Food and Nutrition Sciences
Vol. 2 No. 5 (2011) , Article ID: 5758 , 4 pages DOI:10.4236/fns.2011.25062
Proximate Composition and Fatty Acid Profile of Raw and Roasted Salt-Dried Sardines (Sardinella Brasiliensis)
![]()
1Department of Chemistry, State University of Maringá, Colombo, Brazil, 2Federal Technologic University of Parana, Londrina, Brazil.
Email: *nesouza@utfpr.edu.br
Received April 27th, 2011; revised May 5th, 2011; accepted May 12th, 2011.
Keywords: Sardine, Sardinella Brasiliensis, Raw Sardine, Roasted Sardine, Fatty Acids
ABSTRACT
The proximate composition and fatty acid profile of five lots of samples of raw and roasted salt-dried sardines (Sardinella brasiliensis) bought locally in Maringá, Paraná State, Brazil were determined. Significant differences (P < 0.05) were observed between samples of raw and roasted sardines, both in relation to moisture, total lipids, proteins, and ashes. The major fatty acids in raw and roasted sardine samples were docosahexaenoic acid (DHA, 22:6n-3, 35.98%, 12.46%); palmitic acid (16:0, 37.59%; 24.18%), and eicosapentaenoic acid (EPA, 20:5n-3, 6.62%; 2.95%), respectively. The ratios of polyunsaturated to saturated fatty acid (PUFA/SFA) were 1.32 and 0.33, and the n-6/n-3 ratios were 0.07 and 0.13 in raw and roasted sardines, respectively. The results showed that roasting increased the SFA and reduced the PUFA in sardines, which still were rich in PUFA and remained a low-cost and nutritionally healthy food.
1. Introduction
Besides the high biological value of its proteins and its low cost, this species presents significant amounts of polyunsaturated fatty acids (PUFA) of the omega-3 series, which grants it valuable nutritional and functional characteristics [1-3].
Sardine oil is a rich source of long-chain n-3 polyunsaturated fatty acids (PUFA), Very-long chain fatty acids [4] such as eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3) play important roles in the development and functioning of the brain and the retina. They can also be synthesized from linoleic and alphalinoleic acids present in the diet (20:5n-3). Additionally, these fatty acids have been associated with the reduction of the incidence of inflammatory and cardiovascular disorders [5-7], the level of cholesterol in the blood [8], psoriasis, arthritis, and cancer [9]; they may also be involved in human fertility [10].
Studies with animals have shown that diets deficient in n-3 fatty acids result in a decrease in the concentration of DHA in the brain and retina tissues and increases the amount of DPA. These results evidence that a high degree of unsaturation is required in the brain, as in the absence of DHA and its precursors, the most similar LC-PUFA (long chain polyunsaturated fatty acid), DPA, is synthesized [11].
It is estimated that the ratio of omega-6 (n-6) to omega-3 (n-3) fatty acids of the human diet before the industrialization was around 1:1 to 2:1 due to the abundant consumption of vegetables and seafood high in n-3 fatty acids. With the industrialization, there was a gradual increase in this ratio, mainly due to the consumption of refined oils from oleaginous seeds with a high content of linoleic acid (LA) and the reduction of ingestion of fruit and vegetables. This resulted in diets with inappropriate amounts of n-3 fatty acids. In the last decades, the average ingestion of n-6/n-3 fatty acids in several countries has been round from 10:1 to 20:1, with reports of values up to 50:1 [11]. Some clinical studies have pointed out the need of reducing the n-6/n-3 ratio in the last decade. Among the benefits of a low n-6/n-3 ratio are the reduction of 70% in the mortality ratio in cardiovascular patients, the reduction of inflammation caused by rheumatoid arthritis, and the reduction of the symptoms of asthma [11].
Much of the fish consumed, including sardine, is canned and may contain soybean oil or tomato sauce as a filling, which may alter the fatty acid profile of fish due to the interaction among the lipids, fish, and the respective filling [3]. Salt-dried sardine is largely consumed as well. Salt-drying is one of the oldest food preservation methods. Fish meat has about 0.08% - 1% salt, which is insufficient for its preservation. This content is raised with the application of sodium chloride to preserve meant and prevent its decay [12].
The objective of this study was to evaluate the proximate composition, and the fatty acid profile of raw and roasted salt-dried sardines.
2. Materials and Methods
Three samples of five different lots of Sardines were purchased locally in Maringá, Paraná State.
Before analysis and roasted, the sardines were desalted with five washes in running water, water immersion by 24 hours and left drain water by 2 hours.
