American Journal of Plant Sciences
Vol.4 No.4(2013), Article ID:29834,6 pages DOI:10.4236/ajps.2013.44103
Phenotypic and Molecular Characterization of Phaseolus vulgaris Plants from Non-Cryopreserved and Cryopreserved Seeds
![]()
1Faculty of Agronomy, Universidad de Ciego de Ávila, Ciego de Ávila, Cuba; 2Laboratory for Plant Breeding, Centro de Bioplantas, Universidad de Ciego de Ávila, Ciego de Ávila, Cuba; 3Institut de Recherche pour le Développement, UMR DIADE (Diversité Adaptation et Développement des Plantes), Montpellier, France; 4Department of Agriculture, Division of Plant Genetics and Biotechnology, University of Naples Federico II, Portici, Italy.
Email: florent.engelmann@ird.fr, raversan@unina.it, jclorenzo@bioplantas.cu
Copyright © 2013 Inaudis Cejas et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 24th, 2013; revised March 27th, 2013; accepted April 3rd, 2013
Keywords: Common Bean; Cryostorage; Genotype Variation; Phenotype Variation
ABSTRACT
The objective of this work was to evaluate if cryostorage of Phaseolus vulgaris L. seeds induced variations in regenerated plants at the phenotypic and molecular levels. A series of agricultural traits was measured on plants grown from control, non-cryopreserved and cryopreserved seeds, and the genetic stability of plants of the second generation was analysed at selected microsatellite loci. The phenotype of the second generation plants was evaluated as well. No statistically significant phenotypic differences were observed for the parameters measured, neither in the first nor in the second generations. Averaging both treatments, about 76% of the seeds had germinated 10 days after sowing. At harvest we recorded plants with about 73 cm in height, 13 stem internodes, 25 fruits, 103 grains and 4 grains per fruit. One hundred seeds weighted about 26 g. The genetic analyses performed on the second generation plants using six nuclear Simple Sequences Repeats (SSR) markers revealed no changes in microsatellite length between control and cryopreserved samples, implying that there was no effect of seed liquid nitrogen exposure on genome integrity. The phenotypic and molecular results reported here confirm that cryostorage is an efficient and reliable technique to conserve P. vulgaris seeds and regenerate true-to-type plants.
1. Introduction
Common bean (Phaseolus vulgaris L.) is one of the world’s most important grain legumes. It is consumed as dietary staple worldwide, especially in Latin America and Africa [1]. Beans are especially rich in iron, containing between 50 and 150 mg Fe kg−1 [2]. In addition, bean consumption may reduce the risk for some chronic diseases, including coronary heart disease, diabetes and obesity [3].
To improve the nutritional and agronomic characteristics of common bean varieties, numerous breeding programmes are carried out, which integrate conventional and advanced approaches. At present, the Laboratory for Plant Breeding (University of Ciego de Avila, Cuba) is conducting a research project on P. vulgaris genetic improvement to increase tolerance to soil salinity. We are therefore interested in conserving elite seeds through cryopreservation in liquid nitrogen. Indeed, cryopreservation finds relevant applications not only for long-term storage of non-orthodox seed species, but also for that of orthodox seeds, as demonstrated by various research results [4].
Fundamental and applied research is necessary to assess the biophysical, molecular and genetic stability of plant material at low/ultra low temperatures in the context of “cryobionomics”, the study of cryoinjury and how it affects the genome and genetic stability [5]. Low temperatures induce oxidative stress through the formation of free radicals. The ability of plant cells to survive under cold conditions depends on their capacity to increase the activity of their antioxidant systems including enzymes such as superoxide dismutase, catalase, ascorbate peroxidase and glutathione reductase [6]. Free radicals may damage the nuclear membrane and DNA in cold-susceptible plants [7] indicated a role for peroxiredoxins in protecting DNA and the nuclear machinery of transcription from toxicity caused by free radicals.
The ultimate confirmation of genetic stability should be the reintroduction in the environment and production of whole, true-to-type and fertile plants recovered from cryopreserved germplasm. This implies that only high quality samples are stored in cryobanks, which will require rigorous operational procedures [8].
