Food and Nutrition Sciences
Vol. 4 No. 2 (2013) , Article ID: 27724 , 8 pages DOI:10.4236/fns.2013.42024
Biochemical Composition and Disease Resistance in Newly Synthesized Amphidiploid and Autotetraploid Peanuts
![]()
1International Crops Research Institute for Semi Arid Tropics (ICRISAT), Patancheru, India; 2All India Coordinated Research Project on Groundnut, Main Agricultural Research Station, University of Agricultural Sciences, Raichur, India; 3Department of Plant Sciences, School of Life Sciences, University of Hyderabad, Hyderabad, India; 4Department of Biotechnology, School of Life Sciences, University of Hyderabad, Hyderabad, India.
Email: *N.Mallikarjuna@CGIAR.ORG
Received November 19th, 2012; revised January 11th, 2013; accepted January 18th, 2013
Keywords: Amphidiploid; Autotetraploid; Biochemical; Disease Resistance; Fatty Acid; Late Leaf Spot; Peanut; Peanut Bud Necrosis Disease; Proteinase Inhibitors; Synthetic Peanut
ABSTRACT
Genetic diversity in peanut (Arachishypogaea L.) is narrow due to its evolution and domestication processes. Amphidiploids and autotetraploids (newly synthesized tetraploids) were created to broaden its genetic base. Molecular analysis has shown that the newly synthesized tetraploids had broader genetic base; and were genetically divergent when compared to cultivated peanut. Nutritional composition relative to oil, fatty acid composition, O/L ratio, protein, iodine value and presence of plant proteinase inhibitors such as trypsin and chymotrypsin inhibitors were studied in the synthesized tetraploids. Some of the newly synthesized tetraploids had higher amounts of proteinase inhibitors. Evaluation of newly synthesized tetraploids revealed several lines resistant to late leaf spot (LLS) and peanut bud necrosis disease (PBND).
1. Introduction
Genetic diversity in peanut (Arachishypogaea L.) is narrow due to the nature of its evolution and domestication. This is exemplified by the fact that cultivated peanut originated by the hybridization of two Arachis species with A and B genome, giving rise to a diploid hybrid. Chromosome doubling of the diploid gave rise to tetraploid-amphiploid peanut. During this process, a series of bottlenecks played a major role in the evolution of the crop with a narrow genetic base. At least six bottlenecks can be seen in the evolution of the crop. The first one is that only two species have contributed their genomes for the evolution of the crop when there were at least 24 more closely related Arachis species available. The second is sterility of the diploid hybrid [1]. If diploid hybrid was fertile probably polyploidy would not have taken place. The third bottle neck was the chance event of tetraploidy from diploid hybrid, which can itself be a bottle neck [2]. The fourth one is selection of tetraploid groundnut by the farmers of South America, and the spread of peanut from South America to rest of the world; and by the 14th century traders is the fifth bottleneck. The sixth is the self-pollinating nature of the crop. To undo the consequences of narrow genetic base, a major effort was made at the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) between 2005-2011 to create new sources not only of amphidiploid groundnut by combining different A and B genome species, but a few autotetraploids by combining A with A genome and B with B genome species. Molecular analysis showed that the newly synthesized tetraploids had broader genetic base; and were genetically divergent when compared to the cultivated peanuts [1].
Nutritional composition which is composed of oil, fatty acid composition, O/L ratio, amount of protein, Iodine value and presence of plant proteinase inhibitors such as trypsin and chymotrypsin inhibitors were studied in the synthesized tetraploids. Proteinase inhibitors (PIs) are natural defense related proteins in the seeds which play an important role against predators and pathogens. The defensive role of PIs is based on their inhibitory activities to insects’ digestive enzymes [3]. O/L ratio is an important factor in deciding the stability of the oil. Fatty acid composition is made up of unsaturated fatty acids (TUSF) and saturated fatty acids (TSF) which make up physical and chemical properties of the oil. The synthesized tetraploids were evaluated for a few diseases such as late leaf spot, rust, and peanut bud necrosis disease resistance traits.
