Food and Nutrition Sciences
Vol.4 No.8A(2013), Article ID:35269,7 pages DOI:10.4236/fns.2013.48A009
Antioxidant Properties of Medicinal Plants from Peru
![]()
Faculty of Pharmacy, Medical University of Warsaw, Warsaw, Poland.
Email: *katarzyna.paradowska@wum.edu.pl
Copyright © 2013 Adam Berłowski et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 10th, 2013; revised May 10th, 2013; accepted May 17th, 2013
Keywords: Antioxidant; Total Polyphenols; Medicinal Plants
ABSTRACT
There is a wide diversity of plants and seasonal crops in Peru, due to the presence of many climatic zones. Numerous plants are used to cure or prevent diseases. These plants are promising candidates for functional foods products. The most frequent form in which they are used is an aqueous infusion or decoction. In this study, we compared the antioxidant properties of ten Peruvian plants infusions and investigated their relation to the phenolic content. The studied plants were: Uncaria tomentosa (cat’s claw), Lepidium meyenii (maca), Berberis vulgaris L. (barberry, agracejo), Phyllantus niruri (chanca piedra), Annona muricata L. (graviola, soursop), Gentianella alborosea (hercampure), Geranium dielsianum (pasuchaca), Tabebuia ochracea (tahuari), Notholaena nivea (“cuti cuti”) and Tiquilia paronychioides (“flor de arena”). Infusions of all studied plants have shown antioxidant activity, though there was a large diversity between the results. The antioxidant properties, determined with DPPH and ABTS scavenging assays as well as FRAP test, were strongly correlated with total phenolic content, while there was no correlation with the carotenoid content.
1. Introduction
There is a wide diversity of plants and seasonal crops in Peru, due to the presence of many climatic zones, including the unique areas such as Amazonian rainforest or Andean mountains. Numerous plants are used in medicine, to cure or prevent diseases. Widely known are Uncaria tomentosa (cat’s claw), Phyllantus niruri (chanca piedra) or Lepidium meyenii (maca) which were already shown to have therapeutic or prophylactic potential. These plants are promising candidates for functional foods products.
U. tomentosa, a vine growing in the Amazon region, has been used medicinally by native tribes for at least 2000 years in treating inflammation, arthritis, bone pain, asthma, deep wounds, and cancer [1]. It is probably the best known medicinal plant of South America. Extracts from the bark were extensively studied [2] and found to exhibit immunostimulating, antiinflammatory and antioxidant properties [3].
L. meyenii, a tuber from the central Andes consumed by native Peruvians has also multipharmacological functions such as fertility improvement and the protection of cells against oxidative stress [4,5]. It has been demonstrated that different types of L. meyenii (differentiated by size and colour) have distinct biological properties [6,7]. L. meyenii infusion may act as an antioxidant by radical scavenging or by maintenance of intracellular ATP production in conditions of oxidative stress [8] as well as an immunomodulator [9].
Barberry (Berberis vulgaris L.) is known in Peru under the name “agracejo”. Its fruits, leaves, root and stem are used for ailments of kidneys, liver, urinary and gastrointestinal tract, for the relieve of respiratory tract discomforts, and as a stimulant for the circulatory system [10, 11]. Berberis species were active antioxidants in many tests evaluating antioxidant activity, such as DPPH (2,2- diphenyl-1-picrylhydrazyl) radical scavenging, β-carotene bleaching, prevention of lipid peroxidation and oxyhaemoglobin bleaching, or protection from DNA damage [12,13].
Chanca piedra (P. niruri), a small herb indigenous to the tropical areas is widely used as medicinal plant. In Ayurveda and Traditional Chinese Medicine it was used for treating dysentery, influenza, diabetes, kidney stones, diuretics and tumours, as well as hepatotoxicity and hyperglycaemia [14]. The biologically active compounds of P. niruri are flavonoids, alkaloids, lignans, tannins, coumarins, terpenes, saponins and phenylpropanoids [15,16]. Research on this plant revealed that its aqueous extract inhibited HIV-1 reverse transcriptase [17], as well as reduced urinary calcium in patients with hypercalcuria [18]. It showed antioxidant properties in in vitro tests [19], namely free radical-scavenging, inhibition of reactive oxygen species production and peroxidation of lipids, as well as hepatoprotective properties against the paracetamol-induced injury in mice model [20].
