambient Air,” Polish Journal of Environmental Studies, Vol. 21, No. 5, 2012, pp. 1279- 1285.
- E. López-Villarrubia, C. Iñiguez, N. Peral, M. D. García and F. Ballester, “Characterizing Mortality Effects of Particulate Matter Size Fractions in the Two Capital Cities of the Canary Islands,” Environmental Research, Vol. 112, 2012, pp. 129-138. doi:10.1016/j.envres.2011.10.005
- M. Tainio, K. Juda-Rezler, M. Reizer, A. Warchałowski, W. Trapp and K. Skotak, “Future Climate and Adverse Health Effects Caused by Fine Particulate Matter Air Pollution: Case Study for Poland,” Regional Environmental Change, 2012 (in Press).
- K. T. Whitby, “The Physical Characteristics of Sulfur Aerosol,” Atmospheric Environment, Vol. 12, No. 1-3, 1978, pp. 135-139. doi:10.1016/0004-6981(78)90196-8
- D. Grosjean and J. H. Seinfeld, “Parametrization of the Formation Potential of Secondary Organic Aerosols,” Atmospheric Environment, Vol. 23, No. 8, 1989, pp. 1733- 1147. doi:10.1016/0004-6981(89)90058-9
- E. R. Whitby and P. H. McMurry, “Modal Aerosol Dynamics Modeling,” Aerosol Science and Technology, Vol. 27, No. 6, 1997, pp. 673-688. doi:10.1080/02786829708965504
- W. C. Hinds, “Aerosol Technology. Properties, Behavior, and Measurement of Airborne Particles,” 2nd Edition, John Wiley & Sons, Inc., New York, 1998.
- K. Klejnowski, J. S. Pastuszka, W. Rogula-Kozłowska, E. Talik and A. Krasa, “Mass Size Distribution and Chemical Composition of the Surface Layer of Summer and Winter Airborne Particles in Zabrze, Poland,” Bulletin of Environmental Contamination and Toxicology, Vol. 88, No. 2, 2012, pp. 255-259. doi:10.1007/s00128-011-0452-3
- B. Ostro, W. Y. Feng, R. Broadwin, S. Green and M. Lipsett, “The Effects of Components of Fine Particulate Air Pollution on Mortality in California: Results from CALFINE,” Environmental Health Perspectives, Vol. 115, 2007, pp. 13-19. doi:10.1289/ehp.9281
- N. Englert, “Fine Particles and Human Health—A Review of Epidemiological Studies,” Toxicology Letters, Vol. 149, No. 1-3, 2004, pp. 235-242. doi:10.1016/j.toxlet.2003.12.035
- R. Rückerl, A. Schneider, S. Breitner, J. Cyrys and A. Peters, “Health Effects of Particulate Air Pollution: A Review of Epidemiological Evidence,” Inhalation Toxicology, Vol. 23, No. 10, 2011, pp. 555-592. doi:10.3109/08958378.2011.593587
- W. Zhang, T. Lei, Z. Q. Lin, H. S. Zhang, D. F. Yang, Z. G. Xi, J. H. Chen and W. Wang, “Pulmonary Toxicity Study in Rats with PM10 and PM2.5: Differential Responses Related to Scale and Composition,” Atmospheric Environment, Vol. 45, No. 4, 2011, pp. 1034-1041. doi:10.1016/j.atmosenv.2010.10.043
- J. H. Seinfeld, “Atmospheric Chemistry of Physics of Air Pollution,” Wiley, New York, 1986.
- A. Jaecker-Voirol and P. Mirabel, “Heteromolecular Nucleation in the Sulphuric Acid-Water System,” Atmospheric Environment, Vol. 23, No. 9, 1989, pp. 2053-2057. doi:10.1016/0004-6981(89)90530-1
- J. G. Watson, J. C. Chow, F. Lurmann and S. Musarra, “Ammonium Nitrate, Nitric Acid, and Ammonia Equilibrium in Wintertime Phoenix, AZ,” Journal of the Air & Waste Management Association, Vo. 44, No. 4, 1994, pp. 261-268.
