Journal of Environmental Protection
Vol. 3 No. 6 (2012) , Article ID: 20067 , 10 pages DOI:10.4236/jep.2012.36063
Influencing Factors and Process on in Situ Degradation of Poly(Butylene Succinate) Film by Strain Bionectria ochroleuca BFM-X1 in Soil
![]()
1College of Environmental Science & Engineering, Beijing Forestry University, Beijing, China; 2Key Laboratory for Silviculture and Conservation of Ministry of Education, Beijing Forestry University, Beijing, China; 3Museum of Beijing Forestry University, Beijing Forestry University, Beijing, China.
Email: liangym812@126.com
Received February 29th, 2012; revised March 25th, 2012; accepted April 18th, 2012
Keywords: Poly(Butylene Succinate) (PBS); In Situ Biodegradation; Strain B. ochroleuca BFM-X1; Temperature; Humidity
ABSTRACT
This is the first report on the PBS film degraded by any Bionectria ochroleuca fungal strain. The fungal strain BFM-X1 was isolated from an air environment on a vegetable field and was capable of degrading poly(butylene succinate) (PBS). The taxonomic identity of the strain BFM-X1 was confirmed to be Bionectria ochroleuca (showing a 99% similarity to B. ochroleuca in a BLAST search) through an ITS rRNA analysis. The bio-degradation of the PBS film by strain BFM-X1 was studied. Approximately 97.9% of the PBS film was degraded after strain BFM-X1 was inoculated at 28˚C for 14 days. The degradation efficiency of BFM-X1 against PBS film under different soil environmental conditions was characterized. The results indicated that 62.78% of the PBS film loss was recorded in a 30-d experimental run in a sterile soil environment indoors. On adding strain BFM-X1 to a soil sample, the PBS degradation rate accelerated approximately fivefold. Furthermore, both temperature and humidity influenced the in situ degradation of the PBS by strain BFM-X1, and temperature may be the major regulating factor. The degradation was particularly effective in the warm season, with 90% of weight loss occurring in July and August. Scanning electron microscope observations showed surface changes to the film during the degradation process, which suggested that strain BFM-X1preferentially degraded an amorphous part of the film from the surface. These results suggested that the strain B. ochroleuca BFM-X1 was a new resource for degrading PBS film and has high potential in the bioremediation of PBS-plastic-contaminated soil environment
1. Introduction
Plastics have been widely used in agriculture, industry and construction due to their excellent characteristics and low cost. Unfortunately, the disposal of the waste produced by traditional chemosynthetic plastics has caused many environmental problems. Biodegradable polymers have been developed as a possible solution to these problems [1,2]. Poly(butylene succinate) (PBS), one such chemosynthetic and biodegradable aliphatic polyester, is highly favored due to its relatively low production cost, excellent mechanical properties, good thermal stability and environmental compatibility [3]. PBS is expected to be used widely as an alternative to ordinary plastics. Many related products have been commercialized, such as Bionolle 10,000, which was produced in Japan and included in the the Japan BioPlastic Association’s list of biodegradable plastics [4].
Current studies of PBS have mainly focused on its synthesis, physical properties, and the isolation and purification of degrading bacteria. Theoretically, PBS is easily degraded by microorganisms. However, degradability varies due to differences in copolymer composition and molecular weight [5]. Hayase et al. [6] isolated the Bacillus pumilus strain 1-A from soil, which showed high degradation ability against PBS. Aspergillus fumigatus strain NKCM1706 was also isolated from soil and shown to degrade PBS film at a rate of 10.5 µg·cm–2·h–1 [7]. PBS has good biodegradability under controlled compost conditions and A. versicolor has stronger biodegradability than PBS in a compost environment. Moreover, the rate of PBS biodegradation in compost was affected by the shapes of the PBS sample. PBS powders had a faster degradation rate than film and granules [8].
The biodegradability of PBS has been demonstrated in a culture broth [9,10], in compost [11,12], and in a simulated soil environment [7,13]. However, information about PBS-degradation in a natural soil environment is limited and its detailed in situ degradation by inoculated PBS-degrading microorganisms has not been clarified. Since many kinds of microorganisms coexist and contribute to PBS degradation in complex microbial consortiums, it is essential to identify the factors involved in this degradation by employing a degrading-strain in the field in order to select an appropriate engineering-strain.
In a previous study we isolated the PBS-degrading fungal strain BFM-X1 from the environment [14]. In this paper, we confirmed the possibility of in situ biodegradation of the PBS film by this strain in the soil. Furthermore, we investigated the effects of temperature and humidity on PBS degradation by BFM-X1 and elucidated the degradation pattern of PBS in natural environments.
2. Materials and Methods
2.1. Materials and Chemicals
PBS plastic film (Mw, 2.0 × 105; thickness of 20 μm) was purchased from the Showa Polymer Chemical Company (Japan). PBS pellet (Mw, 1.5 × 105; size: 3-mm diameter × 4 mm) was supplied by the Haier National Engineering Research Center of Engineering Plastics Co Ltd (Beijing, China). The PBS emulsion preparation followed Horowit et al.’s method [15]. All other reagents used were commercially produced and analytical.