Annona muricata L. (graviola, soursop) is a tree widely distributed in most of tropical countries. Its leaves have been traditionally used to treat headaches, hypertension, cough, asthma and as sedative [21,22]. In a model of skin papilloma in mice, the A. muricata leaves extract was able to suppress tumor initiation and tumor promotion even at lower dosage [23]. It showed antioxidant [24,25], antibacterial [26], antifungal [27] and anti-inflammatory [28] properties.
Gentianella alborosea (hercampure) is used in folk medicine for obesity treatment, in liver ailments and as colagogue, coleretic and digestive [29]. The active compounds are flavonoids, alkaloids, saponins and glycosides [30]. The extract exhibited moderate antioxidant activity and apoptotic properties on HeLa cell line [1].
Plants from the Geranium genus, to which Geranium dielsianum (pasuchaca) belongs, have been shown to have anti-influenza virus activity (G. sanguineum L.) [31], as well as antioxidant and radical scavenging capacities (G. macrorrhizum) [32]. Those properties have been attributed to their polyphenolic constituents. In traditional medicine G. dielsianum is used as blood purifier and hypoglycemic herb.
Antioxidant properties were also observed for Tabebuia ochracea (tahuari) [33]. It exhibited also antibacterial activity against Staphylococcus aureus.
Notholaena nivea (cuti cuti) is used in South America mainly as a herbal tea with the hypoglycaemic effect [34]. The lipophilic extract from aerial parts of this plant exhibited antioxidant properties [35].
T. paronychioides (flor de arena) is used in traditional medicine for treating inflammation of the ovaries [36] and the antioxidant mechanism is often at least partly responsible for antiinflammatory action. To our best knowledge, there is no data on its antioxidant properties.
The common feature of all mentioned above plants is their antioxidant activity. It is usually ascribed to the presence polyphenols, since a high content of phenolic compounds in a plant is usually connected with high antioxidant properties [37]. However, popular and the most frequent form in which those plants are used is an aqueous infusion or decoction. Its preparation can induce the degradation of polyphenols. Therefore, in this study, we compared the antioxidant properties of ten Peruvian plants infusions and investigated their relations to the phenolic content.
The antioxidant properties were studied with DPPH and ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) radical scavenging and ferric reducing antioxidant power (FRAP) tests. For DPPH test we have chosen EPR spectroscopy technique, as it gives more reliable results than spectrophotometry [38]. We determined also the carotenoid content of those plants, to check whether this group of antioxidants can be also partly responsible for antioxidant properties.
2. Experimental
2.1. Plant Material
Ten samples of dried Peruvian plants: Uncaria tomentosa (cat’s claw), Lepidium meyenii (maca), Berberis vulgaris L. (barberry, agracejo), Phyllantus niruri (chanca piedra), Annona muricata L. (graviola, soursop), Gentianella alborosea (hercampure), Tabebuia ochracea (tahuari), were obtained from Uncaria Institute (Warsaw, Poland).
2.2. Infusion Preparation
The 2.5 g of dry powdered plant material was weighted and 250 ml of distilled boiling water was added. Then infusions were left for 10 hours at darkness.
2.3. DPPH Scavenging (EPR Test)
100 μl of an infusion was mixed with 1 ml of 1.3 mM DPPH methanolic solution. After vortexing the samples were kept for 30 minutes at darkness and then EPR spectra were recorded. The samples with distilled water (100 μl) in place of an infusion were prepared as intensity standards. The intensity was taken as the double integral of the spectra. Results were expressed as Trolox equivalents (TEAC, milimoles per 100 ml) with the use of previously prepared standard curve. All experiments were performed in triplicate.
ESR measurements were performed on a Miniscope MS200 spectrometer (Magnettech GmbH). Parameters were as follows: central field 334 mT, sweep range 8 mT, sweep time 30 s, microwave power 10 mW, modulation amplitude 0.1 mT.
2.4. ABTS Scavenging
ABTS assay was performed according to Re et al [39] with small modifications. Briefly, 1500 μl of ABTS cation radical solution, prepared by mixing 7 mM ABTS reagent and 2.45 mM potassium persulfate in equal volumes, equlibrating the mixture for 16 hours and diluting with ethanol to absorbance value of 0.70, was added to 15 μl of plant infusion or standard (trolox) solution. The absorbance reading was taken at 734 nm in sixth minute after adding the radical solution. All experiments were performed in triplicate and results were expressed as milimoles of trolox for 100 ml (TEAC) of an aqueous infusion.
