American Journal of Plant Sciences
Vol.4 No.12B(2013), Article ID:41446,7 pages DOI:10.4236/ajps.2013.412A2009
Isolation, Identification and Germplasm Preservation of Different Native Spirulina Species from Western Mexico
![]()
1Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, A. C. Av. Normalistas #800, Colinas de la Normal, Guadalajara, Jalisco, Mexico; 2Biotecnología Mexicana de Microalgas S. A. de C. V. Sta. Maria 136, Conlonia Tepeyac, Zapopan, Jalisco, Mexico.
Email: *khandual@yahoo.com
Copyright © 2013 Rout Nutan Prasad et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received November 19th, 2013; revised December 19th, 2013; accepted December 28th, 2013
Keywords: Germplasm; Cryopreservation; Spirulina; Long Term Preservation
ABSTRACT
Spirulina is an edible algae and has a wide range of pharmaceutical applications in addition to its nutritional value. Isolation and identification of several Spirulina species were conducted in the western part of Mexico especially in the state of Jalisco. The purification strategy consisted of five optimized processing steps: 1) washing and centrifugation, 2) chemical treatment, 3) micromanipulation, 4) serial dilution, and 5) plating. Four species were isolated from different locations and two out of these four species were identified taxonomically up to the species level: Spirulina subsalsa and S. major. For short term conservation (30 days), the strains were maintained in liquid and solid agar medium at 10˚C and 4˚C. For medium term (few months), they were preserved in solid medium under a dried condition as agar flakes and for long term, cryopreservation was employed by using 5% and 10% DMSO, glycerol and methanol as osmoprotectants in liquid nitrogen. For short term preservation nearly 90% liquid and 100% agar recovered strains were viable after one month at both temperatures. In the case of the agar flakes, cells were viable after three months of conservation at room temperature. Cryopreservation did not give any suitable results after three months of conservation. Variable and two-step improved cryopreservation processes are now in progress for conservation.
1. Introduction
Spirulina is a microalgae that has a unique set of biological characteristics which are very useful for a broad range of applications. It has been certified by the FDA (USA Food and Drug Administration) as GRAS (Generally Recognized as Safe). It can be used as a nutritive supplement or pharmaceutical additive with no risks to health. Spirulina is useful for human nutrition, because of the high quality and quantity (60% - 70% of its dry weight) [1] of protein and amino acids [2,3]. Spirulina contains essential amino acids, especially leucine (10.9% of total amino acids), valine (7.5%), and isoleucine (6.8%) [4]. Spirulina has a relatively high provitamin A concentration [5] and harmless b-carotene [6]. Spirulina is a very rich source of vitamin B12, which is important for people who need supplements to treat pernicious anemia [3,5,7].
History of use of the microalgae Spirulina is not accurately known. Ancient Mexicans were the first people in the world to use Spirulina. In 1521, S. maxima used to be harvested from Lake Texcoco, dried, and sold for human consumption in Tenochtitlan (today’s Mexico City) [1]. In 1940, the French phycologist P. Dangeard reported about the consumption of a cake called dihé, by the Kanembu tribe near the African Lake Chad [1,8].
Spirulina plays an important role in the booming new markets for natural ingredients. A 2002 report from the National Centre for Natural Products Research (NCNPR) estimated that the US market is $4 to $5 billion in botanical dietary supplements, since these supplements (herbal remedies) are consumed by millions of people
(http://www.tradekey.com/selloffer_view/id/224168.htm). While there are many companies around the world that produce Spirulina products in high volumes, prior to 2001, there were only a few companies in the USA that produced more than 1450 tons per year [9,10]. On the other hand, advances in conservation technology have led to methods that allow preservation of biological materials like microorganisms, tissues, primary cells, established cell lines, small multicellular organisms, complex cellular structures such as embryos, as well as nucleic acids and proteins. This has a great importance for maintainance of cell or organ culture and is a very laborious and time consuming process [11]. Again, fixation of a biological material at a particular stage of life is essential to study expression of genes, enzymes, proteins, etc. In this manner, in vitro preservation facilitates fixing biological materials and avoiding chances of contamination that occurs normally due to frequent handling of biological material in subcultures. During the freezing process, complex phenomena occur which are not fully understood. Microbial cells, particularly bacteria and yeast can be easily preserved with cryoprotectants at −80˚C and can be recovered in agar plates. There are some reports about cryopreservation of Cyanobacteria, but there is still not much success. Freeze-drying bio-storage methods for microalgae have not been found to be a successful procedure in long term preservation and the viability has been less than 1% [12-14]. There are few established techniques reported for cyanobacterial preservation [15-17]. Most of them are based on batch cultures, agar slants, liquid nitrogen and storage at low temperatures (−80˚C) using protectants such as glycerol, sucrose, etc., to maintain pure cultures for varying periods of time. All these processes require laborious regular transfer to fresh liquid media that enhances high risks of contamination.