The moisture, raw protein, and ash contents were determined according to AOAC [13]. Total lipids were extracted by the Bligh and Dyer [14] method and transesterified by the Hartman and Lago method [15]. The methyl esters were separated in a gas chromatograph (Shimadzu 14-A, Japan) equipped with a flame ionization detector and fused silica capillary column CP Sil-88 (50 m, 0.25 mm i.d.). The gas flow rates were 1.2 mL/min for the carrier gas (H2); 30 mL/min for the auxiliary gas (N2), and 30 and 300 mL/min for H2 and the flame synthetic air, respectively. The sample splitting rate was 1:100. The injector and detector temperatures were 220 and 230˚C, respectively. The column temperature was programmed for 2˚C/min from 180 to 240˚C. The injection volume was 2 μL. The peak areas were determined using an integrator-processor CG-300 (Instrumentos Científicos CG). The fatty acids were identified by comparison of retention times with methyl esters of Sigma (USA) standards.
3. Results and Discussion
There was a significant difference (P < 0.05) between the samples for the different raw and roasted sardine lots, both in proximate composition (Table 1) and fatty acid profile (Table 2).
According to the Brazilian Chart of Food Composition (Tabela Brasileira de Composição de Alimen-tos, TACO) developed by the Núcleo de Estudos e Pesquisas em Alimentação [16] raw sardine has 76.6% moisture, 21.1% protein, and 2.7% lipids.
Both raw and roasted sardines have significant values of ashes (mineral salts) and protein. Tarley et al. [3] re-
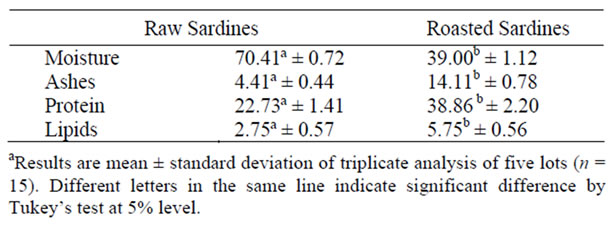
Table 1. Proximate composition (%) of raw and roasted sardine samplesa.
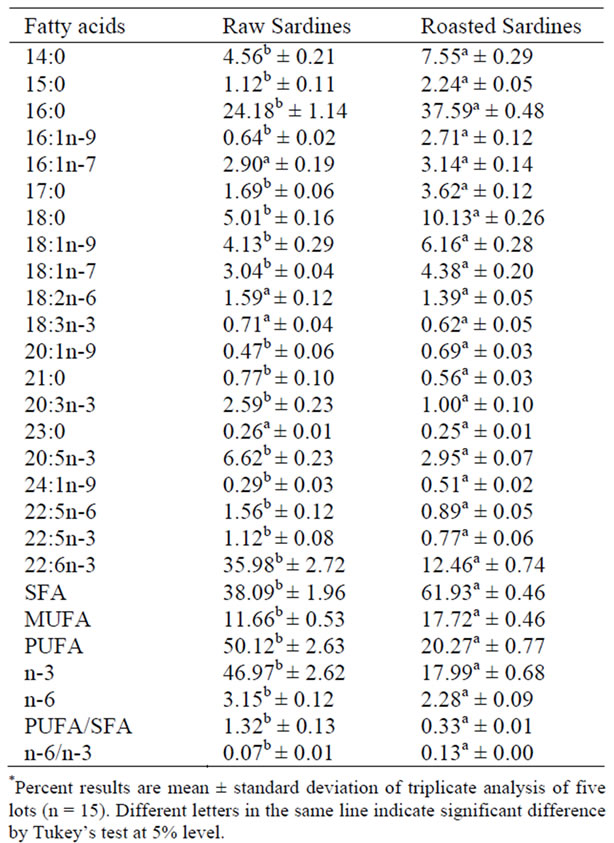
Table 2. Fatty acid composition (%) of raw and roasted salted sardines*.
ported that canned sardines in oil or tomato sauce have 19.8 to 24.4% protein, a value that is close to the protein level of raw sardines.
The low moisture found in roasted sardines, when compared to raw sardine, is due to dehydration by the exposure to heat. Pereira et al. [17] found 71.02% moisture in defrozen fresh sardine.
Tarley et al. [3] found lipid values for canned sardines from 5.30% to 16.8%, which were higher than the values found in this study of salt-dried sardines. The ash contents reported by the same authors in six brands of canned sardines were between 2.65 and 3.11%, which were lower than the values of 4.45 and 14.11% presently found in raw and roasted sardines. Szenttamásy [12] found ash contents ranging from 0.08 to 1% for fresh fish meat. The greater ash content can be attributed to the incomplete desalting of salted raw sardine and to dehydration by high temperature and the consequent concentration of salt in roasted sardine.