2.5. FRAP Assay
FRAP assay was done according to Benzie and Strain procedure [40]. Briefly, 50 μl of plant infusion or 50 μl of freshly prepared FeSO4 standard solution was mixed with 1500 μl of working FRAP reagent, and absorbance reading at 593 nm was taken after 4 minutes of thermostatting at 37˚C. The working FRAP reagent was prepared daily by mixing FeCl3 and TPTZ (2,4,6-Tripyridyl-sTriazine) solutions with acetate buffer (pH 3.6). The results were taken as a mean of three replicates and expressed as milimoles of reduced Fe3+ per 100 ml of plant infusion.
2.6. Total Phenolic Content Total phenolic content (TP) was determined by modified Folin-Ciocalteu colorimetric method [41]. Briefly, to 20 μl of an infusion 1580 μl of Millipore water and 100 μl of Folin-Ciocalteu reagent was added. After 5 minutes at room temperature, 300 μl of 20% sodium carbonate was added, and the reaction mixture was thermostated for 20 minutes at 37˚C and the absorbance at 765 nm was taken using Evolution 60S spectrophotometer (Thermo Scientific). Results were expressed as gallic acid equivalents (GAE [mg/100 ml]) with the use of the standard curve, prepared in parallel with measurements. All experiments were performed in triplicate.
2.7. Total Carotenoid Content
Total carotenoid content (TC) determination was carried out as beforehand reported [42] with small modifications. The extraction was realized by adding 30 mL n-hexaneacetone mixture (6:4) to 2.5 g of the powdered sample. After shaking for 10 minutes it was filtered through a paper filter and the absorbance at 450 nm was measured immediately with Evolution 60S spectrophotometer (Thermo Scientific), with up to 10 fold dilution where appropriate. Results were expressed as μg of carotenoids per 1 g of plant material, calculated with average carotenoid absorbance coefficient A1% of 2500 [43]. All experiments were performed in duplicate.
2.8. Statistical Analysis
Pearson correlation analysis was performed using a Statistica (Statistical Statsoft, Tulsa, OK) software; P-values < 0.05 were considered significant.
3. Results and Discussion
Among studied plants, the best DPPH-scavenging properties were observed for U. tomentosa and G. dielsianum (1.327 ± 0.034 and 1.234 ± 0.031 mmol trolox/100 ml, respectively), followed by N. nivea, T. paronychioides and A. muricata L. (Figure 1(a)). The DPPH-TEAC value obtained for those samples was over three times higher than the value for the weakest DPPH scavengers, i.e. L. meyenii and T. ochracea (0.434 ± 0.005 and 0.413 ± 0.016, respectively). In case of the ABTS radical, G. dielsianum and U. tomentosa still showed the highest scavenging activity (0.645 ± 0.027 and 0.513 ± 0.061 mmol trolox/100 ml, respectively), although all results obtained from this test were lower than those obtained from the DPPH test (Figure 1(b)). However, the best result in the ABTS test (for G. dielsianum) was over six times better than those for the weakest scavengers, i.e. L. meyenii and T. ochracea (0.067 ± 0.010 and 0.079 ± 0.006 mmol trolox/100 ml, respectively). The result for U. tomentosa was also quite high, but for other plants it was distinctly lower. The difference in results from those two tests can be tentatively ascribed to the different structure of those radicals and especially to different charge, since the DPPH molecule has no charge and the ABTS radical is a cation. This can result in different reaction mechanism both for active compounds in plant infusions and for standard antioxidant, i.e. trolox.
For the FRAP test results, the highest value was obtained for T. paronychioides infusion (0.607 ± 0.022 mmol Fe/100 ml), followed by G. dielsianum (0.562 ± 0.017 mmol Fe/100 ml) and U. tomentosa (0.507 ± 0.016 mmol Fe/100 ml) (Figure 1(c)). The lowest result was given by L. meyenii (0.011 ± 0.001 mmol Fe/100 ml), similar to the radical scavenging tests. In this test the variability among studied samples was distinctly bigger than for previous tests, with the value obtained for T. paronychioides over fifty times higher than the value for L. meyenii.