There is no doubt that Spirulina is a Wonder Edible Algae. Mexico was the first country in the world to use Spirulina [1]. Nowadays, other countries have become more technologically advanced than Mexico in the production, use, and commercialization of Spirulina. Research data indicates that Mexico has many species of Spirulina [18-20], and only recently we have isolated and identified the species S. subsalsa for the first time (Rout NP unpublished data). However, at present there are no reports for the isolation of other species nor is there a center for the culture and collection of local Spirulina. In addition to this, all of the species have different physiological responses, so there is a need to standardize the process of in-vitro conservation for each species. The objective of this work was the collection of microalgae to get useful local Spirulina strains, identification, optimization of the short, medium and long term preservation methods to establish a culture collection (Algal Bank) of the algae genus Spirulina from the western part of Mexico.
2. Materials and Methods
2.1. Collection of Algal Samples
The sites for water sampling and isolation of algae were selected according to the published database in the book Algae of Western Mexico [18]. The regions selected for this study were several locations of Sayula, Zacoalco de Torres, Rio Caliente (Hot water spring) in the Primavera forest, the Zapotlán Lake and a water treatment plant in the municipality of Atoyac (Figure 1 and Table 1), all places located in the State of Jalisco (Figure 1). The water samples were taken from a surface depth of 10 and to 50 cm. using plankton net with mesh sizes between 10 - 100 microns during the months of September and November at various points. Then concentrated samples were washed with distilled water and kept in containers of fresh water. During this process, sampling data was taken such as pH and temperature at each locality.
2.2. Isolation of Alga
The isolation and purification strategy consisted of five optimized processing steps 1) Density centrifugation: First, the sample was centrifuged at 800 - 2000 rpm for different phase separation to eliminate solid residues and contamination, then it was cultured in different nutrient media, 2) Chemical treatment: For the purpose of elimination of eukaryotic algae in the mixed algal culture cycloheximide was added at different concentrations (25 - 100 mg/l) for a week and resulted cultures were recovered, 3) Serial dilutions: The sample was centrifuged at 1000 rpm and washed repeatedly 2 - 5 times to eliminate
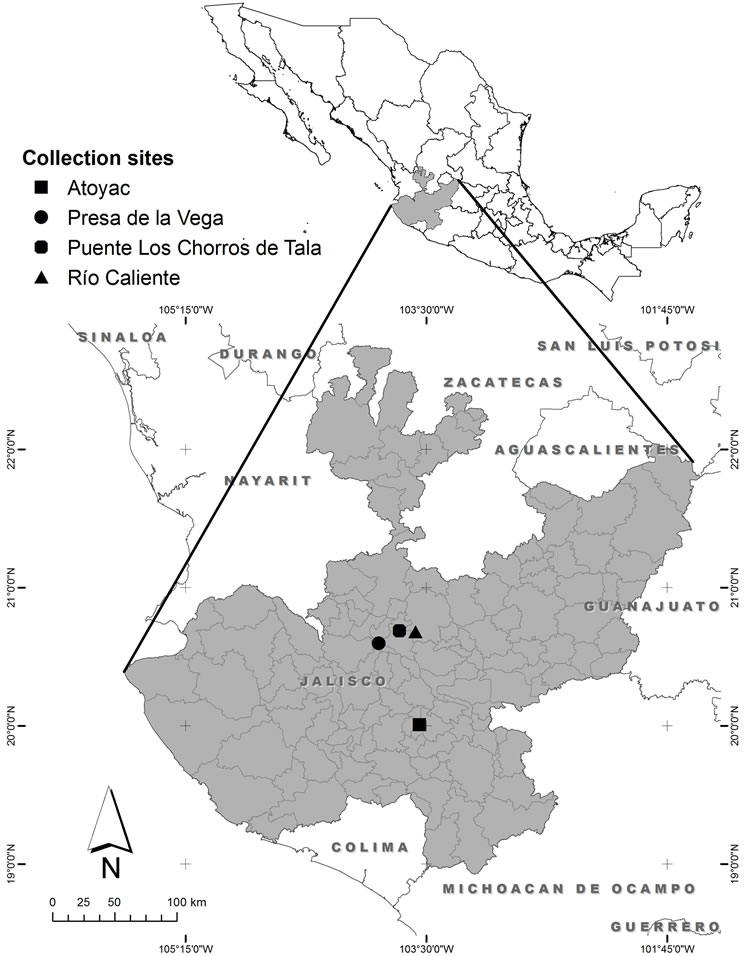
Figure 1. Collection sites of algae in the state of Jalisco, Mexico.
