American Journal of Plant Sciences
Vol. 4 No. 7 (2013) , Article ID: 33995 , 7 pages DOI:10.4236/ajps.2013.47170
Persian Shallot (Allium hirtifolium Boiss) Extract Elevates Glucokinase (GCK) Activity and Gene Expression in Diabetic Rats
![]()
1Department of Biochemistry, Faculty of Medicine, Rafsanjan University of Medical Sciences, Rafsanjan, Iran; 2Department of Biochemistry and Hamadan student research committee, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran; 3Department of Social Medicine, Faculty of Medicine, Rafsanjan University of Medical Sciences, Rafsanjan, Iran; 4Department of immunology, Faculty of Medicine, Rafsanjan University of Medical Sciences, Rafsanjan, Iran; 5Molecular Medicine Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
Email: *seyedo2@yahoo.com
Copyright © 2013 Mehdi Mahmoodi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 6th, 2013; revised February 7th, 2013; accepted May 10th, 2013
Keywords: Persian Shallot; Glucokinase; Gene Expression; Diabetes
ABSTRACT
Hepatic GCK is a key enzyme in glucose homeostasis and, as such, is a potential target for treatment strategies of diabetes. We investigated the effect of Persian shallot (Allium hirtifolium Boiss) hydroalchoholic extract on blood glucose level, plasma insulin level, GCK activity and its gene expression. Thirty two male rats were divided into 4 groups of 8, diabetic groups received 100 and 200 mg/kg Persian shallot extract, diabetic control and normal control received 0.9% saline for 30 days. Investigations of gene expression by Real-Time PCR showed that Persian shallot had led to gently increased GCK gene expression in diabetic rats. GCK activity increased significantly in Persian shallot treated group in dose dependent manner (P < 0.05). These results indicated that Persian shallot exhibited a significant potential as a hypoglycemic agent perhaps via its ability to enhance insulin secretion, GCK gene expression and its activity.
1. Introduction
Diabetes mellitus is found in universal of the world and is rapidly increasing in most parts of the world. As diabetes aggravates and β-cell function deteriorates, the insulin level begins to fall below the body’s requirements and causes prolonged and more severe hyperglycemia [1]. Extensive exposure of pancreatic β-cells to high glucose levels causes β-cell dysfunction that is associated with impaired insulin secretion, function and biosynthesis [2]. Liver is an insulin-sensitive tissue and plays a major role in maintaining glucose homeostasis by regulating the interaction between the glucose utilization and production. Thus, a suitable antidiabetic agent should improve glucose-induced insulin secretion, hepatic glucose metabolism, and peripheral insulin sensitivity [3].
To stimulate pancreatic insulin secretion, glucose must be metabolized in pancreatic β-cell. Glucose must be first be phosphorylated before being utilized by cells. This reaction is catalyzed by a family of enzymes called hexokinases, which are found in different organisms diverse from bacteria to humans [4]. Mammalian hexokinase IV (D), also known as GCK (glucokinase) (ATP: D-hexose 6-phosphotransferase; EC 2.7.1.1), plays a key role in maintaining glucose homoeostasis [5], and is the major glucose-phosphorylating enzyme expressed in hepatocytes and pancreatic β-cells. GCK is a monomeric protein of 465 amino acids and a molecular weight of about 50 kD. There are at least two clefts, one for the active site, binding glucose and MgATP, and the other for a putative allosteric activator that has not yet been identified [6]. GCK is unique amongst hexokinases in that it displays a sigmoidal substrate dose-response curve, demonstrates low affinity and positive cooperativity for substrate glucose, and is not susceptible to product (glucose-6-phosphate) inhibition. These properties are critical to the role GCK plays as the glucose sensor. Also, GCK mutations have been associated with maturity onset diabetes of the young [7]. Given its pivotal role in regulating glucose homeostasis, there has been significant interest in GCK as a target for treating diabetes mellitus. GCK is one of the essential factors for the glucosestimulated insulin secretion [8]. Long-term regulation of hepatic GCK activity is controlled by its mRNA level. Transcription of GCK is regulated differentially by an upstream promoter in pancreatic β-cells and a downstream promoter in hepatocytes. Activation of either one of them leads to the generation of a GCK mRNA and produces active form of GCK [9,10]. In the liver, expression of GCK is very closely dependent on the presence of insulin. Stimulation of transcription of genes encoding GCK, leads to a decreased glucose level [11].