- P. Korhonen, M. Kumala, A. Laaksonen, Y. Viisanen, R. McGraw and J. H. Seinfeld, “Ternary Nucleation of H2SO4, NH; and H2O in the Atmosphere,” Journal of Geophysical Research, Vol. 104, No. D21, 1999, pp. 26349-26353. doi:10.1029/1999JD900784
- G. J. Sun, L. Yao, L. Jiao, Y. Shi, Q. Y. Zhang, M. N. Tao, G. R. Shan and Y. He, “Characterizing PM2.5 Pollution of a Subtropical Metropolitan Area in China,” Atmospheric and Climate Sciences, Vol. 3, No. 1, 2013, pp. 100-110. doi:10.4236/acs.2013.31012
- M. Sillanpää, R. Hillamo, S. Saarikoski, A. Frey, A. Pennanen, U. Makkonen, Z. Spolnik, R. Van Grieken, M. Braniš, B. Brunekreef, M. C. Chalbot, T. Kuhlbusch, J. Sunyer, V. M. Kerminen, M. Kulmala and R. O. Salonen, “Chemical Composition and Mass Closure of Particulate Matter at Six Urban Sites in Europe,” Atmospheric Environment, Vol. 40, Suppl. 2, 2006, pp. S212-S223. doi:10.1016/j.atmosenv.2006.01.063
- T. Lee, X.-Y. Yu, B. Ayres, S. M. Kreidenweis, W. C. Malm and J. L. Collett Jr., “Observations of Fine and Coarse 5 Particle Nitrate at Several Rural Locations in the United States,” Atmospheric Environment, Vol. 42, No. 11, 2008, pp. 2720-2732. doi:10.1016/j.atmosenv.2007.05.016
- Y. Zhao and Y. Gao, “Mass Size Distributions of WaterSoluble Inorganic and Organic Ions in Size-Segregated Aerosols over Metropolitan Newark in the US East Coast,” Atmospheric Environment, Vol. 42, No. 18, 2008, pp. 4063- 4078. doi:10.1016/j.atmosenv.2008.01.032
- W. Rogula-Kozłowska, K. Klejnowski, P. Rogula-Kopiec, B. Mathews and S. Szopa, “A Study on the Seasonal Mass Closure of Ambient Fine and Coarse Dusts in Zabrze, Poland,” Bulletin of Environmental Contamination and Toxicology, Vol. 88, No. 5, 2012, pp. 722-729. doi:10.1007/s00128-012-0533-y
- Z. Y. Meng and J. H. Seinfeld, “On the Source of the Submicrometer Droplet Mode of Urban and Regional Aerosols,” Aerosol Science and Technology, Vol. 20, No. 3, 1994, pp. 253-265. doi:10.1080/02786829408959681
- R. F. Pueshel, “Stratospheric Aerosols: Formation, Properties, Effect,” Journal of Aerosol Science, Vol. 27, No. 3, 1996, pp. 359-382.