The compounds responsible for DPPH and ABTS scavenging, as well as for iron-reducing activity, are mainly polyphenols, as can be seen from coefficients of correlations (r) between results of those tests and polyphenol content (Table 1), ranging from 0.803 to 0.930. It is worth stressing that correlations between FRAP and ABTS tests results and total polyphenols was significant with p < 0.005, and between DPPH test results and polyphenol content with p = 0.005.
The polyphenol amount per 100 ml of an infusion varies from 4.6 GAE/100 ml for L. meyenii to 75.7 GAE for T. paronychioides (Figure 2(a)). The high polyphenol content and good antioxidant properties of U. tomentosa bark infusion is consistent with results obtained by Gon- çalves et al. [1], Pilarski et al. [44] and Ranilla et al. [17]. These works also have shown that potent radical scavenging activity strongly correlated with the presence of proanthocyanidins and phenolic acids. The main phenolic acid was either caffeic acid [1,32] or chlorogenic acid [32].
In our study the total phenolic content obtained for P. niruri (14.9 GAE/100 ml) was lower than that for U.
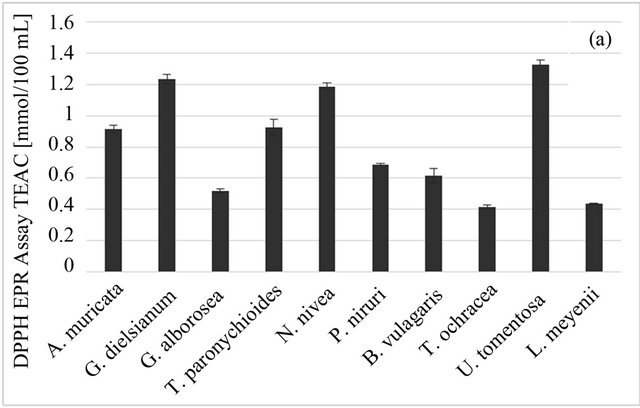
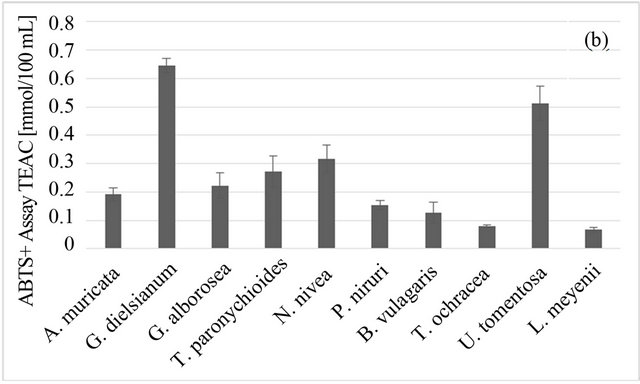
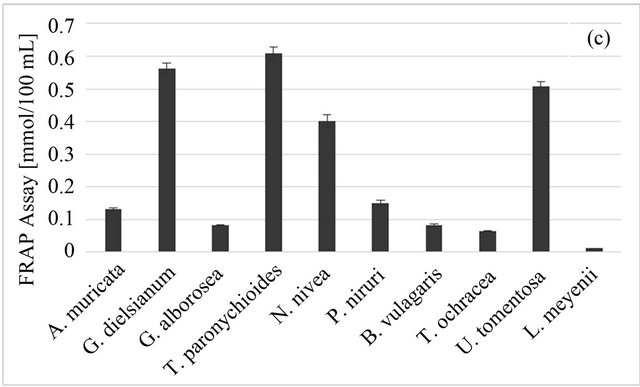
Figure 1. Antioxidant properties of Peruvian plants infusions. (a) DPPH-EPR; (b) ABTS; (c) FRAP. Results are presented as means of three experiments results with standard deviation.
tomentosa (58.9 GAE/100 ml), as opposed to the results of Ranilla [17]. However, this difference can be due to different methods of extraction.
The highest total carotenoid content was observed for A. muricata L., the lowest for T. ochracea (Figure 2(b)). However, it should be noted that for all studied plants the carotenoid content in plant material was low (0.001 - 0.310 μg/1 g). Whatsmore, carotenoids are lipophilic compounds and therefore they would be extracted only in very small part into aqueous infusion. It can be the reason behind the lack of correlation between carotenoids content of plant material and antioxidant properties of aqueous infusions (Table 1).