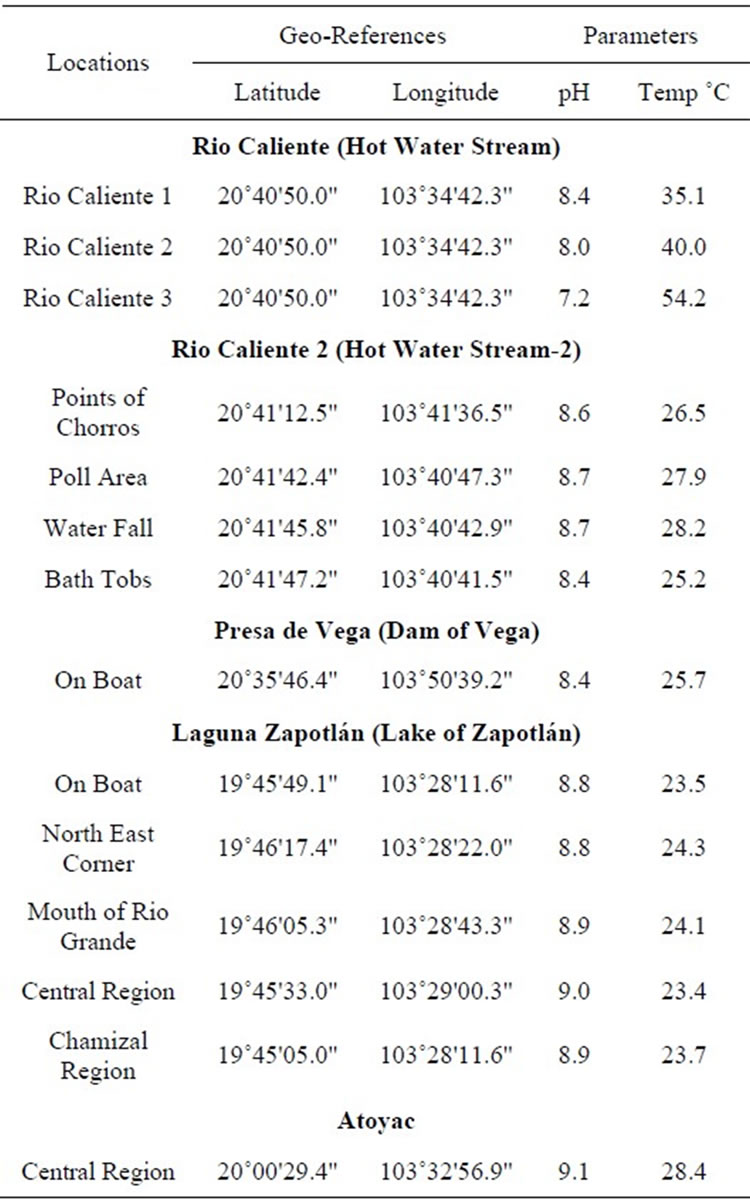
Table 1. Localities, geographical references, pH and temperature of the collection sites.
the small unicellular algae if any and series of dilution were made 10 times to 10 tubes. Then the sample was examined under an Olympus BH-2 microscope coupled to a Leica DFC450 C digital camera to check for the presence of Spirulina, 4) Micromanipulation: Algal filaments were picked by the use of a glass micropipette into one drop of medium on a microscope slide and was examined under microscope from time to time to select the pure culture and discard contaminants and 5) Streak plating: The serial-diluted and micro-manipulated Spirulina samples were spread with a needle on solid plates (with 1.5% agar) and cultured under normal laboratory conditions for 3 - 4 weeks in order to isolate pure colonies.
2.3. Morphological Identification of Spirulina
Pure colonies were observed under a microscope and photographed with a coupled Leica DFC camera 450c at 20X to 100x for taxonomic identification. The Spirulina species were identified taxonomically according to Ciferri (1983) [1] and Tomasseli (1997) [21]. The typical morphology of Spirulina, the degree of spiralization and the arrangement of the spirals were the main taxonomic criteria for Spirulina classification and species differentiation [1,22].
2.4. Short Term Preservation of Spirulina
The two new identified species of Spirulina were grown under laboratory conditions for preservation. UTEX Spirulina medium was used for liquid medium and 2% bacteriological agar with a gelling temperature of 40˚C was supplemented for preparation of solid plates. Diluted samples of Spirulina cells at exponential growth phase were spread on these plates and kept for one week under light (fluorescent light 2000 lux) for proper growth. Direct liquid culture and one week solid streak plates were kept at 10˚C and 4˚C for one to two months and then viability was verified.
2.5. Medium Term Preservation
For preservation of Spirulina for a few months (up to a year) the protocol of Mayashree and Bhattacharjee (2010) (24) was followed. UTEX solid medium was prepared with 2.5% bacteriological agar at a gelling temperature of 40˚C. Spirulina cells at exponential growth phase (10 - 15 days old) were concentrated by centrifugation at 2000 rpm for 5 minutes (100 ml of culture was concentrated to 10 ml). Following this, 10 ml of Spirulina culture were poured into 40 ml of warm nutrient agar solutions (final volume 50 ml). This mixture was spread on flat bottom glass petri dishes.
2.6. Long Term Preservation (Cryopreservation)
Spirulina cultures at exponential growth phase were concentrated by centrifugation at 3000 rpm for 3 min.
The pellet was re-suspended in fresh UTEX culture medium with two different concentrations (5% and 10%) of cryoprotectants like methanol, glycerol and DMSO. Then transferred to sterile vials and kept at 4˚C overnight. Then slowly transferred to −20˚C, then to −80˚C and finally kept in liquid nitrogen until use. Some of the cryoviales were recovered in liquid medium for algal cell survival tests. Cryopreserved Spirulina strains were taken out from the liquid nitrogen for recovery and immediately kept in water bath at 40˚C until the material thawed properly. The liquefied samples were transferred to sterile liquid culture and solid plate culture in a laminar hood [13].
3. Results and Discussion
3.1. Collection of Algal Samples and
Collection points were selected from the book Algas del Occidente de México [18]. The collection points were located in Sayula, Rio Caliente in the Primavera forest and the Zapotlán Lake in the state of Jalisco as shown in (Table 1). There were different species of microalgae associated with Spirulina. All collected samples were contaminated with various solid abiotic wastes along with filamentous and unicellular algal populations. As some hot springs [23] and lakes [24] are source of Spirulina, we found four species of Spirulina in all collections.