Recently there has been a growing interest in hypoglycemic agents from natural products, especially those derived from plants, because plant sources are usually considered to be safer, with fewer side effects than synthetic sources [12-14].
In this line Investigations showed some plants had hypoglycemic effects and mimics insulin, and increased Glucokinase activity and mRNA expression in the liver of rats in a dose dependent manner [15,16]. Persian shallot (Allium hirtifolium Boiss) is a nutritive plant with special taste that belongs to liliacea family. Biochemical analysis of Persian shallot extracts has confirmed its hypoglycemic and hepatoprotective effects [17,18]. The Persian shallot extract is a stronger hypoglycemic agent compared to garlic extract and it could be a useful supplemental remedy in diabetes [19]. However, to our knowledge, there have been no experimental reports regarding the effects of Persian shallot on liver glycolytic in an experimentally induced type 1 diabetes model. Therefore, the present study was designed to investigate the possible anti-diabetic effects of two different doses (100 and 200 mg/kg) of Persian shallot in streptozotocininduced diabetic rats; a suitable model for type 1 diabetes [20]. The diabetogenic agent streptozotocin is selectively toxic to insulin-secreting β-cells of pancreatic islets [21]. In the present study we have evaluated glucose homeostasis, the activity and gene expression of GCK in liver, as well as insulin and FBS level.
2. Methods
2.1. Preparation of Hydroalcoholic Extract of Persian Shallot
Fresh Persian shallot (Allium hirtifolium Boiss) bulbs were obtained from Sanandaj (Kordestan, Iran). The genus and species of the bulbs were confirmed by the botanists (Department of botany, Valiasr University Rafsanjan, Iran). Then, 100 gr of fresh bulbs was well crushed and 400 ml distilled water/ethanol (25/75) was added. After 48 hours incubation, the solution was filtered using a filter paper through a Buchner funnel. The filtered resultant solutions obtained from this stage, concentrated by means of a vacuum distillation and decanted to dry powder, then, needed concentrations prepared [17].
2.2. Induction of Diabetes and Persian Shallot Treatments
In this study 32 male albino Wistar rats weighing 180 - 230 g were recruited. Twenty four rats were injected (intraperitoneal injection) with 45 mg/Kg body weight of streptozotocin (STZ) (diabetic type-1 rats) and eight rats considered as normal group. After being matched according to body weight, the rats were divided into 4 groups of 8 rats per group:
Group 1: diabetic rats received daily 200 mg/kg Persian shallot extract (2 ml) for 30 days.
Group 2: diabetic rats received daily 100 mg/kg Persian shallot extract (2 ml) for 30 days.
Group 3: diabetic rats received daily 0.9% saline (2 ml) for 30 days (diabetic control).
Group 4: normal rats received daily 0.9% saline (2 ml) for 30 days (normal control).
The solutions (2 ml) given to animals by using a gavage syringe. The animals were then housed in cages and had free access to water and standard food. Animal handling was performed with regard to Iranian animal ethics society and local university rules. Following 30 days blood and liver samples were collected.
2.3. Biochemical Analyses
Plasma insulin concentrations were assayed by ELISA method using a commercial kit (Mercodia, Sweden) and FBS was measured by BT-3000 autoanalyzer.
2.4. Hepatic Enzyme Activity
Glucokinase activity was determined from liver samples homogenized in 9 volumes of a buffer containing 50 mmol/L Tris-HCl, pH 7.4, 100 mmol/L KCl, 10 mmol/L mercaptoethanol, and 1 mmol/L EDTA. Homogenates were centrifuged at 100,000 g for 1 h; the postmicrosomal supernatant was used for the spectrophotometric continuous assay as described previously [22], with a slight modification, whereby the formation of glucose-6- phosphate from glucose at 27˚C was coupled to its oxidation by glucose-6-phosphate dehydrogenase and nicotinamide adenine dinucleotide (NAD). One unit of enzyme activity is defined as that activity catalyzing the formation of 1 m mol of product per minute at 30˚C [23].
2.5. Extraction of RNA
For the isolation of tissue RNA, rats were humanly sacrificed and under aseptic situations the liver tissues were removed and immediately frozen in liquid nitrogen. Prior to RNA extraction, Liver samples were homogenized in TRIZOLTM reagent (Invitrogen,) using Mixer 301. Total RNA was extracted according to the manufacturer’s guidance. RNA samples were electrophoresed in agarose gels and visualized with ethidium bromide for quality control, its purity evaluate by spectrophotomery (260/280 nm).