2.2. Sources of PBS-Degradation Strain
For the purposes of this study, strain BFM-X1, which showed a clear zone around the colony on an emulsified PBS agar plate, was used as PBS-degrader. The strain was isolated from the air environment on a vegetable field and stored at 4˚C in the Key Laboratory for the Silviculture and Conservation of Ministry of Education at the Beijing Forestry University in China [14].
2.3. Media and Culture Conditions
Fungi were cultured in a basal medium (pH 7.1; K2HPO4, 1.0 g/L; NaNO3, 2.0 g/L; MgSO4·7H2O, 0.5 g/L; KCl, 0.5 g/L; FeSO2·7H2O, 0.01 g/L; MnSO4, 0.0005 g/L; chloromycetin, 0.05 g/L) or PD medium supplemented with 1% (w/v) emulsified PBS as the sole carbon [7]. In the case of solid media, 2% agar was added. Erlenmeyer flasks (500 mL) containing 250 mL of the basal medium were inoculated with strain BFM-X1 at 5% (v/v) cell concentration and then cultivated at 28˚C on a rotary shaker (120 rpm/min) for a week. The culture broth was used in the follow-up soil experiments.
2.4. Analytical Method for Measuring the PBS Degradation
Two methods were used to investigate the degradation ability of strain BFM-X1 against PBS film under different conditions. PBS degradation on the basal medium plate was monitored by the area-loss method. The residual film following degradation was scanned and the scanned images were analyzed through ImageJ 1.38x. The area loss of each film was calculated by subtracting its area following degradation from its initial area. The PBS degradation in the soil environment was monitored by the weight-loss method [7]. The weight loss of each film was calculated by subtracting its weight following degradation from its original weight. The ratio of the weight loss to its original weight was considered to the degradation rate.
2.5. Identification of a Fungal Strain Capable of Degrading PBS
The total genomic DNAs (gDNAs) of the strain BFM-X1 were extracted from the mycelia by using the DNA extraction kit (Promega, Tokyo, Japan). Primary PCR was performed for cloning the internal transcribed spacer (ITS) and 5.8S rDNA regions by using universal amplification primers ITS1F: 5’-C TTGGTCATTTAGAGGAAGTAA-3’ [16] and ITS4: 5’-TC CTCCGCTTATTGATATGC-3’ [17]. Sequencing was carried out by the Shanghai Sangon Biological Engineering Technology & Service Co., Ltd. (Shanghai, China). After sequencing, the ITS sequence was compared with the GenBank data using the program blast via the NCBI site (http://www.blast.ncbi.nlm.nih.gov/Blast.cgi). Sequences showing high similarity to the ITS gene sequence of strain BFM-X1 were retrieved from the Genbank (http://www.ncbi.nlm.nih.gov) and aligned in Clustal W. The phylogenetic tree was constructed by the neighborjoining method using Molecular Evolutionary Genetics Analysis software PAUP.
2.6. Experiment on PBS Film Biodegradation under Controlled Conditions in Soil
The soil sample used in the indoor degradation analysis was collected from the nursery of the Beijing Forestry University (Beijing, China). Sterile soil (100 g) was scattered onto a test panel (approximately 2-cm thick). PBS films (4 cm × 4 cm) were sterilized by being soaked in ethanol, rinsed with sterile water, and then placed on the soil and covered with another 100 g of sterile soil. In addition, 200 mL of prepared culture broth was sprayed on the soil. The surface of the test panel was covered by gauze to inhibit water evaporation. The panel was incubated at 30˚C and sprayed quantitatively with sterile water daily to maintain relative humidity at 60%. The residual films were recovered every 5 d. After being carefully washed with water, which ensured that their surface structure wasn’t destroyed, the films were dried to a constant weight and then weighed. The control experiment was performed by using autoclaved soil without the culture broth. Another control experiment was performed by using unsterilized soil lacking strain BMF-X1. All experiments were conducted in duplicate.
2.7. Experiment on PBS Film Biodegradation in Natural Soil
The degradation experiment was performed in the nursery of the Beijing Forestry University (Beijing, China). There, the soil was scraped into ridges of 30 cm width and the PBS films (6 cm × 6 cm) were placed in a double row on the ridges. The distance between the films was 2 cm and the distance between two ridges was 25 cm. Further, 400 mL of prepared culture broth was sprayed on the soil and then covered with a 5-cm-thick layer of soil. The bed of one ridge was watered artificially every second day to preserve moisture; the other was kept in a natural state. The residual films were recovered every 5 d, washed carefully with water, and weighed after the films were dried to a constant weight. The control experiment was performed by using 400 mL of basal medium solution. The above mentioned experiments were conducted in different seasons. One was completed in the cold season (March-April), hereafter called the “low-temperature phase” and the other was conducted in the warm season (July-August), hereafter called the “high-temperature phase”. All experiments were conducted in duplicate.