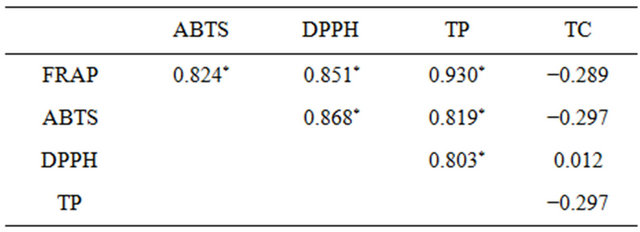
Table 1. Pearson’s correlation coefficients. An asterisk indicates significant correlations.
Overall, the studied plant infusions could be divided into three groups: good antioxidant sources (U. tomentosa, P. niruri, T. paronychioides and N. nivea), which
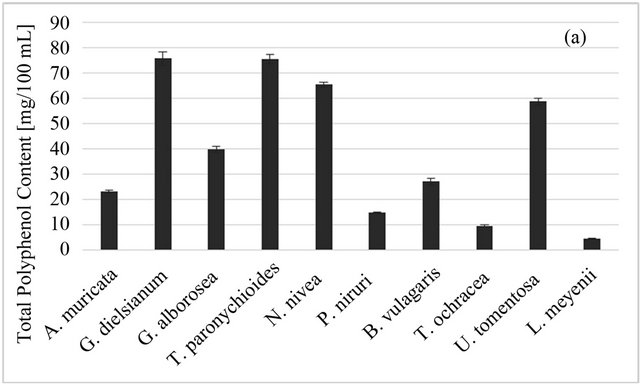
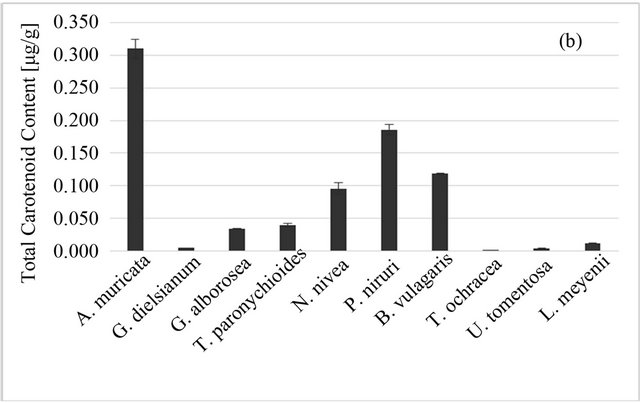
Figure 2. Total phenolic (a) and total carotenoid; (b) content of Peruvian plants infusions and in plant material, respectively. Results are presented as means of three or two respectively experiment results with standard deviation.
gave high results in all antioxidant tests (DPPH, ABTS and FRAP), weak antioxidant sources, with low values obtained from all those tests (L. meyenii, T. ochracea, B. vulgaris L., P. niruri) and selective antioxidant sources, which had high or at least moderate values only in one or two tests (A. muricata L., G. alborosea). Good scavengers have also a high content of total polyphenols, while the phenolic content of plants infusions with low TEAC values is also low. The exception is hercampuri (G. alborosea), which is a weak scavenger of ABTS radical and has low FRAP value despite its relatively high polyphenol content.
4. Conclusions
Infusions of all ten studied plants have shown antioxidant activity, though there was a large diversity between the results. Therefore, they can be used as a valuable antioxidant component of human diet not only in areas where they are endemic to, but in the whole world.
The antioxidant properties were correlated with polyphenols content; there was no correlation with the carotenoids content. It supports the hypothesis that the polyphenol group is the dominating group of antioxidants in those plants, at least in their most popular serving form, i.e. aqueous infusion.