The objectives of the study was to characterize the evaluate the newly synthesized amphidiploids and autotetraploids for some of the biochemical traits such as the presence of PIs, protein contact, fatty acid composition, O/L ratio and disease resistance to rust, late leaf spot, and peanut bud necrosis disease. Characterization and evaluation data would give better insight to the nature of the material generated. The results of the study are presented and discussed in this communication.
2. Materials and Methods
2.1. Plant Material
Synthetic tetraploids used in the study were developed at the ICRISAT in Patancheru, India, by crossing Arachis species from section Arachis and developing diploid hybrids. Tetraploids were synthesized from the diploid hybrids; and the details of the procedure are given in Mallikarjuna et al. [1]. Arachis species were obtained from the ICRISAT Genetic Resources Unit, ICRISAT and the details of the material used in the study, are given in Table 1.
Table 1. Details of the amphidiploids and autotetraploids used in the study.


Extraction of fatty acids: Fatty acids were esterified by the saponification-trans-esterification method followed by Metcalf et al. [4] with 35 g of peanut seeds were ground in a Krups grinder. An aliquot of 300 mg (approx.) of the powder was taken into a large glass culture tube. To this, 15 ml of petroleum ether was added, and the contents agitated to mix on a tube rotator for 30 min. After 30 min of mixing, the contents were centrifuged for 5 min at 4000 rpm. Five ml of the supernatant was transferred to a culture tube; and allowed the solvent to evaporate under a stream of nitrogen gas. 1.3 ml of 0.5 N NaOH in methanol was added, and the mixture was heated on a boiling water bath for 5 min. The mixture was cooled and to this 2 ml of 12% boron trifluoride in methanol was added, and heated for 5 min on a boiling water bath. The mixture was cooled and 2 ml of saturated solution of NaCl was added and agitated for 10 min on a tube rotator. Two ml of petroleum ether was added and the contents mixed again on a tube rotator for 5 min followed by centrifuging at 4000 rpm for 5 min. The supernatant petroleum ether layer containing the fatty acid methyl esters was transferred into the automatic sampler vial and stored in a freezer for GLC analysis.
2.2. Fatty Acid Analysis by GLC
A gas chromatograph, model GC-9 A GLC unit equipped with programmable oven, flame ionization detector and connected to electronic CR3-A integrator (shimadzu, Kyoto, Japan) was used to separate the fatty acid methyl esters. The Glass column which was 6 feet long with 3 mm inner diameter packed with GP 3% SP-2310/2%, SP-2300 on 100/120 Chromasorb W-AW DMCS (80 - 100 mesh) (SupelcoInc). The Initial column temperature was set at 195˚C and held for 5 min followed by a step up of 10˚C/min to reach a final temperature of 250˚C for 2 min. The Injection port and flame ionization and detector temperatures were set at 260˚C. The flow rates for carrier gas Helium (or nitrogen) 50 ml/min (Primary pressure 6 kg/cm2) and hydrogen gas flow to 0.6 kg/cm2 and air to 0.5 kg/cm2. A shimadzu C-R4 A Chromatopac Integrator (Shimadzu Corporation, Kyoto, Japan) was used to record the retention time and peak area of fatty acids. A standard fatty acid methyl esters mixture Nu Check 21 A (Nu Check Prep, Inc. P. O. Box 172, Elysian, MN 56028) was used to identify the fatty acids in the samples.
The fatty acid methyl esters eluted in the order: palmitic (16:0), stearic (18:0), oleic (18:1), linoleic (18:2), arachidic (20.0), eicosenoic (20:1), behenic (22:0), and lignoceric (24:0), when the above mentioned column is used. From the fatty acid estimation, the following quality parameters were calculated [5] 1) Iodine content (% oleic acid × 0.8601 + % linoleic acid × 1.7321); 2) total saturated fatty acids (%; TSF) = % palmitic acid + % stearic acid + % arachidic acid + % beheniccaid + % lignoceric acid; 3) total unsaturated fatty acids = % oleic acid + % linoleic acid.