2.8. Microscopic Observations
During the process of microbial degradation, surface changes in the PBS film were observed under a scanning electron microscope (SEM, voltage 10 kV; S-3400N, Japan-Hitachi Ltd). The samples were coated with gold prior to SEM observation.
3. Results
3.1. Characterization and Identification of Fungal Strain BFM-X1
The alignment of the ITS region genes’ nucleotide sequences including the 5.8S region of the PBS-degrading fungal strain, was analyzed by a BLAST search of the NCBI. The lab BLAST analysis showed that the strain BFM-X1 (DDBJ nucleotide sequence accession number: AB470910) was closely related to Bionectria ochroleuca, with the ITS region identity value at 99% within the GeneBank database. A phylogenetic tree (Figure 1) depicts the position of strain BFM-X1 with the genus Bionectria sp. Based on colony morphology, microscopic features, and ITS sequence analysis, BFM-X1 was classified into B. ochroleuca.
3.2. Degradation of PBS Film by Strain BFM-X1
To examine the biodegradation ability of strain BFM-X1 to the PBS film, BFM-X1 and PBF film were co-cultured at 28˚C on basal plates containing 1 g/L PBS emulsion with the initial PH 4.0, and the area-loss of PBS film was recorded. The area loss of the film tended to escalate with incubation time (Figure 2). Strain BFM-X1 degraded the PBS film very efficiently and showed complete degradation within only 16 days. There were three phases in the degradation process: the lag phase, exponential phase and fast phase. The area loss of the film was a slow process during the lag phase and the degradation ratio was only 5.6% on the 6 d. After 6 days, the degradation had partially accelerated. After 14 d, approximately 97.9% of the film area was lost.
3.3. Contribution of Strain BFM-X1 to PBS Degradation in Soil Environments
A soil-buried test of the PBS film was performed under controlled indoor conditions (Figure 3) to investigate the contribution of BFM-X1 to the PBS film degradation in soil conditions. The PBS film degradation rate increased quickly after 10 days post inoculation (dpi) in the soil appended with the strain. The weight of the film lost was approximately 60% at 30 dpi. There were many visible holes on the surface at 10 dpi and the original form of the specimen collapsed due to the formation of craters during further incubation. The weight loss of the PBS film was <10% at 30 dpi in the soil without the strain, which was considerably lower than the weight loss in the soil appended with the strain. There was no significant weight loss in the autoclaved soil at 30 dpi. The PBS degradation rate efficiency accelerated approximately fivefold in soil with the strain BFM-X1 added. Our results suggested that PBS was degraded only when it came into contact with degrading microorganisms such as the strain BFMX1 under particular conditions of temperature and humidity.
3.4. In Situ Degradation Capabilities of the Strain BFM-X1 for PBS under Different Temperatures
In order to test the influence of temperature on biodegradation in natural field soil, we performed the soil-buried
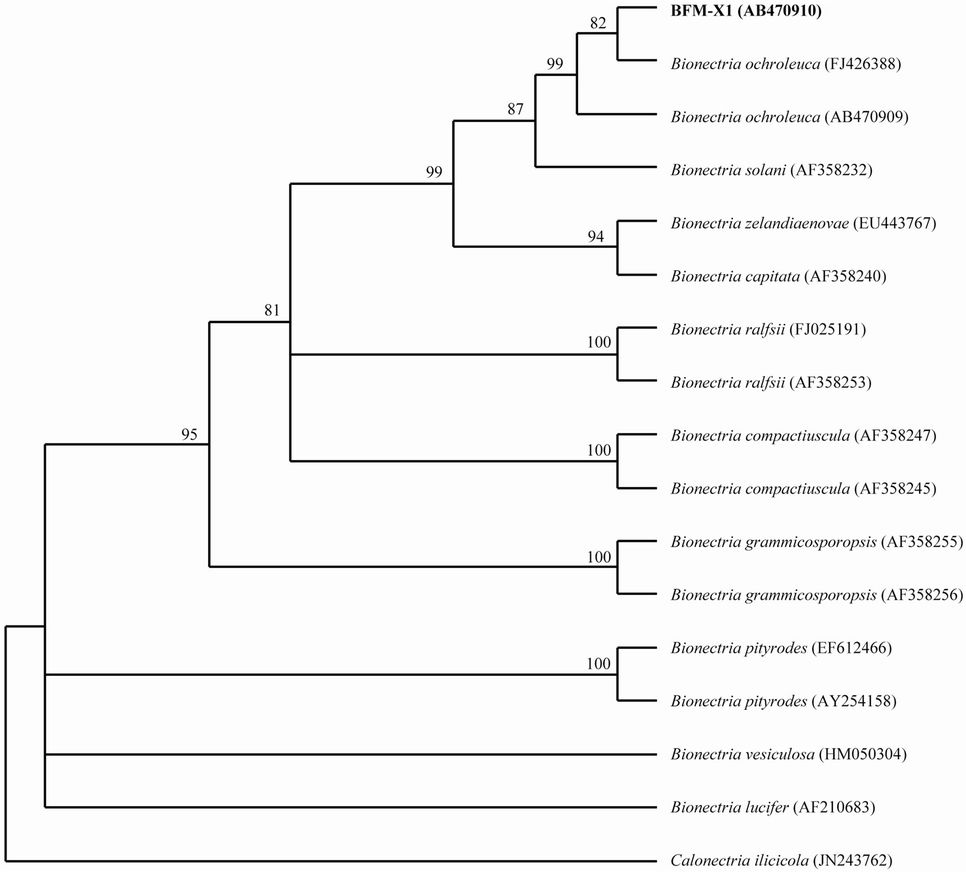
Figure 1. Neighbor-joining phylogenetic tree from analysis of nuclear ribosomal internal transcribed spacer (ITS, include 5.8S region) sequences date from strain BFM-X1 and related species. Levels of bootstrap support were obtained for 1000 repetitions and indicated at all nodes. Calonectria iliaicola was selected as an outlier to root the tree.