- K. Klejnowski, W. Rogula-Kozłowska and A. Krasa, “Structure of Atmospheric Aerosol in Upper Silesia (Poland)-Contribution of PM2.5 to PM10 in Zabrze, Katowice and Częstochowa in 2005-2007,” Archives of Environmental Protection, Vol. 35, No. 2, 2009, pp. 3-13
- D. K. Deshmukh, Y. I. Tsai, M. K. Deb and P. Zarmpas, “Characteristics and Sources of Water-Soluble Ionic Species Associated with PM10 Particles in the Ambient Air of Central India,” Bulletin of Environmental Contamination and Toxicology, Vol. 89, No. 5, 2012, pp. 1091-1097. doi:10.1007/s00128-012-0806-5
- N. Chuersuwan, S. Nimrat, S. Lekphet and T. Kerdkumrai, “Levels and Major Sources of PM2.5 and PM10 in Bangkok Metropolitan Region,” Environment International, Vol. 34, No. 5, 2008, pp. 671-677. doi:10.1016/j.envint.2007.12.018
- W. Rogula-Kozłowska and K. Klejnowski, “Submicrometer Aerosol in Rural and Urban Backgrounds in Southern Poland—Primary and Secondary Components of PM1,” Bulletin of Environmental Contamination and Toxicology, Vol. 90, No. 1, 2013, pp. 103-109. doi:10.1007/s00128-012-0868-4
- C. Hüeglin, R. Gehrig, U. Baltensperger, M. Gysel, C. Monn and H. Vonmont, “Chemical Characterization of PM2.5, PM10 and Coarse Particles at Urban, Near-City and Rural Sites in Switzerland,” Atmospheric Environment, Vol. 39, No. 4, 2005, pp. 637-651. doi:10.1016/j.atmosenv.2004.10.027
- D. Temesi, A. Molnár, E. Mészáros, T. Feczkó, A. Gelencsér, G. Kiss and Z. Krivácsy, “Size Resolved Chemical Mass Balance of Aerosol Particles over Rural Hungary,” Atmospheric Environment, Vol. 35, No. 25, 2001, pp. 4347-4355. doi:10.1016/S1352-2310(01)00233-3
- P. Salvador, B. Artíñano, X. Querol, A. Alastuey and M. Costoya, “Characterisation of Local and External Contributions of Atmospheric Particulate Matter at a Background Coastal Site,” Atmospheric Environment, Vol. 41, No. 1, 2007, pp. 1-17. doi:10.1016/j.atmosenv.2006.08.007
- M. Viana, X. Querol and A. Alastuey, “Chemical Characterisation of PM Episodes in NE Spain,” Chemosphere, Vol. 62, No. 6, 2006, pp. 947-956. doi:10.1016/j.chemosphere.2005.05.048
- M. Sillnapää, S. Saarikoski, R. Hillamo, A. Pennanen, U. Makkonen, Z. Spolnik, R. Van Grieken, T. Koskentalo and R. O. Salonen, “Chemical Composition, Mass Size Distribution and Source Analysis of Longe-Range Transported Wildfire Smokes in Helsinki,” Science of the Total Environment, Vol. 350, No. 1-3, 2005, pp. 119-135. doi:10.1016/j.scitotenv.2005.01.024
- K. Ravindra, M. Stranger and R. Van Grieken, “Chemical Characterization and Multivariate Analysis of Atmospheric PM2.5 Particles,” Journal of Atmospheric Chemistry, Vol. 59, No. 3, 2008, pp. 199-218. doi:10.1007/s10874-008-9102-5
- M. Čačković, V. Vađić, K. Šega and I. Bešlić, “Acidic Anions in PM10 Particle Fraction in Zagreb Air, Croatia,” Bulletin of Environmental Contamination and Toxicology, Vol. 83, No. 2, 2009, pp. 188-192. doi:10.1007/s00128-009-9641-8
- G. Spindler, E. Brüggemann, T. Gnauk, A. Grüner, K. Müller and H. Herrmann, “A Four-Year Size-Segregated Characterization Study of Particles PM10, PM2.5 and PM1 Depending on Air Mass Origin at Melpitz,” Atmospheric Environment, Vol. 44, No. 2, 2010, pp. 164-173. doi:10.1016/j.atmosenv.2009.10.015
- I. Kopanakis, N. Lydakis-Simantiris, E. Katsivela, D. Pentari, P. Zarmpas, N. Mihalopoulos and M. Lazaridis, “Size Distribution and Chemical Composition of Airborne Particles at Akrotiri Research Station, Crete, Greece,” Global Nest Journal, Vol. 12, No. 1, 2010, pp. 54-62.