2.3. Determination of Oil Content by Soxhlet Extraction Method
Oil content was determined by grinding about 10 g of groundnut seeds in a krups, KM 75 blender and the groundnut meal was extracted with n-hexane for 18 hours in a soxhlet apparatus. Hexane was evaporated in a hot sand bath and oil residue was weighed to calculate the percentage of the sample. Oil content was also checked by using the NMR method.
2.4. Protein Estimation
Protein content was determined by Folins-Ciocalteau method using BSA as a standard [6]. The working solution consist of 4% Na2CO3 in 0.2 N NaOH: 2% Sodium potassium tartarate: 1% CuSo4 which were mixed freshly (23:1:1) before adding to the sample to be tested.
2.5. Assay for Proteinase Inhibitors: Proteinases and Substrates
Bovine pancreatic trypsin and bovine pancreatic a-chymotrypsin were procured from Sisco Research Laboratory, Mumbai, India. N-a-Benzoyl-dl-arginine-p-nitroanilide (BAPNA) and n-glutaryl-l-phenylalanine-p-nitroanilide (GLUPHEPA) were procured from Sigma.
The seed extract enriched with crude protein [proteinase inhibitor (PI) sample] was prepared from mature dry seeds of amphidiploids andtetraploids as described in [7]. Trypsin or chymotrypsin inhibitor activity was determined by using appropriate volumes of crude extract or purified protein that resulted in 40% - 60% decrease in corresponding enzyme activity. Assay mixture (1.0 ml) consisted of PI sample and assay buffer, (50 mM TrisHCL containing 20 mM CaCl2 either at pH 8.2 for trypsin or pH 7.8 for chymotrypsin). 10 μg of trypsin or 80 μg of chymotrypsin was added to the assay mixture and incubated for 15 min at 37˚C. Residual proteinase activiity in the above assay mixture was determined after incubating for 45 min at 37˚C using 1 mM BAPNA (1.0 ml) as a substrate for trypsin [8] and 1 mM GLUPHEPA (1.0 ml) as a substrate for chymotrypsin [9]. The reaction was terminated by adding 0.2 ml of 30% acetic acid. The activity of proteinase inhibitors was expressed as trypsin inhibitors (TI) units/mg protein or chymotrypsin inhibitor (CI) units/mg protein. One TI or CI unit was defined as the amount of inhibitor required to inhibit 50% of the corresponding enzyme activity. BAPNA and GLUPHEPA were dissolved in dimethyl sulphoxide.
2.6. Field Screening Technique for Peanut Bud Necrosis Disease (PBND)
The method of infector row technique was followed to screen peanut entries against PBND. For the purpose of screening, each entry was sown in single row of 5 m length flanked by susceptible check KRG-1 at every fourth row, with a spacing of 45 cm between rows and 10 cm between plants. A susceptible variety was sown after a set of 4 test entries as an infector row. Five lines of KRG-1 were sown all along the border of screening plot 15 days prior to sowing of test entries. Screening was done under natural disease incidence conditions as the Raichur center in Karnataka, India, has been identified “hot spot” for PBND [10]. The final observations on the disease incidence were recorded a week before harvest of the crop.
Randomly collected samples showing typical PBND symptoms from the screening block were collected and mechanically inoculated on cowpea seedlings (C-152) to confirm the the presence of peanut bud necrosis virus in the screening block. For further confirmation, ELISA was also carried out and results showed positive reaction for PBNV.
To record the disease incidence, total number of plants and number of PBND plants in each entry were counted. Finally, percent disease incidence was worked out using the formula given below.

The entries were grouped into different categories following 0 - 5 resistance/susceptible scale [11].
2.7. Field Screening Technique for Late Leaf Spot and Rust
The disease incidence of late leaf spot and rust is severe on variety KRG-1 during the kharif (rainy) season at AICRP Raichur centre under natural disease incidence conditions. The method of infector row technique was followed to screen the entries against late leaf spot and rust. Test lines were planted in 5 m rows (45 × 10 cm). Susceptible variety (KRG-1) was sown after a set of 4 test entries, as an infector row. Five lines of KRG-1 were sown all along the border of screening plot 15 days prior to sowing of test entries. The final observations on the disease incidence were recorded a week before harvest of the crop with a modified 9 point field scale [12]. The entries were classified as immune (1 grade), resistant (2 - 3 grade), moderately resistant (4 - 5 grade), susceptible (6 - 7 grade) and highly susceptible (8 - 9 grade) [13].