test of PBS film for different months in the Beijing Forest University’s nursery. The low-temperature phase had an average temperature of approximately 16˚C and the high-temperature phase had an average temperature of approximately 28˚C. The weight loss of the PBS film increased with time (Figure 4). There was a lag phase of about 15 dpi in the high-temperature phase, prior to the exponential degradation phase, and weight loss was >80% after 75 dpi. In contrast, the PBS film was not effectively biodegraded in the low-temperature phase, and only 15% of the PBS film’s weight was lost within 60 dpi. However, there was an increased weight loss in the low-temperature phase after 60 dpi due to the increasing temperature. There was very little weight loss in the control groups for both the highand low-temperature phases. Temperature was thus an important factor affecting the biodegradation efficiency of PBS film in natural field soil.
3.5. In Situ Degradation Capability of PBS by Strain BFM-X1 at Different Humidities
Humidity was studied in addition to temperature. In this study, a soil-buried test of the PBS film was performed at different humidities at high and low temperatures (Figsure 5 and 6). In the high-temperature phase, the weight loss of PBS film quickly increased at 25 dpi both in the artificially water ridges and the ridges with natural hu-
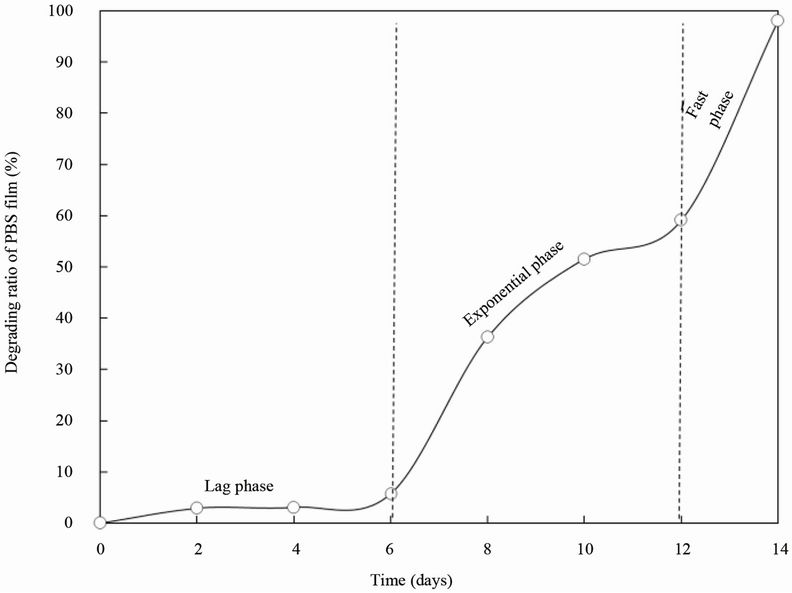
Figure 2. Degradation of the PBS film by strain BFM-X1. The PBS film (20 × 20 mm) was incubated with strain BFM-X1 on basal medium plates at 28˚C.
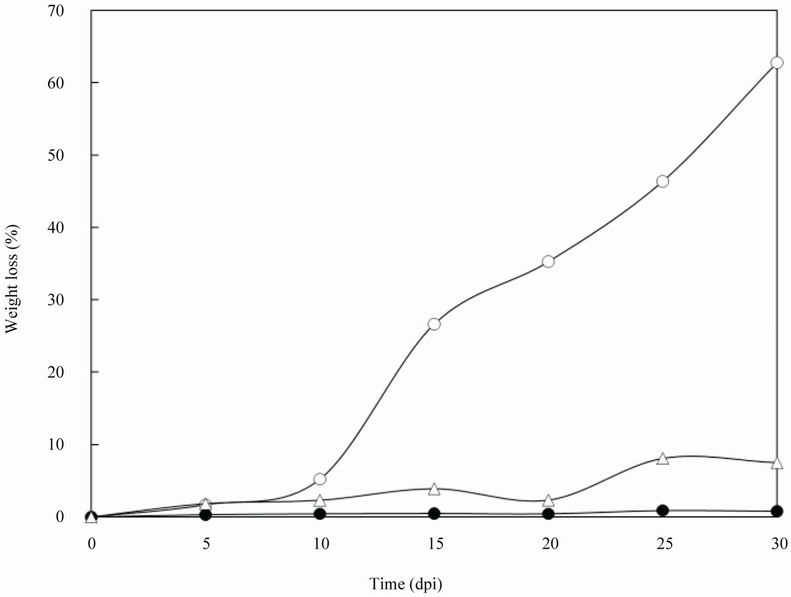
Figure 3. Indoor degradation of PBS film in soil samples. Weight loss of the PBS film in the presence of strain BMF-X1 (○), control (unsterilized soil lacking strain BMF-X1) (∆), and negative control (sterilized) (●).