REFERENCES
- K. Keplinger, G. Laus, M. Wurm, M. P. Dierich and H. Teppner, “Uncaria tomentosa (Willd.) DC.-Ethnomedicinal Use and New Pharmacological, Toxicological and Botanical Results,” Journal of Ethnopharmacology, Vol. 64, No. 1, 1999, pp. 23-34. doi:10.1016/S0378-8741(98)00096-8
- M. E. Heitzman, C. C. Neto, E. Winiarz, A. J. Vaisberg and G. B. Hammond, “Ethnobotany, Phytochemistry and Pharmacology of Uncaria (Rubiaceae),” Phytochemistry, Vol. 66, No. 1, 2005, pp. 5-29. doi:10.1016/j.phytochem.2004.10.022
- C. Gonçalves, T. Dinis and M. T. Batista, “Antioxidant Properties of Proanthocyanidins of Uncaria tomentosa Bark Decoction: A Mechanism for Anti-Inflammatory Activity,” Phytochemistry, Vol. 66, No. 1, 2005, pp. 89-98. doi:10.1016/j.phytochem.2004.10.025
- Y. Wang, Y. Wang, B. McNeil and L. M. Harvey, “Maca: An Andean Crop with Multi-Pharmacological Functions,” Food Research International, Vol. 40, No. 7, 2007 pp. 783-792. doi:10.1016/j.foodres.2007.02.005
- G. F. Gonzales, C. Gonzales and C. Gonzales-Castañeda. “Lepidium meyenii (Maca): A Plant from the Highlands of Peru—From Tradition to Science,” Forsch Komplementmed, Vol. 16, No. 6, 2009, pp. 373-380. doi:10.1159/000264618
- C. Gonzales, J. Rubio, M. Gasco, Y. Nieto, S. Yucra and G. F. Gonzales, “Effect of Short Term and Long Term Treatments with Three Ecotypes of Lepidium meyenii (maca) on Spermatogenesis in Rats,” Journal of Ethnopharmacology, Vol. 103, No. 3, 2006, pp. 448-454. doi:10.1016/j.jep.2005.08.035
- J. Rubio, M. Caldas, S. Dávila, M. Gasco and G. F. Gonzales, “Effect of Three Different Cultivars of Lepidium meyenii (maca) on Learning and Depression in Ovariect-Omized Mice,” BMC Complementary & Alternative Medicine, Vol. 6, No. 1, 2006, pp. 23-30. doi:10.1186/1472-6882-6-23
- M. Sandoval, N. N. Okuhama, F. M. Angeles, V. M. Melchor, L. A. Condezo, J. Lao and M. J. S. Miller, “Antioxidant Activity of the Cruciferous Vegetable Maca (Lepidium meyenii),” Food Chemistry, Vol. 79, No. 2, 2002, pp. 207-213. doi:10.1016/S0308-8146(02)00133-4
- K. J. Lee, K. Dabrowski, J. Rinchard, C. Gomez, L. Guz and C. Vilchez, “Supplementation of Maca (Lepidium meyenii) Tuber Meal in Diets Improves Growth Rate and Survival of Rainbow Trout Oncorhynchus Mykiss (Walbaum) Alevins and Juveniles,” Aquaculture Research, Vol. 35, No. 3, 2004, pp. 215-223. doi:10.1111/j.1365-2109.2004.01022.x
- M. Blumenthal, W. R. Busse, A. Goldbert, J. Gruenwald, T. Hall, S. Klein, C. W. Riggins and R. S. Rister, “The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicine,” American Botanical Council, Austin, 1998.
- J. Brunetton, “Pharmacognosy, Phytochemistry, Medicinal Plants,” Lavoisier Publishing, Paris, 1999.
- P. Hanachi, S. H. Kua, R. Asmah, G. Motalleb and O. Fauziah, “Cytotoxic Effect of Berberis vulgaris Fruit Extract on the Proliferation of Human Liver Cancer Line (HepG2) and Its Antioxidant Properties,” International Journal of Cancer Research, Vol. 2, No. 1, 2006, pp. 1-9. doi:10.3923/ijcr.2006.1.9
- S. Schaffer and M. Heinrich, “Understanding Local Mediterranean Diets: A Multidisciplinary Pharmacological and Ethnobotanical Approach,” Pharmacological Research, Vol. 52, No. 4, 2005, pp. 353-366. doi:10.1016/j.phrs.2005.06.005
- R. N. Chopra, S. L. Nayar and I. C. Chopra, “Glossary of Indian Medicinal Plants,” Catholic Press, Ranchi, CSIR, New Delhi, 1986.
- M. L. Dhar, M. M. Dhar, B. N. Dhawan, B. N. Mehrotra and C. Ray, “Screening of Indian Plants for Biological Activity: Part 1,” Indian Journal of Experimental Biology, Vol. 6, No. 4, 1968, pp. 232-247.
- G. Bagalkotkar, S. R. Sagineedu, M. S. Saad and J. Stanslas, “Phytochemicals from Phyllantus niruri L. and Their Pharmacological Properties: A Review,” Journal of Pharmacy and Pharmacology, Vol. 58, No. 12, 2006, pp. 1559- 1570. sdoi:10.1211/jpp.58.12.0001
- A. D. Naik and A. R. Juvekar, “Effect of Alkaloidal Extract of Phyllantus niruri on HIV Replication,” Indian Journal of Medical Science, Vol. 57, No. 9, 2003, pp. 387-393.