Phenotypic and molecular studies on the effects of cryostorage have been carried out [4,5]. Phenotypic assessment reflects genome interactions, which affect the phenotype. Plants are subject to phenotypic plasticity, and mutations/deletions in non-coding regions may go undetected. Meanwhile, molecular markers are not environmentally regulated and are detectable at any stage of plant growth. Analytical techniques produce characteristic polymorphic marker profiles, which consist typically of DNA fragments within a molecular weight range [9-12].
From our knowledge, genetic stability studies in relation to cryopreservation have been carried out mostly on in vitro cultured material. No modification has been observed at the phenotypical, biochemical, chromosomal or molecular level that could be attributed to cryopreservation [13]. In a recent work performed on cryopreservation of P. vulgaris seeds, we observed that, during the early stages of germination, no phenotypic changes were observed visually in seedlings recovered from cryopreserved seeds [14]. However, several significant effects of seed liquid nitrogen exposure were recorded at the biochemical level, including a decrease in protein and phenolics content and an increase in aldehyde contents in stems, and a decrease in phenolics contents in roots. In general, roots were more affected by cryostorage compared with other plant parts, while leaves were the least affected. The effects of seed cryopreservation seemed to decline progressively along with seedling growth.
The work presented in this paper is a continuation of the study performed on the early stages of P. vulgaris seed germination described above. It aimed at evaluating the effect of seed cryopreservation on the production of true-to-type plants. The main agricultural traits of plants grown from cryopreserved seeds were compared to those measured on non-cryopreserved seeds. Moreover, the genetic stability of second generation plants was analysed with a set of nuclear Simple Sequences Repeats (SSR) markers developed by [15] from expressed sequence tags and located on the consensus genetic map of P. vulgaris L. The phenotype of the second generation plants was evaluated as well. As far as we know, this is the first report on the phenotypic and genetic characterization of P. vulgaris L. plants originating from cryopreserved seeds.
2. Materials and Methods
P. vulgaris L. seeds (cv. Milagro villaclareño) were used. Seeds were stored at 4˚C in the dark for 4 months after harvesting, in air-tight containers. Seeds contained 12% moisture content (fresh weight basis) at the beginning of the experiment. One batch of seeds was placed in 5 ml cryovials, immersed in liquid nitrogen and stored at −196˚C for 2 weeks. Another batch remained in the dark at 4˚C for the same duration (control treatment). The rewarming procedure consisted of letting the samples warm under ambient laboratory temperature (20˚C - 25˚C) to equilibrium.
From each treatment, 90 seeds were randomly selected and sown in a plant bed as described in Figure 1(a). Technical instructions provided by the Cuban Ministry for Agriculture to cultivate P. vulgaris L. were applied for culture of plants regenerated from germinated seeds. The percentage of seed germination was recorded 10 days after sowing. The following parameters were measured at harvest (78 days after sowing): plant height, number of stem internodes, number of fruits per plant, number of grains per plant, number of grains per fruit and mass of 100 seeds. Border plants, which had more space to grow, were not considered. The Statistical Package for Social Sciences (Version 17.0 for Windows, SPSS Inc.) was used to perform t-tests (p ≤ 0.05) for analysis of results.
To evaluate the genetic stability of plants of the second generation, five seeds were randomly selected on different plants from each experimental treatment. They were germinated in vitro and DNA was extracted for analyses. DNA extraction was carried out using the Qiagen Plant DNeasy Kit according to the manufacturer’s instructions (Qiagen, Valencia, CA, USA). Simple Sequence Repeat (SSR) analyses were carried out with six nuclear genetagged microsatellite (SSR) primer pairs (Table 1), previously developed by Yu [15]. They were chosen based on quality criteria, genome coverage, and locus-specific information content. PCR reactions were performed in a 20 µL volume containing 1 × reaction buffer with 2.5 mM MgCl2, 0.2 mM of each dNTP, 25 pM FAM- labelled forward SSR primer, 15 pM reverse SSR primer, 1 unit of Taq polymerase, and 30 ng of genomic DNA. PCR parameters were drawn up according to [16]. PCR products were separated on an ABI PRISM® 3130 DNA Analyzer. Size calibration was performed with the molecular weight ladder GenScanTM 500 ROXTM Size Standard. Data were collected, and allele sizes determined using the PeakScanner v. 1.2 software (Applied Biosystems, Foster City, California). Moreover, 90 seeds of the second generation were randomly selected, sown in a plant bed and evaluated as described above.