Figure 4. Degradation of PBS film in natural field soil for different seasons. Weight loss of PBS film in the high-temperature phase (○), low-temperature phase (●), control in high-temperature phase (∆), and control in low-temperature phase (▲).

Figure 5. Degradation of PBS film in natural field soil at different humidities in the high-temperature phase. Weight loss of the PBS film watered artificially (○), at natural humidity (●), control (watered artificially) (∆), and control (at natural humidity) (▲).
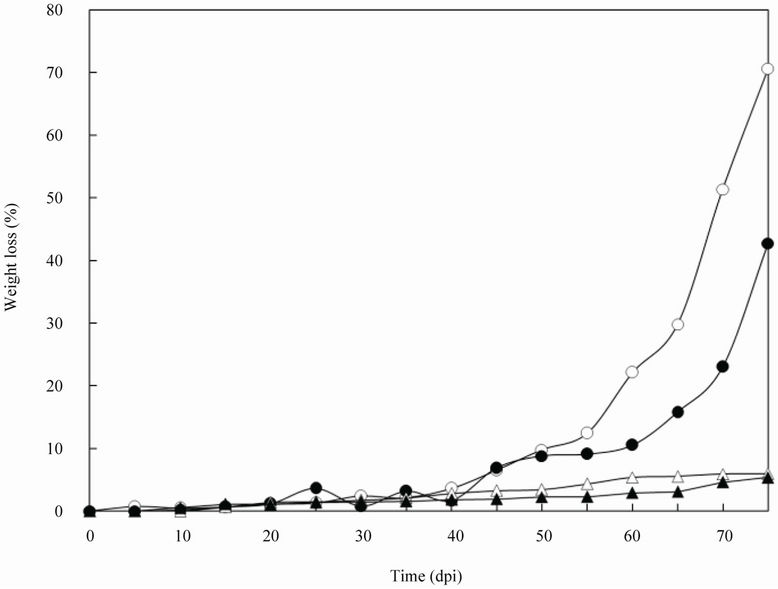
Figure 6. The degradation of PBS film in natural field soil at different humidities in the low-temperature phase. Weight loss of the artificially watered PBS film (○), at natural humidity (●), control (watered artificially) (∆), and control (at natural humidity) (▲).
midity. At 75 dpi of strain BFM-X1, the films in different ridges lost 100% and 80.78% of their weights, respectively. In comparison, the weight loss in the low-temperature phase was <10% up to 50 dpi and then increased. Therefore, the weight loss of the group watered artificially was higher than that maintained in the natural state for both warm and cold phases. Suitable humidity should therefore promote PBS degradation by strain BFM-X1.
3.6. Degradation Process of the PBS Film by Strain BFM-X1
SEM was used to investigate the degradation processes of the PBS film and showed the surfaces of PBS films at various times during degradation (Figure 7). The PBS film’s degree of degradation increased gradually after inoculation with strain BFM-X1. The surface of the virgin film was extremely smooth (A), but exhibited cavities interspersed with smooth areas at 10 dpi with strain BFM-X1. At 20 dpi, morphology from the spherulites covered the entire surface; some lamella crystal structure was observed; and the original outward form of the film had totally disappeared.
The surface change of the PBS film in the unsterilized soil without strain BFM-X1 differed markedly from the surface change with BFM-X1. At 10 dpi, the surface was smooth and little change was observed. At 20 dpi, a marked eroded trace of diameter 5 ± 1 µm appeared on the film surface, while many parts remained smooth. At 30 dpi, the surface was rougher, the eroded trace was deeper, and there were few holes. However, the surface of the film remained smooth at 30 dpi after treatment in autoclaved soil lacking strain BFM-X1 (micrographs not shown).
4. Discussion
The strain BFM-X1 was identified as B. ochroleuca Based on ITS rRNA gene sequence analyses. This was the first time that Bionectria has been reported as a PBSdegrading microorganism. The area-loss method was used in plate experiments to investigate Bionectria’s degradation ability. The PBS film’s area was reduced by lost 97.9% after being degraded by the strain BFM-X1 for 14 days. The resulting degradation ratio based on the area-loss method was relatively conservative in comparison to the weight-loss method.