- J. L. Nishiura, A. H. Campos, M. A. Boim, I. P. Heilberg and N. Schor, “Phyllantus niruri Normalizes Elevated Urinary Calcium Levels in Calcium Stone Forming (CSF) Patients,” Urological Research, Vol. 32, No. 5, 2004, pp. 362-366. doi:10.1007/s00240-004-0432-8
- L. G. Ranilla, Y.-I. Kwon, E. Apostolidis and K. Shetty, “Phenolic Compounds, Antioxidant Activity and in Vitro Inhibitory Potential against Key Enzymes Relevant for Hyperglycemia and Hypertension of Commonly Used Medicinal Plants, Herbs and Spices in Latin America,” Bioresource Technology, Vol. 101, No. 12, 2010, pp. 4676- 4689. doi:10.1016/j.biortech.2010.01.093
- S. M. Sabir and J. B. T. Rocha, “Water-Extractable Phytochemicals from Phyllanthus niruri Exhibit Distinct in Vitro Antioxidant and in Vivo Hepatoprotective Activity against Paracetamol-Induced Liver Damage in Mice” Food Chemistry, Vol. 111, No. 4, 2008, pp. 845-851. doi:10.1016/j.foodchem.2008.04.060
- L. Taylor, “Technical Data Report for Graviola, Annona Muricata,” Sage Press, Austin, 2002.
- C. A. Lans, “Ethnomedicines Used in Trinidad and Tobago for Urinary Problems and Diabetes Mellitus,” Journal of Ethnobiology and Ethnomedicine, Vol. 2, No. 52, 2006, pp. 45-55. doi:10.1186/1746-4269-2-45
- H. Sulaiman, A. H. Roslida, O. Fezah, K. L. Tan, Y. S. Tor and C. I. Tan, “Chemopreventive Potential of Annona Muricata L. Leaves on Chemically-Induced Skin Papillomagenesis in Mice,” Asian Pacific Journal of Cancer Prevention, Vol. 13, No. 6, 2012, pp. 2533-2539. doi:10.7314/APJCP.2012.13.6.2533
- J. G. de Melo, T. A. de Sousa Araújo, V. T. N. de Almeida e Castro, D. L. de Vasconcelos Cabral, M. do Desterro Rodrigues, S. C. do Nascimento, E. L. Cavalcanti de Amorim, U. P. de Albuquerque, “Antiproliferative Activity, Antioxidant Capacity and Tannin Content in Plants of Semi-Arid Northeastern Brazil,” Molecules, Vol. 15, No. 12, 2010, pp. 8534-8542. doi:10.3390/molecules15128534
- R. Baskar, V. Rajeswari and T. S. Kumar, “In Vitro Antioxidant Studies in Leaves of Annona Species,” Indian Journal of Experimental Biology, Vol. 45, No. 5, 2007, pp. 480-485.
- K. Sundarrao, I. Burrows and M. Kuduk, “Preliminary Screening of Anti-Bacterial and Anti-Tumor Activities of Papuan New Guinean Active Medicinal Plants,” Pharmaceutical Biology, Vol. 31, No. 1, 1993, pp. 3-6. doi:10.3109/13880209309082909
- M. Heinrich, M. Kuhnt and C. W. Wright, “Parasitological and Microbiological Evaluation of Mixe Indian Medicinal Plants (Mexico),” Journal of Ethnopharmacology, Vol. 36, No. 1, 1992, pp. 81-85. doi:10.1016/0378-8741(92)90063-W
- O. V. de Sousa, G. D. V. Vieira, J. J. R. G. de Pinho et al., “Antinociceptive and Anti-Inflammatory Activities of the Ethanol Extract of annona muricata L. Leaves in Animal Models,” International Journal of Molecular Sciences, Vol. 11, No. 5, 2010, pp. 2067-2078. doi:10.3390/ijms11052067
- K. Żurowska, “Amazonian and Andean Phytotherapy,” Tower Press, Gdańsk, 2001.