3.2. Isolation of Alga
3.2.1. Density Centrifugation
The collected samples contained lots of suspended abiotic contaminants, bacteria and a large amount of diverse microorganisms. Repeated low-speed centrifugation (500 - 1000 rpm) removed up to 90% of the suspended materials and unicellular microorganisms. Spirulina was found at the upper phase in the floating mass along with other 12 blue-green and green algae species. Those species were used for the mixed culture in liquid medium (Figure 2).
After culturing the mixed algal sample in different culture media [25], it was found that the most suitable medium was the UTEX Spirluina medium for its high pH value (9.2) to reduce other algal population in the mix culture. Then after one week it was observed that there were around 3 more species growing with Spirulina in UTEX medium. Density gradient is one of the rapid and sensitive techniques for algal separation [26,27] and most of the algal contaminations were eliminated by this method.
3.2.2. Chemical Treatment
Cycloheximide is a chemical which inhibits eukaryotic protein synthesis and eliminates eukaryotes algae from culture in very minor concentration [28]. Cyloheximide was used at different concentrations (25 - 100 ug/ml) to eliminate any eukaryotic algae. It was found effective for eliminating the brown algae at 50 ug/ml (Figure 3), but not able to eliminate one of the unicellular algae contaminations. So it was suspected as cyanobacteria.
3.2.3. Serial Dilution
A process of serial dilutions was effective in our experiments [29]. The percentage of unicellular algae decreased 95% but could not be totally eliminated.
3.2.4. Micromanipulation
After micromanipulation under the microscope the filamentous algae was always found associated with a small unicellular microalgae.
3.2.5. Streak Plating
Solid plating was tried several times repeatedly with the dilution up to 10−5 and it was found suitable for separation of individual colonies. Finally, single colonies from the plate were selected and used for monocultures of the individual Spirulina species (Figure 4).
3.3. Identification of Spirulina
The first species of Spirulina was identified according to Ciferri (1983) [1] and Tomasseli (1997) [21]. After observation under the microscope at different magnifications and according to the taxonomic guide, one of the species was identified as Spirulina subsalsa (Figure 5) [30]. The guide for identification was trichomes width, cell length, pointed calyptras, type of coil and helixshape [30]. The second algae identified to the species level was Spirulina major (Figure 6). It was identified according to the guideline published in the references of Gomont (1892) [31], Anagnostidis & Golubic (1966) [32], Cohen-Bazire Guglielmi & (1982) [33], Rethmeier (1995) [34] and Komárek (1992) [35].
3.4. Short Term Preservation of Spirulina
Short term preservation is for up to 2 months for regular laboratory maintenance. The liquid and solid (2% bacteriological agar plates) media were used for short term preservation at 10˚C and 4˚C. All the cultures were recovered more than 50% after 2 months. The recovery time was longer than a regular culture but effective (Figure 7).
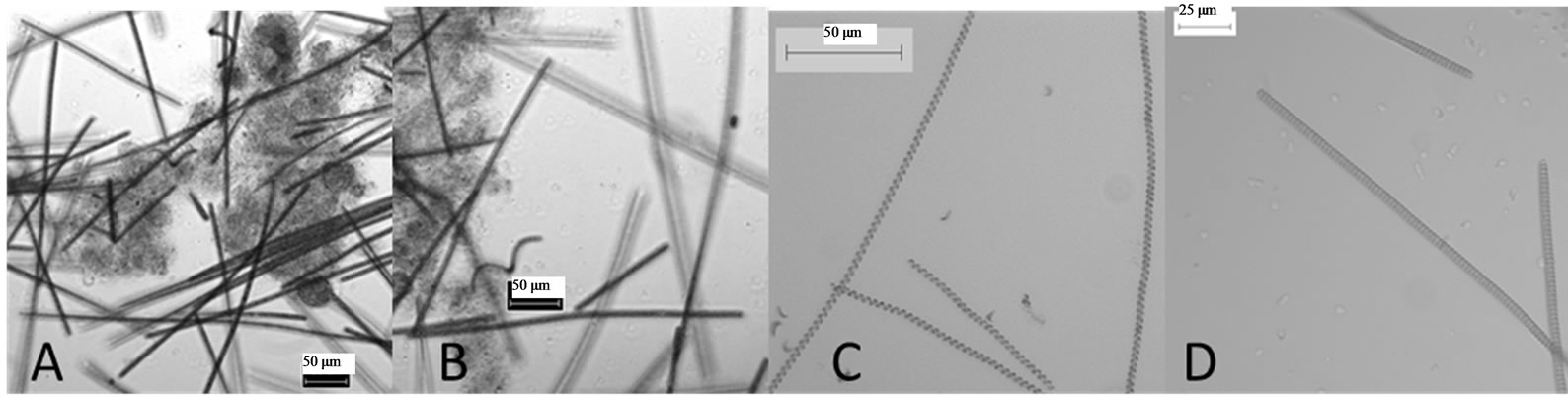
Figure 2. Mixed partial purified cultures. (A) Around 12 species. (B) Around 6 - 8 and (C) Around 3 species. All the samples were observed under 20× magnifications.