Among the microorganisms that have been reported to be PBS degraders strain Alternaria sp. HJ03 has showed the best degradation ability with the ratio at 79.4% after 18 days [18]. However, our strain BFM-X1 obviously
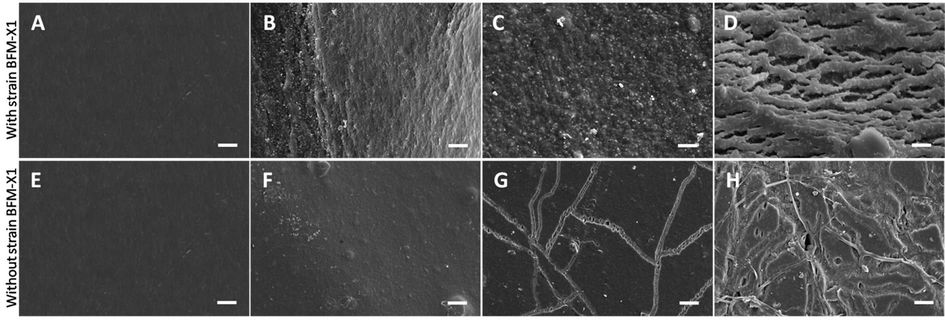
Figure 7. Scanning electron micrographs of the PBS film surfaces before (A, E) and after degradation indoors at 10 (B, F), 20 (C, G), and 30 dpi (D, H). The white bars indicate a length of 20 µm.
degraded PBS for powerfully. “On the face of it” suggests that “appeared to degrade” might be more accurate than “obviously degraded”. Further, although some microorganisms have been reported to be PBS degraders [6,7,12], there have been no reported studies using these microorganisms to degrade PBS film in situ for soil. This suggested that the PBS-degrading microorganisms which remain active in a natural soil environment may be limited strains. However, these microorganisms could be of value when play a role in natural environments. Therefore, we analyzed that whether the degradation ability of the strain BFM-X1 remained in a natural soil environment.
The results of the indoor experiment suggested that strain Bionectria ochroleuca BFM-X1 showed a high degradation activity against PBS film and that the degradation rate accelerated approximately fivefold compared to controls. The results were similar to those of Ishii et al., who used a glass bottle with a PBS film, a microorganism strain and soil [7]. The degradability of strain Bionectria ochroleuca BFM-X1 against PBS in a sterile soil environment was much stronger than that of the Fusarium solani strain WF-6 isolated by Abe et al., which only degraded 2.8% of PBS after 14 d in a sterile soil environment [19].
The degradation rate of PBS film in soil obviously differed between indoor and outdoor conditions, indicating that the complex soil environment and climate conditions affected the degradation of the PBS film by strain Bionectria ochroleuca BFM-X1. This result was also supported by Kasuya et al., who reported that the environmental biodegradation rate of PBS was much lower than that of other biodegradable plastics such as poly (ε-caprolactone) (PCL) or poly(3-hydroxybutyrate) (P (3HB)) and also depended on the ambient environmental conditions [20]. Abe et al. suggested that the degradeability of F. solani strain WF-6 strongly depended on the cell density level of the indigenous microorganisms and that the degradation was remarkably diminished when the cell concentration level increased [19]. Our study revealed that the in situ degradation of PBS film was slow in comparison to an indoor soil environment. This phenomenon might be due to the inhibition of growth and/or the degrading activity of plastic-degrading microorganisms in complex natural environments where many kinds of microorganisms coexist.
Temperature was probably a major factor that regulated the in situ biodegradation of the PBS film. Irrespective of other conditions, the degradation of PBS film was greater in the high-temperature phase (average temperature approximately 28˚C) than in the low-temperature phase (average approximately 16˚C). There are two reasons for the considerably greater degradation rate in the high-temperature phase. The first is that microbial growth and activity are increased in warm conditions. The second is that the PBS film becomes a more favorable carbon and/or energy source for strain Bionectria ochroleuca BFM-X1 at that temperature. Södergård et al. reported that the flexibility of the polymer chain increases at temperatures higher than the glass transition temperature [21]. In addition, water absorption into the polymer matrix increases at high temperatures [22], which may not only accelerate chemical hydrolysis, but also facilitate the attachment of microbes and enzymes to the polymer surface by increasing polymer hydrophilicity. It is well known that PBS is hydrolyzed by lipase [23]. The Chromobacterium viscosum lipase [24] and the Rhizopus delemar lipase [23,25] can degrade PBS by extracellular enzymes that were excreted by degradation bacteria. In our study, the average outdoor temperature was about 16˚C and even lower at night in the low-temperature phase. The enzymes that were excreted into the soil from the culture broth would therefore not work well due to low temperature. In contrast, the enzymes could play a more direct and effective role in the high-temperature phase due to the relatively high temperatures.
Humidity was another important factor affecting microbial growth, metabolism and enzymatic activity. The present results showed that humidity affected the PBS film degradation and that increasing the humidity within a certain range could increase the degradation rate.