- N. Acero, F. Llinares, A. Galán de Mera, B. Oltra and D. Muñoz-Mingarro, “Apoptotic and Free Radical Scavenging Properties of the Methanolic Extract of Gentianella alborosea,” Fitoterapia, Vol. 77, No. 8, 2006, pp. 475- 477. doi:10.1016/j.fitote.2006.05.020
- J. Serkedjieva and A. J. Hay, “In Vitro Anti-Influenza Virus Activity of a Plant Preparation from Geranium sanguineum L.,” Antiviral Research, Vol. 37, No. 2, 1998, pp. 221-230. doi:10.1016/S0166-3542(97)00067-3
- M. Sokmena, M. Angelova, E. Krumova, S. Pashova, S. Ivancheva, A. Sokmen and J. Serkedjieva, “In Vitro Antioxidant Activity of Polyphenol Extracts with Antiviral Properties from Geranium sanguineum L.,” Life Sciences, Vol. 76, No. 25, 2005, pp. 2981-2993. doi:10.1016/j.lfs.2004.11.020
- A. F. Ospina, J. P. C. Guerrero, Y. C. O. Buendía, I. B. P. Bolívar and F. D. Castillo, “Antiinflammatory, Antioxidant and Antibacterial Activity of Two Species of Tabebuia Genus,” Revista Cubana de Plantas Medicinales, Vol. 18, No. 1, 2013, pp. 34-46.
- R. W. Bussmann and A. Glenn, “Traditional Knowledge for Modern Ailments—Plants Used for the Treatment of Diabetes and Cancer in Northern Peru,” Journal of Medical Plants Research, Vol. 5, No. 31, 2011, pp. 6916- 6930.
- G. Cioffi, P. Montoro, O. Lock de Ugaz, A. Vassallo, L. Severino, C. Pizza and N. de Tommasi, “Antioxidant Bibenzyl Derivatives from Notholaena Nivea Desv,” Molecules, Vol. 16, No. 3, 2011, pp. 2527-2541. doi:10.3390/molecules16032527
- R. W. Bussmann and A. Glenn, “Medicinal Plants Used in Northern Peru for Reproductive Problems and Female Health,” Journal of Ethnobiology and Ethnomedicine, Vol. 6, No. 1, 2010, pp. 30-41. doi:10.1186/1746-4269-6-30
- M. P. Kähkönen, A. I. Hopia, H. J. Vuorela, J. P. Rauha, K. Pihlaja, T. S. Kujala and M. Heinonen, “Antioxidant Activity of Plant Extracts Containing Phenolic, Compounds,” Journal of Agricultural and Food Chemistry, Vol. 47, No. 10, 1999, pp. 3954-3962. doi:10.1021/jf990146l
- D. Sanna, G. Delogu, M. Mulas, M. Schirra and A. Fadda, “Determination of Free Radical Scavenging Activity of Plant Extracts through DPPH Assay: An EPR and UVVis Study,” Food Analytical Methods, Vol. 5, No. 4, 2012, pp. 759-766. doi:10.1007/s12161-011-9306-1
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, “Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay,” Free Radical Biology and Medicine, Vol. 26, No. 9-10, 1999, pp. 1231-1237. doi:10.1016/S0891-5849(98)00315-3
- I. F. Benzie and J. J. Strain, “The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘Antioxidant Power’: The FRAP Assay,” Analytical Biochemistry, Vol. 239, No. 1, 1996, pp. 70-76. doi:10.1006/abio.1996.0292
- V. Singleton, R. Orthofer and R. M. Lamuela-Raventos, “Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent,” Methods in Enzymology, Vol. 299, No. 39, 1999, pp. 152-175. doi:10.1016/S0076-6879(99)99017-1
- I. Ferreira, E. Aires, J. C. M. Barreira and L. Estevinho, “Antiooxidant Activity of Portuguese Honey Samples: Different Contributions of the Entire Honey and Phenolic Extract,” Food Chemistry, Vol. 114, No. 4, 2009, pp. 1438- 1443. doi:10.1016/j.foodchem.2008.11.028
- Current Protocols in Food Analytical Chemistry, “Detection and Measurement of Carotenoids by UV/VIS Spectrophotometry,” 2001.
- R. Pilarski, H. Zielinski, D. Ciesiolka and K. Gulewicz, “Antioxidant Activity of Ethanolic and Aqueous Extracts of Uncaria Tomentosa (Willd.) DC,” Journal of Ethnopharmacology, Vol. 104, No. 1-2, 2006, pp. 18-23. doi:10.1016/j.jep.2005.08.046
NOTES
*Corresponding author.