3. Results and Discussion
No statistically significant phenotypic differences were observed between plants originating from non-cryopreserved and from cryopreserved seeds (Figures 1(b) and (c); Table 2). Averaging both treatments, about 76% of seeds had germinated 10 days after sowing, and at harvest (78 days after sowing), we measured plants about 73 cm in height, with thirteen stem internodes, twenty-five fruits, one hundred and three grains and four grains per fruit. One hundred seeds weighted about 26 g.
Genetic analysis of second generation plants was carried out with six gene-tagged SSR markers. Due to their hypervariable nature and extensive genome coverage, these markers offer several advantages over many others (e.g., RAPD, RFLP, AFLP, ISSR) to assess genetic variation at the molecular level [17]. In this study, five out of the six primer combinations tested allowed detecting one allele per individual and they were consequently considered homozygous at the locus assayed (Table 1). By contrast, the primer combination PH5B5 revealed two alleles at the corresponding locus, highlighting the heterozygous status of the marker associated with the endochitinase gene. None of the SSR loci studied showed microsatellite length changes between cryostored seed derived plants and non-cryopreserved ones. The phenotypic evaluation of the second generation plants did not show any statistically significant difference between both treatments (t-test, p > 0.05, data not shown but similar to Table 2).
Therefore, our data not only confirmed that second generation plants were true-to-type after cryostorage but also revealed that no structural changes due to liquid nitrogen exposure affected the P. vulgaris genome at the loci considered. Similar results were found in many plant species using a variety of different molecular markers including SSR [18,19].
Cryopreservation imposes a series of stresses to the plant material, which are susceptible of inducing modifications in cryopreserved explants and regenerated plants [20]. It is thus necessary to verify that genetic stability of cryopreserved material is not altered before routinely using this technique for long-term conservation of plant genetic resources [21].
There are very few reports of phenotypic variations occurring during in vitro culture of plant materials recovered from cryopreservation. One example concerns
Table 1. SSR markers used to assess the genetic stability of Phaseolus vulgaris L. plants from non-cryopreserved and cryopreserved seeds.
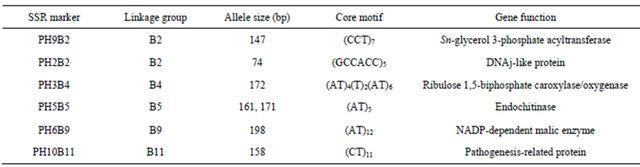
Table 2. Phenotypic characterization of Phaseolus vulgaris L. plants from non-cryopreserved and cryopreserved seeds.
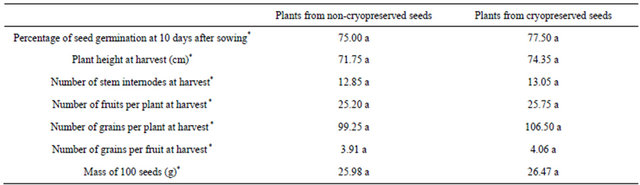
*Results with the same letter are not statistically different (t-test, p > 0.05).
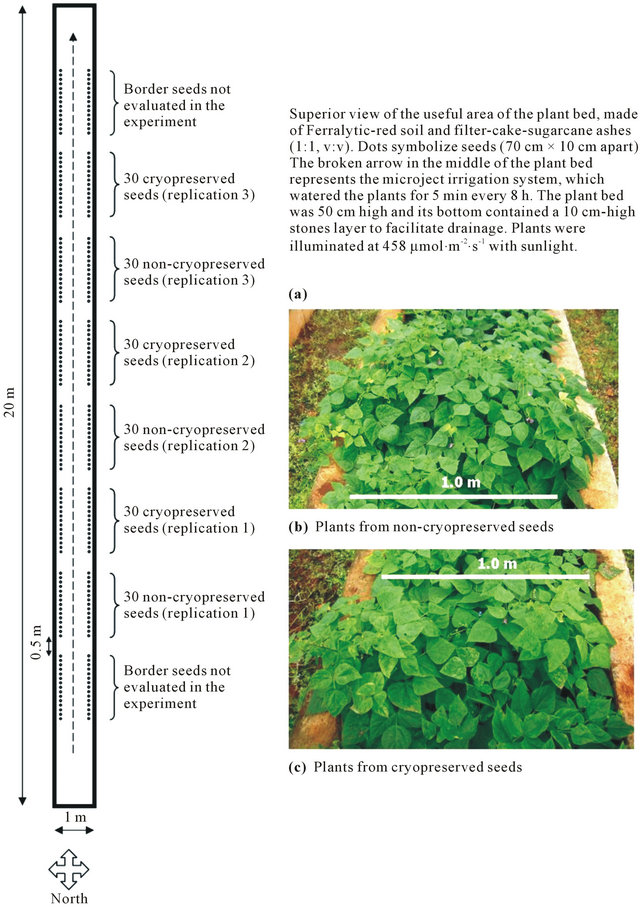
Figure 1. Experimental design in the plant bed (a) and effect of cryopreservation of Phaseolus vulgaris seeds on ex vitro plant growth (60 days) (b), (c).