3. Results
Fatty acid composition of synthetic peanut together with a few wild Arachis species revealed that peanuts in general are made up of oleic and linoleic acids (>70%). There was not much difference between cultivated peanut (75% - 79%) and the synthesized tetraploids (69% - 78%) with respect to oleic and linoleic acid content. Although a few of the test lines had slightly lower amounts of the component. A similar range was observed in the Arachis species used in the study. Some difference was however observed in percent oil content in the cultivated (~44% oil) and synthesized tetraploids (varied from 45% to 57%, with majority of the lines having above 50%). One of the Arachis species namely A. magna had 57%. O/L ratio is an important factor and the ratio was approximately 1.0 in the peanut cultivars. In Arachis species and synthesized tetraploids it varied from 0.8% to 1.3% (Table 2).
Total saturated fatty acids varied from 21% to 24% in cultivated peanuts used in the study. It varied from 22% - 31% in the Arachis species, and a similar trend was observed in the synthesized tetraploids. With respect to the components of fatty acids, cultivated peanut had 1% - 2% lignoceric acid. A similar trend was observed in the Arachis species, except for A. cardenasii accession ICG 11548 and synthesized tetraploid ISATGR 9 A, which had above 4.0%. Behenic acid content in cultivars varied from 4% to 5%. In the Arachis species used in the study, it varied from 2% to 8 % and a similar trend was observed in the synthesized tetraploids. Stearic acid content varied from 3% to 4 % in the cultivars, and a similar trend was observed in the Arachis species and the synthesized tetraploids. Synthesized tetraploids had 11 to 14% palmitic acid and a similar trend was observed in the Arachis species. In cultivated peanut, palmitic acid content did not exceed 12% (Table 2). In most of the tetraploids, Iodine value varied from 95 to 103, and similar trend was observed in the Arachis species.
Total protein concentration in cultivated peanut accessions ranged from 65 mg/g to 85 mg/g compared to 140 mg/g in A. kempffmercadoi ICG 8959, the Arachis species used in the study (Figure 1). In the synthesized tetraploids, protein concentration varied from a minimum of 43 mg/g in ISATGR 5 B to a maximum of 134 mg/g in ISATGR 9 A. In all the other tetraploids, it was above 70 mg/g protein in the seeds (Figure 1).
Trypsin or Chymotrypsin inhibitor activity was assayed in the synthesized tetraploids. ISATGR 52 B, ISATGR 278 18, ISATGR 268-5 A, ISATGR 9 A had TI activity of 11 to 15 units/mg protein, two varieties ISATGR-1212 and ISATGR 10 B had TI activity of 17.46 and 18.59 units/mg protein, three varieties ISATGR 265-5, ISATGR 34 B and ISATGR 206 B had TI activity varied between 21 - 25 TI units/mg protein, one tetraploid viz., ISATGR 5 B has the least TI activity of 8.2 units/mg protein respectively (Figure 2). The culti
Table 2. Fatty acid composition in newly synthesized tetraploids.

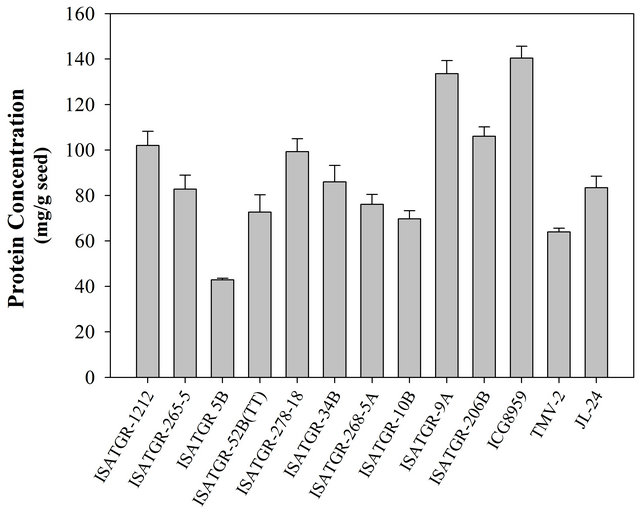
Figure 1. Protein concentration in some newly synthesized amphidiploid/tetraploids, Arachis species and cultivars of peanut.