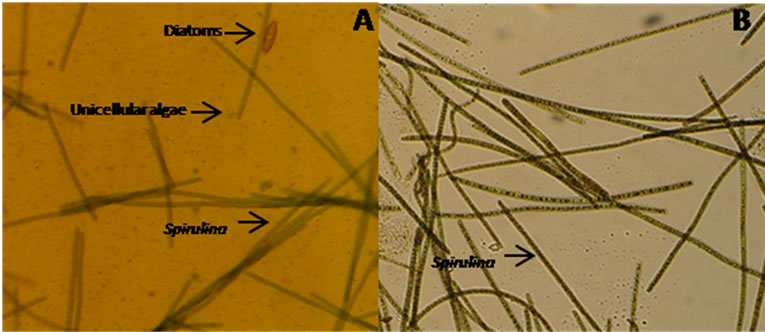
Figure 3. Cycloheximide treatment for the cleaning of Spirulina cultures. All the samples were observed under 20× magnifications.
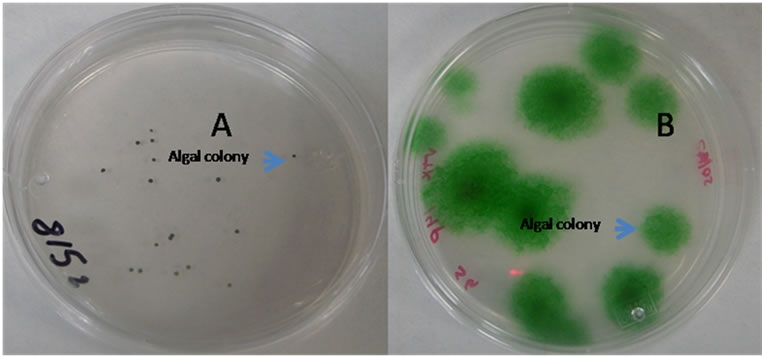
Figure 4. Formation algal colony in agar plates. (A) dilution at 10−1, (B) dilution at 10−5.
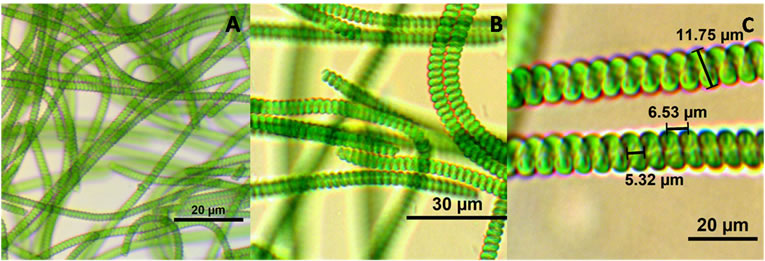
Figure 5. Spirulina subsalsa (A) 40× (25 um scale), (B) 100×.
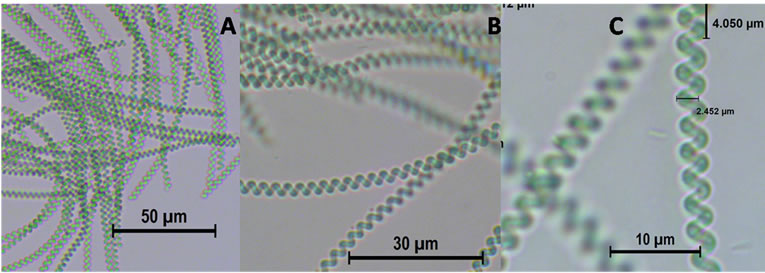
Figure 6. Spirulina major: Photograph at different scale.
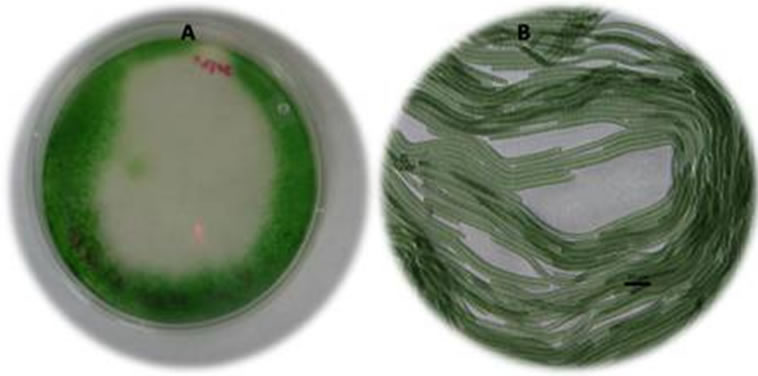
Figure 7. Spirulina subsalsa in agar medium, (A) whole agar plate, (B) Photograph at 20×.
3.5. Medium Term Preservation of Spirulina
In this method the strains were prepared mixed with agar medium and dried to form agar flakes. This method serves very well for Spirulina strain preservation. As the method includes drying the algal material along with agar agar, it can be preserved at room temperature for up to six months and the revival percentage was 70% - 80% and can be kept at 4˚C for one to two years [36]. In our experiments we found that, after three months in agar flakes both of the strains were well recovered. Nearly 80% of viability was found on our study (Figure 8).