phenotypic alterations in Chrysanthemum flower colouring after regeneration of 106 cryopreserved apices [22]. On the other hand, [23] found differences in strawberry fruit production when studied 50 plants derived from cryopreserved apices.
Recent studies comparing the vegetative and floral development in the field of plants originating from control and cryopreserved material performed with several species including oil palm (about 52 plants studied per clone, [24]), potato (120 apices per variety per batch, [25]), and banana [26] did not reveal any differences in the characters studied. In our laboratory, we studied the field performance of sugarcane plants originating from control and cryopreserved embryogenic calluses, compared to plants produced using classical macropropagation (100 plants per treatment, [27,28]). The results showed only transitory differences between plants originating from in vitro cultured materials, irrespective of their cryopreservation status and in vivo plants. Stems produced from in vitro cultured materials had a smaller diameter and a shorter height compared to those produced from macropropagated buds. These differences were not observed anymore after 12 months of stool field growth.
Although the effects of the cooling/warming cycle on the genome are unknown, the potential variations observed may not be due to cryopreservation per se but may be the result of the whole culture-cryoprotectionregeneration process [5,20]. Walters commented that variability in ageing kinetics within a seed species couldn’t be accounted for by water content and temperature only. [29] The effect of genotype × environment interactions during plant growth and seed formation before cryostorage is also relevant. These authors suggested that the basis for this variability is not only genetic, but due to environmental factors and cautioned that seed genebank operators cannot assume a particular accession would exhibit average deterioration kinetics.
Another important finding resulting from thermodynamic studies, which contradicts the classical rule of thumbs that “the lower the storage temperature and the moisture content of a seed, the longer the viability” is that the optimal water content for storage of seeds already in the glassy state increases with decreasing temperature [29,30]. Clearly, the physiological status of germplasm before it is cryobanked has important implications for its long-term stability and viability.
In the genebank context and from an agronomic point of view, the effect of LN exposure on seed viability and germination should be tested for each plant material before using cryopreservation for long-term storage. As far as we know, there is no published report on the impact of LN exposure on viability and germination of common bean seeds. The results presented in this paper confirm at the phenotype and molecular levels the effectiveness of P. vulgaris L. seed cryostorage to preserve and regenerate true-to-type plants, although experiments with higher number of plants are required to screen potential mutants. We understand that we did not use a molecular technique allowing very in-depth study of DNA. Moreover, in general, molecular techniques cannot explore the complete genome. For this reason, the study of relevant morphological characteristics and agronomic traits of common bean plants represents the most important part of the paper. The molecular study presented here provides additional information but is not the core of our research.
4. Acknowledgements
This research was supported by the Cuban Ministry for Superior Education. We are grateful to Mrs. Julia Martínez, Mrs. Alitza Iglesias, Mrs. Yaima Pino, Mrs. Sandra Llanes Fiallo, Mrs. Yudelmis de la Pera Borrego, Mr. Yosvany Palmero, Dr. Guillermo Perez and Mr. Diego Martinez for their excellent technical assistance.
REFERENCES
- FAOSTAT, 2010. http://faostat.fao.org/
- R. M. Welch, W. A. House, S. Beebe and Z. Cheng, “Genetic Selection for Enhanced Bioavailable Levels of Iron in Bean (Phaseolus vulgaris L.) Seeds,” Journal of Agricultural and Food Chemistry, Vol. 48, No. 8, 2000, pp. 3576-3580. doi:10.1021/jf0000981
- P. B. Geil and J. W. Anderson, “Nutrition and Health Implications of Dry Beans: A Review,” Journal of the American College of Nutrition, Vol. 13, No. 6, 1994, pp. 549-558.