vated varieties viz., TMV-2 and JL-24 had the TI activity of 21.65 and 8.19 units/mg protein respectively (Figure 2). ISATGR 5 B, ISATGR 278-18, ISATGR 268-5 A, ISATGR 9 A and ISATGR 206 B had the CI activity of 8 to 10 CI units/mg protein, three varieties ISATGR 1212, ISATGR 265-5, ISATGR 10 B had the CI activity of 12 to 17 CI units/mg protein, two varieties ISATGR 52 B and ISATGR 34 B, had the CI activity of 20.58 and 25.65 units/mg protein respectively (Figure 2). The cultivar TMV-2 showed undetectable CI activity and cultivar JL-24 had the CI activity of 14 CI units/mg protein (Figure 2).
Evaluating the synthesized tetraploids for peanut bud necrosis disease (PBND) showed that seven lines had no disease incidence and were classified as immune. Incidence of disease in some lines varied from 5% to 13%, and these were classified as resistant. One line had 25% disease incidence and it was classified as moderately resistant. Two lines had high disease incidence (50%) which was more than what was observed in the local susceptible check (44%) and they were classified as highly susceptible (Table 3).
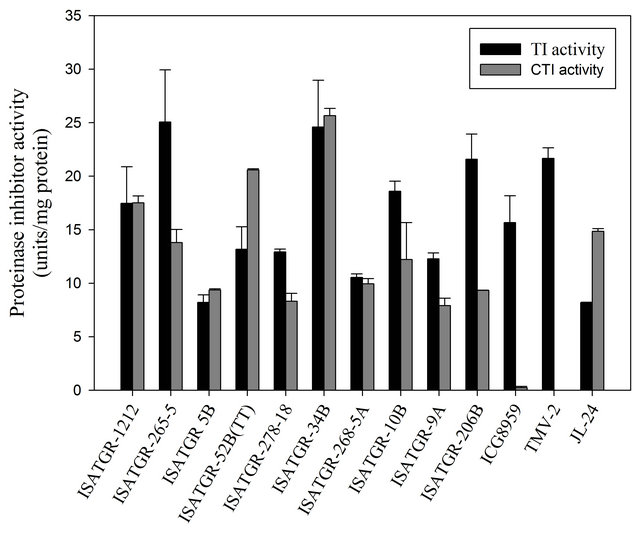
Figure 2. Concentration of TI and CI in synthesized amphidiploids/tetraploids, Arachis species and peanut cultivars.
Table 3. Screening some synthesized tetraploids for PBND, LLS and rust.
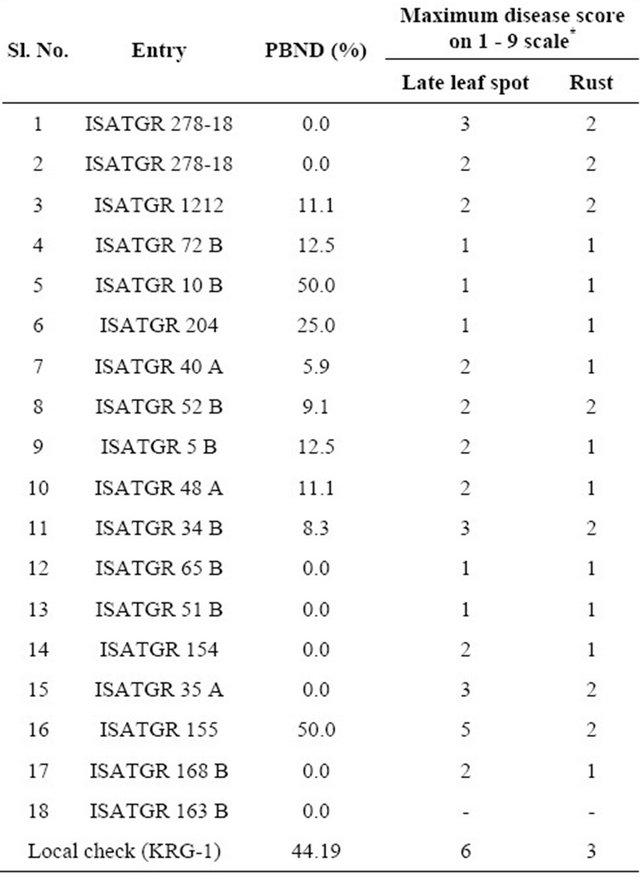
*Data on screening amphidiploids and autotetraploids for PBND, LLS and rust.