3.6. Long Term Preservation (Cryopreservation) of Spirulina
DMSO is well known for preserving cyanobacteria [37- 42]. DMSO has been used as a cryoprotective agent because it reduces crystal formation during freezing and avoids cell breakage. The results of the present study are consistent with those of Davis et al. (1978) [43] and Madigan et al. (2003) [44] (Figures 9(a)-(c)). The two Spirulina species treated in different ways for cryopreservation using methanol, glycerol and DMSO as osmoprotectants. It was found that after thawing the sample from liquid nitrogen only in 5% DMSO treated samples showed low survival (less than 1%) after 3 months of preservation, it partially agrees with the findings of Rippka et al. (1981) [28]. All the treatments with methanol and glycerol did not show any survival of algae (Figure 10). Long term preservation and two steps cryopreservation using methanol, glycerol and DMSO is in progress.
4. Conclusion
The algal strains are normally found in contaminated water bodies and salty lakes. They are generally contaminated with many diverse microorganisms, rotifers and toxic cyanobacterias along with suspended abiotic particles. So here we used simple strategies to establish monocultures of two local Spirulina species: S. subsalsa and S.
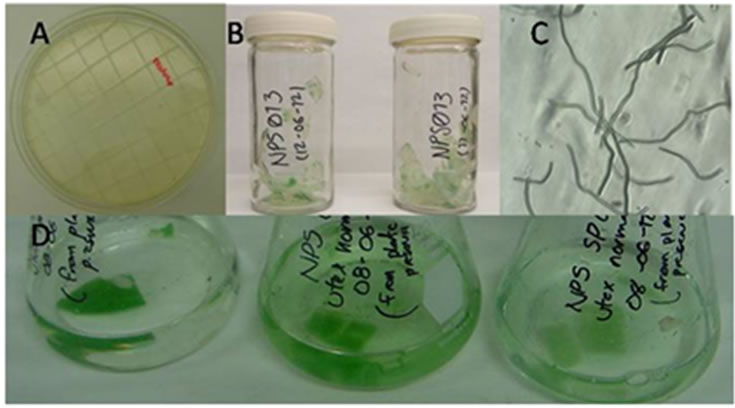
Figure 8. Cryopreservation of Spirulina, (A) Preparation of agar slice, (B) Dried agar flakes with algae, (C) Microscopic observation of embedded Spirulina. (D) Reactivation in UTEX medium.
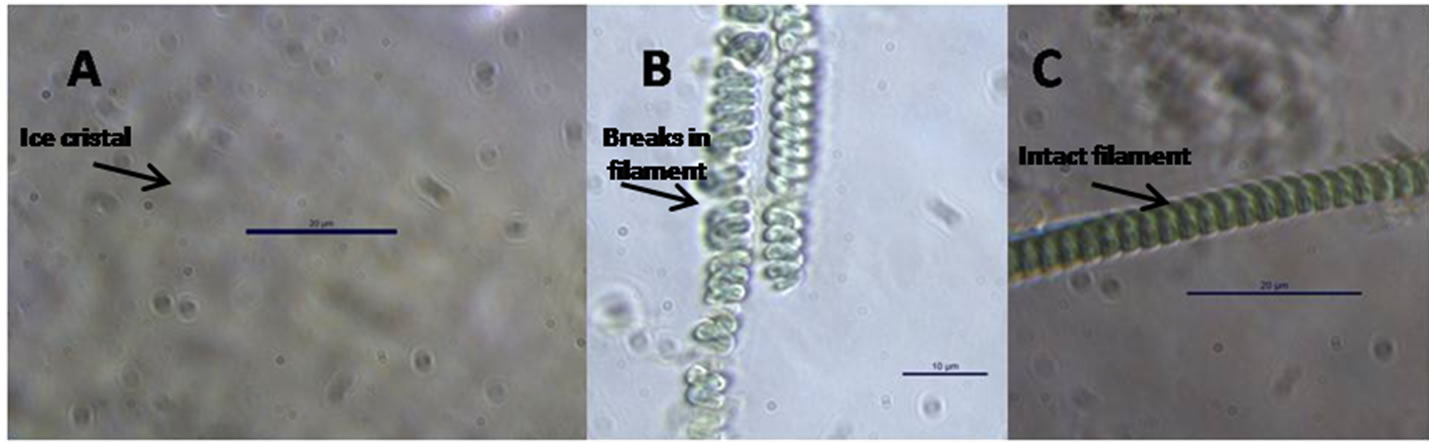
Figure 9. Effect of cryopreservation. (A) Formation of crystal, (B) Broken cell of S. subsalsa, (C) 5% DMSO treatment.

Figure 10. Long-term cryopreservation. Only cells conserved in 5% DMSO and in controls grew at different rates ((A) & (B)), (C) and (D) are the glycerol and methanol treatments.
major which seem to be good local species from Mexico for further utilization and commercialization. Again, to preserve these species in short and long term basis, we standardized the methodology as cyanobacterial germplasm conservation is difficult and recovery percentage is very low. Here we report good recovery by using the dry agar flakes preservation method. The cryopreservation method is not concluded and is currently in progress. The isolation and conservation methods standardized in this work will be of great help for the isolation and germplasm preservation of Spirulina.
5. Acknowledgements
We deeply acknowledge Biotecnologia Mexicana de Microalgas, S. A. and Consejo Nacional de Ciencia y Tecnologia (CONACYT), Mexico for financial support.