The present results show that the major fatty acids in raw and roasted salt dried sardines were docosahexaenoic acid (DHA), 22:6n-3 (35.98%), and palmitic acid, 16:0 (37.59%), respectively. Palmitic acid (22.4%) and DHA (22.5%) were also majoritaty in sardines from the shore of Santos, São Paulo, State, in a study by Visentainer et al. [18]. Visentainer et al. [19] analyzed two parts of sardines, the steak and the eye, finding 13.77% DHA in steaks and 14.81% in eyes.
Roasted sardine samples had lower contents of DHA, 12.46%., with a difference (p < 0.05) in relation to raw sardines. This difference in fatty acid composition between the samples can be attributed to lipid oxidation [12]. Associated with heat, salt dehydrates meat, provoking a reduction in moisture and the activity of water. However, in these conditions, meat can deteriorate by oxidation, resulting in lipid rancidity [20].
Differences (p < 0.05) were observed for EPA. (6.62 and 2.95%) between raw and roasted sardines. The DHA contents of both raw and roasted sardines were greater than the EPA contents, according to Visentainer et al. [18], who found greater DHA contents.
The SFA with the highest content was 16:0 in all the analyzed samples, as was also reported by Tarley et al. [3] in canned sardine samples. This finding is considered typical because palmitic acid is a key metabolite in fish [4].
Myristic acid (14:0) presented a content of 7.55% in roasted sardine and stearic acid (14:0) of 5.01% in raw sardine, values similar to those reported by Visentainer et al. [18], 7.5% myristic acid and 5.2% stearic acid.
The PUFA/SFA ratios found in this work for raw and roasted sardines were 1.32 and 0.33, respectively, with a significant difference (p < 0.05). According to the Health Department of England [21], this ratio is beneficial for human health when it is higher than 0.45. In this way, roasting contributed to the saturation of unsaturated fatty acids, thus decreasing the PUFA/SFA ratio.
The n-6/n-3 ratios found were 0.07 and 0.13 in roasted and raw sardines, respectively. It is important to point out that the intake of linoleic and alpha-linoleic acids must be balanced. The Health Department of England [21] suggests a maximum intake ratio of omega-6 to omega-3 of 4. Simopoulos (1999) suggests that the ratio must be between 5 and 10. In France and Switzerland, the recommended ratio is 5:1, converging to values between 4-5:1 [11].
Based on the above findings, it can be seen that regardless of the processing method, sardines are an excellent source of essential fatty acids.
4. Conclusions
The Brazilian Sardine (Sardinella brasiliensis), the main fishing resource in Brazil in terms of volume, has valuable nutritional functional characteristics due to its high polyunsaturated fatty acid content, especially of EPA and DHA, with higher values of DHA in raw sardine. Additionally, the n-6/n-3 ratios are within the recommended values by some researchers, thus constituting a healthy food and an excellent and inexpensive calorie source.
5. Acknowledgements
The authors are grateful to CAPES, CNPq for the financial support.
REFERENCES
- M. Vasconcellos, “An Analysis of Harvest Strategies and Information Needs in the Purse Seine Fishery for the Brazilian Sardine,” Fisheries Research, Vol. 59, No. 3, 2003, pp. 363-378. doi:10.1016/S0165-7836(02)00026-7
- Acqua Forum 2007. http://www.fundacentro.sc.gov.br/acquaforum
- C. R. T. Tarley, J. V. Visentainer, M. Matsushita and N. E. de Souza, “Proximate Composition, Cholesterol and Fatty Acids Profile of Canned Sardines (Sardinella Brasiliensis) in Soybean Oil and Tomato Sauce,” Food Chemistry, Vol. 88, No. 1, 2004, pp. 1-6. doi:10.1016/j.foodchem.2004.01.016
- A. D. Andrade, A. F. Rubira, M. Matsushita and N. E. Souza, “N-3 Fatty Acids in Feshwater Fish from South Brazil,” Journal of American Oil Chemical Society, Vol. 72, No. 10, 1995, pp. 1207-1210. doi:10.1007/BF02540990
- L. D. Peterson, N. N. Jeffery, F. Thies, P. Sanderson, E. A. Newsholme and P. C. Calder, “Eicosapentaenoic and Docosahexaenoic Acids Alter Rat Spleen Leukocyte Fatty Acid Composition and Prostaglandin and Production But Have Different Effects on Lymphocyte Functions and Cell Mediated Immunity,” Lipids, Vol. 33, No. 2, 1998, pp. 171-180. doi:10.1007/s11745-998-0193-y
- B. J. Hunter and D. C. K. Roberts, “Potencial Impact of the Fat Composition of Farmed Fish on Human Health,” Nutrition Research, Vol. 20, No. 7, 2000, pp. 1047-1058. doi:10.1016/S0271-5317(00)00181-0
- C. von Schacky, “N-3 Fatty Acids and the Prevention of Coronary Atherosclerosis,” American Journal of Clinical Nutrition, Vol. 71, No. 1, 2000, pp. 224S-227S.