All the lines had less rust incidence than the local check i.e. in KRG-1, which had a score of 3. Seven lines had a score of 2, and 10 lines had a score of 1. Lines with a score of 1 can be classified as resistant. With respect to LLS, the local check had a score of 6. Only one synthetic tetraploid (ISATGR 155) had a score of 5. Eight lines had a score of 2, and 5 lines had a score of 1. Lines with a score of 1 to 3 are categorized as resistant (Table 3).
4. Discussion
Fatty acid composition of any oil yielding crop is important as it decides the nature of the oil as to whether it is healthy to human consumption or not. Peanut oil is considered as one of the nutritious oils for human consumption as it has higher amount of TUSF (total unsaturated fatty acid) contents compared to TSF (total saturated fatty acid) contents.
Oleic acid (mono unsaturated omega-9 fatty acid) is known to hinder the progression of adrenoleukodystrophy (ALD), a fatal disease that affects the brain and adrenal glands. Linoleic acid is an unsaturated n-6 fatty acid. It also belongs to the class of essential fatty acid as human body needs it for various biological processes. Deficiency of linoleic acid causes scaling of the skin and hair loss. Both these fatty acid fractions are classified as polyunsaturated (n-9 and n-6). These polyunsaturated fatty acids do not form plaque in the blood vessels. These acids form the major component of peanut oil as well as the synthesized tetraploid peanuts. Oleic acid content ranged from 37% to 38% in the three groundnut cultivars, and it varied from 33% to 43% in the tetraploids. Similar variation in oleic acid content was observed in some of the Arachis species used in the study. Linoleic acid content in the tetraploids varied from 32% to 42% which varied between 39% - 41% in the cultivars and Arachis species used in the study. The ratio of oleic to linoleic acid is a measure of oil stability. The ratio varied from 0.9 to 1.0 in the cultivars used in the study. In the tetraploids the O/L ratio varied from 0.8 to 1.3 with majority of the lines with more than 1.0. Ratio of >1 shows that oleic acid is more than linoleic, hence more stable and long shelf life [14].
Iodine value (I V) is a measure of the degree of oil unsaturation; and has been commonly used as an indicator for predicting shelf life [15]. Low-I V oils are more saturated with fewer double-bonds (lard, tallow, palm oil, coconut oil etc). High-I V oils are more unsaturated with more double-bonds (linseed oil, some fish oils etc). Coconut oil has an Iodine value of 10 and soybean oil has a value of 130. Moderately saturated (monounsaturated) olive oil has an iodine value of 78 - 88. Iodine value of 88 to 105 was observed in the synthesized tetraploids and Arachis species. Cultivated peanut has an I V of 90 - 95. Compared to many of the saturated oils such as palm oil, peanut oil is healthier with higher degree of un-saturation.
Proteinase inhibitors (PIs) prevent the target insect from digesting protein by competitively binding to the active site of protein, which is the actual binding site of proteinase. As the insect cannot digest protein, it is subjected to starvation and/or death. PIs also cause increased levels of insect deformity, due to the potential inhibition of the proteinases involved in metamorphosis of the larvae [7]. A new role for PI in the modulation of apoptosis or programmed cell death has been identified in soybean [16]. The presence of PI in the synthesized tetraploids, shows that they may play a major role in disease and pest resistance. Although PI were also present in cultivated peanuts used in the study, S. Marri and K. Padmashree (unpublished data) observed that the molecular components of those present in cultivated and the synthesized tetraploidswere different, and these components have a differential role with respect to insect midgut trypsin-like proteinases of Spodopteralitura.