REFERENCES
- O. Ciferri, “Spirulina, the Edible Micro-Organism,” Microbiological Reviews, Vol. 47, 1983, pp. 551-578.
- J. C. Dillon and P. A. Phan, “Spirulina as a Source of Proteins in Human Nutrition,” In: F. Doumengue, H. DurandChastel and A. Toulemont, Eds., Spirulinealgue de vie. MuséeOcéanographique. Bulletin de l’InstitutOcéanographique Monaco, Numérospécial, Vol. 12, 1993, pp. 103- 107.
- A. Richmond, “Mass Culture of Cyanobacteria,” In: N. Mann and N. Carr, Eds., Photosynthetic Prokaryotes, 2nd Edition, Plenum Press, New York and London, 1992, pp. 181-210. http://dx.doi.org/10.1007/978-1-4757-1332-9_6
- Z. Cohen, “The Chemicals of Spirulina,” In: A. Vonshak, Ed., Spirulina platensis (Arthrospira) Physiology, CellBiology and Biotechnology, Taylor and Francis, London, 1997, pp. 175-204.
- A. Belay, “Mass Culture of Spirulina Outdoors: The Earthrise Farms Experience,” In: A. Vonshak, Ed., Spirulina platensis (Arthrospira), Physiology, Cell Biology and Biotechnology, Taylor & Francis, London, 1997, pp. 131- 158.
- R. Henrikson, “Spirulina: Food of the Future,” 2nd Edition, Barcelona Urano S.A., 1994, p. 222.
- E. W. Becker, “Nutritional Properties of Microalgal Potentials and Constraints,” In: A. Richmond, Ed., Handbook of Microalgal Mass Culture, CRC Press, Inc., Boca Ratón, 1984, pp. 339-408.
- J. Leonard, “The 1964-65 Belgian Trans-Saharan Expedition,” Nature, Vol. 209, 1966, pp. 126-128. http://dx.doi.org/10.1038/209126a0
- A. Vonshak, “Appendices,” In: A. Vonshak, Ed., Spirulina platensis (Arthrospira): Physiology, Cell Biology and Biotechnology. Taylor and Francis, London, 1997, pp. 213- 226.
- M. Sánchez, J. Bernal-Castillo, C. Rozo and I. Rodríguez, “Spirulina (Arthrospira): An Edible Microorganism. A Review. Revista Universitas Scientiarum,” 2003.
- M. Lorenz, T. Friedl and J. G. Day, “Perpetual Maintenance of Actively Metabolizing Microalgal Cultures,” In: R. A. Andersen, Ed., Algal Culturing Techniques, Academic Press, New York, 2005, pp. 145-156.
- M. S. Mc. Grath, P. Daggett and S. Dilworth, “Freeze-Drying of Algae: Chlorophyta and Chrysophyta,” Journal of Phycology, Vol. 14, 1978, pp. 521-525. http://dx.doi.org/10.1111/j.1529-8817.1978.tb02480.x
- J. G. Day, I. M. Priestley and G. A. Codd, “Storage, Recovery and Photosynthetic Activities of Immobilized Algae,” In: C. Webb and F. Mavituna, Eds., Plant and Animal Cells, Process Possibilities, Ellis Horwood, Chichester, 1987, pp. 257-261.
- O. Holm-Hansen, “Factors Affecting the Viability of Lyophilized Algae,” Cryobiology, 1967, Vol. 4, pp. 17-23. http://dx.doi.org/10.1016/S0011-2240(67)80182-2
- D. Smith, “Emerging Tools and Technologies for Isolation, Conservation and Preservation of Microorganisms,” Proceedings in NBAIM-CAB International, UK Joint Workshop, New Delhi, 2004.
- J. Acreman, “Algae and Cyanobacteria: Isolation, Culture and Long-Term Maintenance,” The Journal of Industrial Microbiology, Vol. 13, 1994, pp. 193-194. http://dx.doi.org/10.1007/BF01584008
- M. B. Syiem, “Entrapped Cyanobacteria: Implications for Biotechnology,” Indian Journal of Biotechnology, Vol. 4, 2005, pp. 209-215.
- M. Navarro, V. García, V. Rodríguez and H. Herrera, “Algas del Occidente de México: Florística y Ecología,” Universidad de Guadalajara, 2006.
- J. Komárek, “Contribution to the Knowledge of Planktic Cyanoprokaryotes from Central Mexico,” Vol. 74, 2002, pp. 207-233.
- J. G. Rodríguez and R. L. Tavera, “Fitoplancton del Lago Zempoala,” Bol Soc Bot Mex, Vol. 63, 1998, pp. 85-100.
- Tomaselli, “Morphology, Ultrastructure and Taxonomy of Arthrospira (Spirulina) maxima and Arthospira. (Spirulina) platensis,” In: A. Vonshak, Ed., Spirulinaplatensis (Arthrospira): Physiology, Cell-Biology and Biotechnology, Taylor and Francis, London, 1997, pp. 1-16.
- R. Lewin, “Uncoiled Variants of Spirulina Platensis (Cyanophyceae: Oscillatoriaceae),” Archiv für Hydrobiologie, Supplement, Vol. 60, 1980, pp. 48-52.