The degradation process of the PBS film by strain Bionectria ochroleuca BFM-X1 was also examined by SEM, which showed that the specimen surface initially became rough and that some lamella crystal structures appeared on the surface with time. We presume that the enzyme preferentially degraded the amorphous part of the film from the surface. Similar microbial degradation processes on the surface were also observed for other semi-crystalline biodegradable polyesters, such as P(3HB) [26] and poly(ethylene succinate) (PESu) [27]. These degradation patterns may be explained by the size and preference of polyester-degrading enzymes, which can hydrolyze ester bonds in amorphous regions at a considerable higher rate than ester bonds in crystalline regions [26]. However, Zhang et al. suggested that the erosion of the film by microorganisms in the activated sludge of a wastewater plant began at the surface crystalline regions and extended to the internal film structure [28]. During the degradation process in non-sterile soil lacking strain BFM-X1, a large number of hyphae first attached to the film surface and some then became embedded in the PBS film’s fragments of PBS film. Typical eroded traces of mycelia growth were also observed.
5. Acknowledgements
This work was supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry and the Ministry of Science and Technology Forest Culture Collection Platform Program of China (No. 2005DKA21207-8).
REFERENCES
- T. Fujimaki, “Processability and Properties of Aliphatic Polyesters, ‘BIONOLLE’, Synthesized by Polycondensation Reaction,” Polymer Degradation and Stability, Vol. 59, No. 1-3, 1998, pp. 209-214. doi:10.1016/S0141-3910(97)00220-6
- R. A. Gross and B. Kalra, “Biodegradable Polymers for the Environment,” Science, Vol. 297, No. 5582, 2002, pp. 803-807. doi:10.1126/science.297.5582.803
- H. S. Kim, H. J. Kim, J. W. Lee and I. G. Choi, “Biodegradability of Bio-Flour Filled Biodegradable Poly(Butylene Succinate) Bio-Composites in Natural and Compost Soil,” Polymer Degradation and Stability, Vol. 91, No. 5, 2006, pp. 1117-1127. doi:10.1016/j.polymdegradstab.2005.07.002
- M. Kunioka, F. Ninomiya and M. Funabashi, “Biodegradation of Poly(Butylene Succinate) Powder in a Controlled Compost at 58˚C Evaluated by Naturally-Occurring Carbon 14 Amounts in Evolved CO2 Based on the ISO 14855-2 Method,” International Journal of Molecular Sciences, Vol. 10, No. 10, 2009, pp. 4267-4283. doi:10.3390/ijms10104267
- J. J. Ge, “Biodegradable Macromolecule Materials & Application,” Chemical Industry Press, Beijing, 2002.
- N. Hayase, H. Yano, E. Kudoh, C. Tsutsumi, K. Ushio, Y. Miyahara, S. Tanaka and K. Nakagawa, “Isolation and Characterization of Poly(Butylene Succinate-Co-Butylene Adipate)-Degrading Microorganism,” Journal of Bioscience and Bioengineering, Vol. 97, No. 2, 2004, pp. 131-133.
- N. Ishii, Y. Inoue, T. Tagaya, H. Mitomo, D. Nagai and K. Kasuya, “Isolation and Characterization of Poly(Butylene Succinate)-Degrading Fungi,” Polymer Degradation and Stability, Vol. 93, No. 5, 2008, pp. 883-888. doi:10.1016/j.polymdegradstab.2008.02.005
- J. H. Zhao, X. Q. Wang, J. Zeng and Q. Yan, “Study on the Biodegradation of Poly(Butylene Succimate) under Compost Conditions,” Journal of Functional Polymers, Vol. 17, No. 4, 2004, pp. 666-170.
- H. Pranamuda, Y. Tokiwa and H. Tanaka, “Microbial Degradation of an Aliphatic Polyester with a High Melting Point, Poly(Tetramethylene Succinate),” Applied and Environmental Microbiology, Vol. 61, No. 5, 1995, pp. 1828-1832.
- H. Li, J. Hang, A. Cao and J. Wang, “In Vitro Evaluation of Biodegradable Poly(Butylene Succinate) as a Novel Biomaterial,” Macromolecular Bioscience, Vol. 5, No. 5, 2005, pp. 433-440. doi:10.1002/mabi.200400183
- H. S. Yang, J. S. Yoon and M. N. Kim, “Effect of Storage of a Mature Compost on Its Potential for Biodegradation of Plastics,” Polymer Degradation and Stability, Vol. 84, No. 3, 2004, pp. 411-417. doi:10.1016/j.polymdegradstab.2004.01.014
- J. H. Zhao, X. Q. Wang, J. Zeng, G. Yang, F. H. Shi and Q. Yan, “Biodegradation of Poly(Butylene Succinate) in Compost,” Journal of Applied Polymer Science, Vol. 97, No. 6, 2005, pp. 2273-2278. doi:10.1002/app.22009
- T. Hirotsu, T. Tsujisaka, T. Masuda and K. Nakayama, “Plasma Surface Treatments and Biodegradation of Poly (Butylene Succinate) Sheets,” Journal of Applied Polymer Science, Vol. 78, No. 5, 2000, pp. 1121-1129. doi:10.1002/1097-4628(20001031)78:5<1121::AID-APP210>3.0.CO;2-H
- X. L. Mei, Y. M. Liang, C. M. Tian, Q. Dong and Q. Sun, “Study on Screening and Degradation Characteristics of High Efficient Degrading Fungi for Poly(Butylenes Succinate),” Microbiology China, Vol. 38, No. 3, 2011, pp. 348-354.