Peanut bud necrosis disease is an economically important disease causing yield losses up to 89 million USD in India alone [17]; and the sources of high levels of resistance are lacking in the cultivated germplasm but many wild Arachis species have resistance [18]. Synthesized tetraploids were screened for PBND in disease sick plot at Raichur (India) and many of them were found to range from immune to resistant. Since tetraploids can be easily crossed with cultivated peanut, sources of resistance in tetraploids opens up new opportunities to breed PBND resistant peanuts.
Cultivated groundnut is susceptible to late leaf spot (LLS) caused by Phaeoisariopsispersonata [(Berk. & M. A. Curtis) Aex]; and the resistance is low to moderate in the primary gene pool of groundnut [19]. Closely related wild species in the secondary gene pool are highly resistant to the disease [20]. Since diploid Arachis species are difficult to use for the introgression of LLS resistance, synthesized tetraploids were screened for LLS in disease hot spot location at Raichur. All the tetraploids, except for ISATGR 155 showed resistance to the disease. Initial screening experiments utilizing one of the sources (ISATGR 265-5) was crossed with cultivated peanut and the progenies were screened for LLS. Screening results showed that some of the progenies had LLS resistance (Sudini H and Mallikarjuna N, unpublished data), thus showing that synthesized tetraploids are good sources of LLS resistance. Mallikarjuna et al. [1] studied the components of LLS resistance such as incubation period, leaf area damage, lesion number and diameter, latent period of infection and infection frequency in some of the synthesized tetraploids using detached leaf techniques. The studies gave a clearer picture of LLS resistance in the synthesized tetraploids.
These studies revealed the potential for multiple disease resistance in synthesized tetraploids coupled with the presence of desirable biochemical traits. Concerted efforts in the utilization of these sources by the peanut community will not only broaden the genetic base but will open up new vistas in peanut breeding.
REFERENCES
- N. Mallikarjuna, S. Senthilvel and D. Hoisington, “Development of Synthetic Groundnuts (Arachishypogaea L.) to Broaden the Genetic Base of Cultivated Groundnut,” Genetic Resources and Crop Evolution, Vol. 58, No. 6, 2011, pp. 889-907 . doi:10.1007/s10722-010-9627-8
- J. C. Sanford, “Ploidy Manipulations,” In: J. N. Moore and J. Janick, Eds., Methods in Fruit Breeding, Purdue University Press, West Lafayette, 1983, pp. 100-123.
- C. A. Ryan, “Protease Inhibitors in Plants: Genes for Improving Defenses against Insects and Pathogens,” Annual Review of Phytopathology, Vol. l28, 1990, pp. 425-449. doi:10.1146/annurev.py.28.090190.002233
- L. P. Metcalf, A. A. Schmitz and J. K. Pelka, “Rapid Preparation of Fatty Acid Esters from Lipids for Gas Chromatographic Analysis,” Analytical Chemistry, Vol. 38, No. 3, 1966, pp. 514-515. doi:10.1021/ac60235a044
- R. W. Mozingo, T. A. Coffelt and J. C. Wynne, “Quality Evaluation of Virginia-Type Peanut Varieties Released from 1944-1985,” Southern Cooperative Series Bulletin No. 335, 1988, pp. 1-28.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, “Protein Measurement with the Folin Phenol Reagent,” The Journal of Biological Chemistry, Vol. 193, No. 1, 1951, pp. 265-275.