- R. Uauy and A. Valenzuela, “Marine Oils: The Health Benefits of n-3 Fatty Acids,” Nutrition, Vol. 16, No. 7, 2000, pp. 680-684.
- A. P. Simopoulos, “Essentiality and Recommended Dietary Intakes for Omega-6 and Omega-3 Fatty Acids,” Annals of Nutrition and Metabolism, Vol. 43, No. 2, 1999, pp. 127-130. doi:10.1159/000012777
- J. A. Conquer, J. B. Martin, I. Tummon, L. Watson and F. Tekpetey, “Effetct of DHA Supplementation on DHA Status and Sperm Motility in Asthenozoospermic Males,” Lipids, Vol. 35, No. 2, 2000, pp. 149-154. doi:10.1007/BF02664764
- C. A. Martin, V. V. Almeida, M. R. Ruiz, J. E. L. Visentainer, M. Matshushita, N. E. de Souza and J. V. Visentainer, “Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Importance and Occurrence in Foods,” Revista de Nutrição, Vol. 19, No. 6, 2006, pp. 761-770.
- E. R. Szenttamásy, S. M. V. B. Barbosa, M. Oetterer and I. A. M. Moreno, “Tecnologia Do Pescado De Água Doce: Aproveitamento Do Pacu (Piaractus Mesopotamicus),” Scientia Agricola, Vol. 50, No. 2, 1993, pp. 303-310.
- P. A. Cunniff, “Official Methods of Analysis of AOAC International,” 16th Edition, Association of Official Analysis Chemists, Arlington, 1998.
- E. G. Bligh and W. J. Dyer, “A Rapid Method of Total Lipid Extraction and Purification,” Canadian Journal of Biochemistry, Vol. 37, 1959, pp. 911-917. doi:10.1139/o59-099
- L. Hartman and R. C. A. Lago, “Rapid Preparation of Fatty Acid Methyl from Lipids,” Laboratory Practice, Vol. 22, 1973, pp. 475-481.
- NEPA-UNICAMP, “TACO (Brazilian Food Composition Table),” Coordinated by the Center for Studies and Re-Searches in Food (NEPA) of UNICAMP and Established by the Ministry of Health (MS), Campinas, 2009.
- A. A. F. Pereira and A. Tenuta-Filho, “Avaliação de condições de consumo da sardinha Sardinella brasiliensis,” Ciência e Tecnologia de Alimentos, Vol. 25, No. 4, 2005, pp. 720-725. doi:10.1590/S0101-20612005000400015
- J. V. Visentainer, M. D. Noffs, P. O. Carvalho, V. V. Almeida, C. C. Oliveira and N. E. Souza, “Lipid Content and Fatty Acid Composition of 15 Marine Fish Species from the Southeast Coast of Brazil,” Journal of the American Oil Chemists’ Society, Vol. 84, No. 6, 2007, pp. 543-547.
- J. V. Visentainer, P. O. Carvalho, M. Ikegaki and Y. K. Park, “Concentração de ácido eicosapentaenóico (EPA) e ácido docosahexaenóico (DHA) em peixes marinhos da costa brasileira,” Ciência e Tecnologia de Alimentos, Vol. 20, No. 1, 2000, pp. 90-93. doi:10.1590/S0101-20612000000100017
- A. G. Silva Sobrinho, N. M. B. L. Zeola, H. B. A. Souza and T. M. A. Lima, “Qualidade Da Carne Ovina Submetida Ao Processo De Salga,” Ciência e Tecnologia de Alimentos, Vol. 24, No. 3, 2004, pp. 369-372. doi:10.1590/S0101-20612004000300011
- HMSO, Report on Health and Social Subjects, Department of Health, Nutritional Aspects of Cardiovascular Disease, London, Vol. 46, 2001, pp. 37-46.