- F. Engelmann and V. R. Rao, “Major Research Challenges and Directions for Future Research,” In: M. N. Normah, H. F. Chin and B. M. Reed, Eds., Conservation of Tropical Plant Species, Springer Verlag, Berlin, 2012.
- K. Harding, “Genetic Integrity of Cryopreserved Plant Cells: A Review,” Cryoletters, Vol. 25, No. 1, 2004, pp. 3-22.
- P. Revilla, A. Butrón, M. E. Cartea, R. A. Malvar and A. Ordás, “Breeding for Cold Tolerante,” In: M. Ashraf and P. J. C. Harris, Ed., Abiotic Stresses: Plant Resistance through Breeding and Molecular Approaches, Food Products Press, New York, 2005, pp. 301-400.
- K. J. Dietz, “Plant Peroxidoxins,” Annual Review of Plant Biology, Vol. 54, 2003, pp. 93-107. doi:10.1146/annurev.arplant.54.031902.134934
- P. Berjak, P. Bartels, E. Benson, K. Harding, D. J. Mycock, N. W. Pammenter and J. Wesley-Smith, “Cryoconservation of South African Plant Genetic Diversity,” In Vitro Cellular & Developmental Biology, Vol. 47, No. 1, 2012, pp. 65-81.
- L. M. Díaz and M. W. Blair, “Race Structure within the Mesoamerican Gene Pool of Common Bean (Phaseolus vulgaris L.) as Determined by Microsatellite Markers,” Theoretical and Applied Genetics, Vol. 114, No. 1, 2006, pp. 143-154. doi:10.1007/s00122-006-0417-9
- L. R. Hanai, T. Campos, L. E. A. Camargo, L. L. Benchimol, A. P. Souza, M. Melotto, S. A. M. Carbonell, A. F. Chioratto, L. Consoli, E. F. Formighieri, M. V. B. M. Siqueira, S. M. Tsai and M. L. C. Vieira, “Development, Characterization, and Comparative Analysis of Polymorphism at Common Bean SSR Loci Isolated from Genic and Genomic Sources,” Genome, Vol. 50, No. 3, 2007, pp. 266-277. doi:10.1139/G07-007
- M. W. Blair, L. M. Díaz, H. F. Buendía and M. C. Duque, “Genetic Diversity, Seed Size Associations and Population Structure of a Core Collection of Common Beans (Phaseolus vulgaris L.),” Theoretical and Applied Genetics, Vol. 119, No. 6, 2009, pp. 955-972. doi:10.1007/s00122-009-1064-8
- T. Campos, P. R. Oblessuc, D. A. Sforça, J. M. K. Cardoso, R. M. Baroni, A. C. B. Sousa, S. A. M. Carbonell, A. F. Chioratto, A. A. F. Garcia, L. B. Rubiano and A. P. Souza, “Inheritance of Growth Habit Detected by Genetic Linkage Analysis Using Microsatellites in the Common Bean (Phaseolus vulgaris L.),” Molecular Breeding, Vol. 27, No. 4, 2011, pp. 549-560. doi:10.1007/s11032-010-9453-x
- F. Engelmann, “Use of Biotechnologies for Conserving Plant Biodiversity,” Acta Horticulturae, Vol. 812, 2009, pp. 63-82.
- I. Cejas, K. Viveas, T. Laudat, J. González-Olmedo, F. Engelmann, M. E. Martínez-Montero and J. C. Lorenzo, “Effects of Cryopreservation of Phaseolus vulgaris L. Seeds on Early Stages of Germination,” Plant Cell Reports, Vol. 31, No. 11, 2012, pp. 2065-2073. doi:10.1007/s00299-012-1317-x
- K. Yu, S. J. Park, V. Poysa and P. Gepts, “Integration of Simple Sequence Repeat (SSR) Markers into a Molecular Linkage Map of Common Bean (Phaseolus vulgaris L.),” Journal of Heredity, Vol. 91, No. 6, 2000, pp. 429-434. doi:10.1093/jhered/91.6.429
- D. Sicard, L. Nanni, O. Porfiri, D. Bulfon and R. Papa, “Genetic Diversity of Phaseolus vulgaris L. and P. coccineus L. Landraces in Central Italy,” Plant Breeding, Vol. 124, No. 5, 2005, pp. 464-472. doi:10.1111/j.1439-0523.2005.01137.x
- R. K. Kalia, M. K. Rai, S. Kalia, R. Singh and A. K. Dhawan, “Microsatellite Markers: An Overview of the Recent Progress in Plants,” Euphytica, Vol. 177, No. 3, 2011, pp. 309-333. doi:10.1007/s10681-010-0286-9
- K. Harding and E. E. Benson, “The Use of Microsatellite Analysis in Solanum tuberosum L. in Vitro Plantlets Derived from Cryopreserved Germplasm,” Cryo Letters, Vol. 22, No. 3, 2001, pp. 199-208.