- F. K. Hinda, “Thermal Microorganisms from a Hot Spring on the Coast of Lake Bogoria, Kenya,” Nova Hedwigia, Beiheft, Vol. 123, 2001, pp. 77-93.
- O. A. Owino, J. O. Oyugi, O. O. Nasirwa and L. A. Bennun, “Patterns of Variation in Water Bird Numbers on Four Rift Valley Lakes in Kenya,” Hydrobiologia, Vol. 458, 2001, pp. 45-53. http://dx.doi.org/10.1023/A:1013115724138
- C. Zarrouk, “Contribution al’etuded’unecyanophycee. Influence de Divers Facteurs Physiques Etchimiquessurlacroissance et Photosynthese de Spirulina maxima,” (Setch et Gardner) Geitler. Ph.D. Thesis, University of Paris, Paris, 1966, pp. 4-5.
- C. A. Ascaso, “Rapid Method for the Quantitative Isolation of Green Algae from Lichens,” 1980, pp. 45-483.
- T. Omata and N. Murata, “Isolation and Characterization of the Cytoplasmic Membranes from the Blue-Green Alga Cyanobacterium) Anacystis nidulans,” Plant Cell Physiology, Vol. 24, No. 6, 1983, pp. 1101-1112.
- R. Rippka, J. Deruelles, J. B. Waterbury, M. Herdman and R. Y. Stanier, “Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria,” Journal of Geneneral Microbiology, Vol. 111, 1979, pp. 1-61. http://dx.doi.org/10.1099/00221287-111-1-1
- C. P. Gopinathan, “Live Feed Culture-Mlcro-Algae,” Bud. CMFRI, No. 48, 1996, pp. 110-116.
- A. Ballot, P. Dadheech and L. Krienitz, “Phylogenetic Relationship of Arthrospira, Phormidium, and Spirulina strains from Kenyan and Indian Waterbodies,” Agra University Journal of Research: Science, Vol. 113, 2004, pp. 37-56. http://dx.doi.org/10.1127/1864-1318/2004/0113-0037
- M. M. Gomont, “Monographie des Oscillariées (Nostocacées Homocystées),” Annals of Scientific Natural Botany, Series, Vol. 7, 1892, pp. 263-368.
- K. Anagnostidis and S. Golubic, “Uber die Okologie Einiger Spirulina-Arten. Nova Hedwigia,” Vol. 11, 1966, pp. 309-335.
- Guglielmgi and C. Bazirge, “Structure et Distribution des pores et des Perforations de l’enveloppe de Peptidoglycane chez Quelques Cyanobactkries,” Protistologica, Vol. 18, 1982, pp. 151-165.
- J. Rethmeier, “Untersuchungen zur O X kologie und zum Mechanismus der Sul®dadaption mariner Cyanobakterien der Ostsee,” PhD Thesis, 1995.
- J. Komárek, “Diversita a Moderní Klasifikace Sinic (Cyanoprocaryota) [Diversity and Modern Classification of Cyanobacteria (Cyanoprokaryota),” Inaugural Dissertation Not Published, 1992.
- B. S. Mayashree and A. Bhattacharjee, “An Efficient Protocol for Long-Term Preservation of Cyanobacteria,” Journal of Advanced Laboratory Research in Biology, Vol. 1, No. 1, 2010, pp. 41-45.
- K. Bodas, C. Brenning, K. R. Diller and J. J. Brand, “Cryopreservation of Blue-Green and Eukaryotic Algae in the Culture Collection at the University of Texas at Austin,” Cryo-Letters, Vol. 16, 1995, pp. 267-274.
- J. G. Day, “Cryo-Conservation of Microalgae and Cyanobacteria. Cryo-Letters Supplement,” Vol. 1, 1998, pp. 7- 14.
- F. Mori, M. Errata and M. M. Watanabe, “Cryopreservation of Cyanobacteria and Green Algae in the NIES-Collection,” Microbiology and Culture Collections, 2002, pp. 45-55.
- J. G. Day, J. Muller, K. Comte, T. Friedl, R. Rippka, T. Proeschold, K. Harding and E. E. Benson, “Phenotypic and Genotypic Stability of Cryopreserved Algal and Cyanobacterial Cultures: A Prerequisite for Taxonomic and Systematic Studies,” Cryobiology, Vol. 53, 2006, pp. 430-431. http://dx.doi.org/10.1016/j.cryobiol.2006.10.153
- O. Kiyoshi, M. Megumi and I. Masahiro, “Cryopreservation of Cyanobacteria,” Genes & Genetic Systems, Vol. 80, 2005, p. 487.
- H. K. Park, “Long-Term Preservation of Bloom-Forming Cyanobacteria by Cryopreservation,” Algae-Inchon., Vol. 21, 2006, pp. 125-132. http://dx.doi.org/10.4490/ALGAE.2006.21.1.125
- D. B. Davis, R. Dulbecco, N. H. Eisen, S. H. Ginsberg, B. W. Wood and M. Mc Carty, “Tratado de Microbiología con Inclusión en Inmunología y Genética Molecular,” 2nd Edition, Salvat, 1978.
- M. T. Madigan, J. M. Martinko, B. Parker and J. P. Brock, “Biology of Microorganisms,” Pearson Education, Inc., 2003.
NOTES
*Corresponding author.