- E. R. Prasad, A. Dutta-Gupta and K. Padmasree, “Insecticidal Potential of Bowman-Birk Proteinase Inhibitors from Red Gram (Cajanuscajan) and Black Gram (Vignamungo) against Lepidopteran Insect Pests,” Pesticide Biochemistry and Physiology, Vol. 98, No. 1, 2010, pp. 80- 88. doi:10.1016/j.pestbp.2010.05.003
- B. F. Erlanger, N. Kokowsky and W. Cohen, “The Preparation and Properties of Two New Chromogenic Substrates of Trypsin,” Archives of Biochemistry and Biophysics, Vol. 95, No. 2, 1961, pp. 271-278. doi:10.1016/0003-9861(61)90145-X
- R. Mueller and J. K. P. Weder, “Isolation and Characterization of Two Trypsin-Chymotrypsin Inhibitors from Lentil Seeds (Lens culinaris Medik.),” Journal of Food Biochemistry, Vol. 13, No. 1, 1989, pp. 39-63. doi:10.1111/j.1745-4514.1989.tb00384.x
- M. S. Basu, “Peanut Bud Necrosis Disease: Activities in the Indian National Programme,” In: A. A. M. Buiel, J. E. Parlevliet and J. M. Lenne, Eds., Recent Studies on Peanut Bud Necrosis Disease, International Crops Research Institute for the Semi-Arid Tropics, Patencheru, pp. 61- 63.
- G. Sunkad, P. V. Kenchanagoudar and V. B. Naragund, “Identification of Sources for Field Resistance to Peanut Bud Necrosis Disease in Groundnut,” Karnataka Journal of Agricultural Sciences, Vol. 15, No. 4, 2001, pp. 646- 648.
- P. V. Subbarao, P. Subrahmanyam and P. M. Reddy, “A Modified Nine Point Disease Scale for Assessment of Rust and Late Leafspot of Groundnut,” 2nd International Congress of French Phytopathological Society, 28-30 November 1990, Montpellier, p. 25.
- P. Subramanyam and D. McDonald, “Groundnut Rust Disease: Epidemiology and Control. ICRISAT (International Crops Research Institute for Semi Arid Tropics), 1987. Groundnut Rust Disease,” Proceedings of a Discussion Group Meeting, 24-28 September 1984, ICRISAT Center, Patancheru, India.
- C. T. Young and G. R. Waller, “Rapid Oleic/Lenoleic Micro-Analytical Procedure for Peanuts,” Journal of Agricultural and Food Chemistry, Vol. 20, No. 6, 1972, pp. 1116-1118. doi:10.1021/jf60184a009
- L. C. Mercer, J. C. Wynne and C. T. Young, “Inheritance of Fatty Acid Content in Peanut Oil,” Peanut Science, Vol. 17, No. 1, 1990, pp. 17-21.
- R. M. Kosslak, M. A. Chamberlin, R. G. Palmer and B. A. Bowen, “Programmed Cell Death in the Root Cortex of Soybean Root Necrosis Mutants,” The Plant Journal, Vol. 11, No. 4, 1997, pp. 729-745. doi:10.1046/j.1365-313X.1997.11040729.x
- Anonymous, “The Medium Term Plan, 1994-1998, (Limited Distribution),” International Crops Research Institute for Semi Arid Tropics, Patancheru, 1992.
- A. S. Reddy, L. J. Reddy, N. Mallikarjuna, M. D. Abduahman, Y. V. Reddy, P. J. Bramel and D. V. R. Reddy, “Identification of Resistance to Peanut Bud Necrosis Virus (PBNV) in Wild Arachis Germplasm,” Annals of Applied Biology, Vol. 137, No. 2, 2000, pp. 135-139. doi:10.1111/j.1744-7348.2000.tb00045.x
- S. L. Dwivedi, S. Gurtu and S. N. Nigam, “ALFP Diversity among Selected Foliar Diseases Resistant Groundnut (Arachishypogaea L.) Germplasm,” Indian Journal of Genetic Resource, Vol. 15, No. 1, 2000, pp. 46-50.
- S. Pande and J. Narayan Rao, “Resistance of Wild Arachis Species to Late Leaf Spot and Rust in Greenhouse Trials,” Plant Diseases, Vol. 85, No. 8, 2001, pp. 851- 855. doi:10.1094/PDIS.2001.85.8.851
NOTES
*Corresponding author.