- N. R. F. Castillo, N. V. Bassil, S. Wada and B. M. Reed, “Genetic Stability of Cryopreserved Shoot Tips of Rubus germplasm,” In Vitro Cellular & Developmental Biology-Plant, Vol. 46, No. 3, 2010, pp. 246-256.
- E. Benson, “Cryopreservation of Phytodiversity: A Critical Appraisal of Theory & Practice,” Critical Reviews in Plant Sciences, Vol. 27, No. 3, 2008, pp. 141-219. doi:10.1080/07352680802202034
- F. Engelmann, “Use of Biotechnologies for the Conservation of Plant Biodiversity,” In Vitro Cellular and Developmental Biology-Plant, Vol. 41, No. 7, 2011, pp. 5-16.
- S. Fukai, M. Goi and M. Tanaka, “The Chimeric Structure of the Apical Dome of Chrysanthemum (Dendranthema grandiflorum (Ramat.) Kitamura) Is Affected by Cryopreservation,” Scientia Horticulturae, Vol. 57, No. 4, 1994, pp. 347-351. doi:10.1016/0304-4238(94)90117-1
- J. J. Medina, I. Clavero-Ramírez, M. E. González-Benito, J. Gálvez-Farfán, J. M. López-Aranda and C. Soria, “Field Performance Characterization of Strawberry (Fragaria × ananassa Duch.) Plants Derived from Cryopreserved Apices,” Scientia Horticulturae, Vol. 113, No. 1, 2007, pp. 28-32. doi:10.1016/j.scienta.2007.01.030
- E. K. Konan, T. Durand-Gasselin, Y. J. Koadio, A. C. Niamké, D. Dumet, Y. Duval, A. Rival and F. Engelmann, “Field Development of Oil Palms (Elaeis guineensis Jacq.) Originating from Cryopreserved Stabilized Polyembryonic Cultures (SPCs),” Cryo Letters, Vol. 28, No. 5, 2007, pp. 377-386.
- G. Mix-Wagner, H. M. Schumacher and R. J. Cross, “Recovery of Potato Apices after Several Years of Storage in Liquid Nitrogen,” Cryo Letters, Vol. 24, No. 1, 2003, pp. 33-41.
- F. X. Côte, O. Goue, R. Domergue, B. Panis and C. Jenny, “In-Field Behavior of Banana Plants (Musa AA sp.) Obtained after Regeneration of Cryopreserved Embryogenic Cell Suspensions,” Cryo Letters, Vol. 21, No. 1, 2000, pp. 19-24.
- M. E. Martínez-Montero, M. T. González-Arnao, C. Borroto-Nordelo, C. Puentes-Diaz and F. Engelmann, “Cryopreservation of Sugarcane Embryogenic Callus Using a Simplified Freezing Process,” Cryo Letters, Vol. 19, No. 3, 1998, pp. 171-176.
- M. E. Martínez-Montero, E. Ojeda, A. Espinosa, M. Sánchez, R. Castillo, M. T. González-Arnao, F. Engelmann and J. C. Lorenzo, “Field Performance of Cryopreserved Callus-Derived Sugarcane Plants,” Cryo Letters, Vol. 23, No. 1, 2002, pp. 21-26.
- C. Walters, L. J. Wheeler and P. C. Stanwood, “Longevity of Cryogenically Stored Seeds,” Cryobiology, Vol. 48, No. 3, 2004, pp. 229-244. doi:10.1016/j.cryobiol.2004.01.007
- C. W. Vertucci and E. E. Roos, “Theoretical Basis of Protocols for Seed Storage II. The Influence of Temperature on Optimal Moisture Levels,” Seed Science Research, Vol. 3, No. 3, 1993, pp. 201-213. doi:10.1017/S0960258500001793