- D. M. Horowit and J. K. M. Sanders, “Amorphous, Biomimetic Granules of Polyhydroxybutyrate: Preparation, Characterization, and Biological Implications,” Journal of the American Chemical Society, Vol. 116, No. 7, 1994, pp. 2695-2702. doi:10.1021/ja00086a001
- M. Gardes and T. D. Bruns, “ITS Primers with Enhanced Specificity for Basidiomycetes-Application to the Identification of Mycorrhizae and Rusts,” Molecular Ecology, Vol. 2, No. 2, 1993, pp. 113-118. doi:10.1111/j.1365-294X.1993.tb00005.x
- T. J. White, T. Bruns, S. Lee and J. Taylor, “Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics,” In: M. A. Innis, D. H. Gelfand, J. J. Sninsky and T. J. White, Eds., PCR Protocols a Guide to Methods and Applications, Academic Press, San Diego, 1990.
- Q. Sun, Y. M. Liang, C. M. Tian and C. A. Zhang, “Screening and Characterization of Poly(Butylenes Succinate)-Degrading Fungi,” Journal of Beijing Forestry University, Vol. 32, No. 6, 2010, pp. 130-134.
- M. Abe, K. Kobayashi, N. Honma and K. Nakasaki, “Microbial Degradation of Poly(Butylene Succinate) by Fusarium Solani in Soil Environments,” Polymer Degradation and Stability, Vol. 95, No. 2, 2010, pp. 138-143. doi:10.1016/j.polymdegradstab.2009.11.042
- K. Kasuya, K. Takagi, S. Ishiwatari, Y. Yoshida and Y. Doi, “Biodegradabilities of Various Aliphatic Polyesters in Natural Waters,” Polymer Degradation and Stability, Vol. 59, No. 1-3, 1998, pp. 327-332. doi:10.1016/S0141-3910(97)00155-9
- A. Södergård, J. F. Selin and J. H. Näsman, “Hydrolytic Degradation of Peroxide Modified Poly(L-Lactide),” Polymer Degradation and Stability, Vol. 51, No. 3, 1996, pp. 351-359. doi:10.1016/0141-3910(95)00271-5
- G. L. Siparsky, K. J. Voorhees, J. R. Dorgan and K. Schilling, “Water Transport on Polylactic Acid (PLA), PLA/ Polycaprolactone Copolymers, and PLA/Polyethylene Glycol Blends,” Journal of Polymers and the Environment, Vol. 5, No. 3, 1997, pp. 125-136.
- M. Itävaara, S. Karjomaa and J. F. Selin, “Biodegradation of Polylactide in Aerobic and Anaerobic Thermophilic Conditions,” Chemosphere, Vol. 46, No. 6, 2002, pp. 879- 885. doi:10.1016/S0045-6535(01)00163-1
- Y. Tokiwa and T. Suzuki, “Hydrolysis of Polyesters by Lipases,” Nature, Vol. 270, No. 5632, 1997, pp. 76-78. doi:10.1038/270076a0
- E. Kitakuni, K. Yoshikawa, K. Nakano, J. Sasuga, M. Nobiki, H. Naoi, Y. Yokota, R. Ishioka and Y. Yakabe, “Biodegradation of Poly(Tetramethylene Succinate-CoTetramethylene Adipate) and Poly(Tetramethylene Succinate) through Water-Soluble Products,” Environmental Toxicology and Chemistry, Vol. 20, No. 5, 2001, pp. 941- 946.
- Y. Tokiwa and T. Suzuki, “Hydrolysis of Polyesters by Rhizopus Delemar Lipase,” Agricultural and Biological Chemistry, Vol. 42, No. 5, 1978, pp. 1071-1072. doi:10.1271/bbb1961.42.1071
- N. Ishii, K. Shimada, Y. Tezuka, H. Mitomo and K. Kasuya, “Fungal Degradation of Poly(Ethylene Succinate),” Polymer Degradation and Stability, Vol. 92, No. 1, 2007, pp. 44-52. doi:10.1016/j.polymdegradstab.2006.09.014
- M. Zhang, X. X. Wang, B. J. Liu, X. X. Liu, L. Wang and J. H. Qiu, “Study on Biodegradable Behavior of Polyesters in the Soil of Shanxi Local,” Polymer Materials Science and Engineering, Vol. 24, No. 1, 2008, pp. 91-93.