2.6. cDNA Synthesis and Quantitative Real-Time PCR
Three micrograms of RNA were reverse transcribed with reverse transcriptase for 1 h at 37˚C for synthesis of cDNA. Quantitative changes in mRNA expression were assessed with Real-Time PCR (Bio-Rad CFX) using SYBR-Green detection consisting of SYBR Green PCR Master Mix (Aria-tous, Iran). The PCR master mix was made up by 0.5 U of Taq polymerase, 2 µL of each primer and 3 µL of each cDNA samples in a final volume of 20 µL. All amplifications were repeated three times. Oligonucleotide primer sequences are illustrated in Table 1. β2-microglobulin was used as endogenous control, and each sample was normalized on the basis of its β2- microglobulin content. Relative quantification of the mRNA expression levels of target genes was calculated using the 2-ΔΔCt method (Table 2).
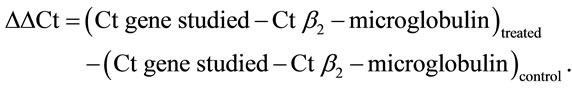
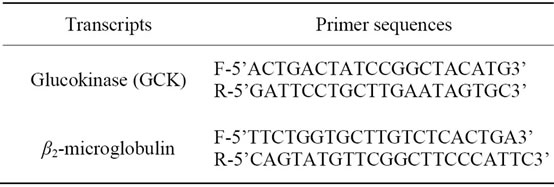
Table 1. Primers sequences and product size.
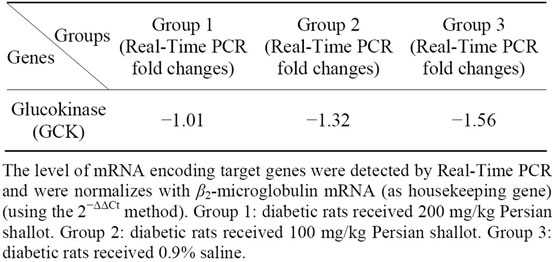
Table 2. Real-time PCR results for selected genes.
2.7. Statistical Analysis
Results are presented as mean ± SD. Statistical difference between the means of the various groups were analyzed using one way analysis of variance (ANOVA) followed by Tukey’smultiple test. Data were considered statistically significant if P < 0.05.
3. Results
3.1. FBS and Insulin
The FBS concentrations of four groups of rats during experimental period are shown in Figure 1. There was a significant difference in FBS level among all groups and Persian shallot consumption reduced significantly FBS level in diabetic treated groups in dose dependent manner (P < 0.05).
As illustrated in Figure 2, diabetic groups showed statistically lower insulin levels in compare to normal control. Although Persian shallot consumption increase slightly insulin level in diabetic rats but this elevation wasn’t significant.
3.2. Hepatic Enzyme Activities
The diabetic rats showed low activity of GCK (Figure 3). Treatment by the Persian shallot significantly increase glucokinase activity when compared with the control group in dose dependent manner (P < 0.05). The 200 mg/kg concentration of Persian shallot had most effect on GCK activity in diabetic group.
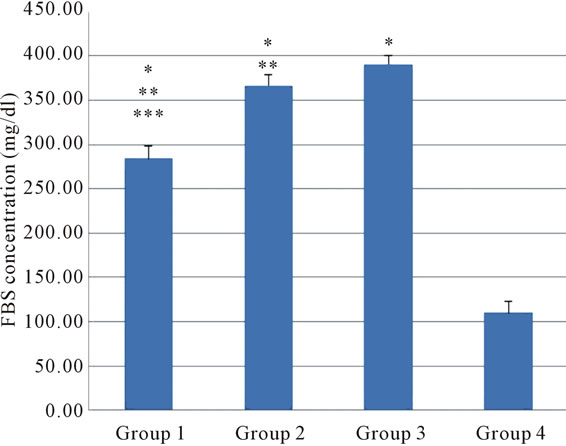
Figure 1. The effect of different concentration of Persian shallot on FBS level (mg/dl) (Mean ± SD) (P < 0.05). Group 1: diabetic rats received 200 mg/kg Persian shallot. Group 2: diabetic rats received 100 mg/kg Persian shallot. Group 3: diabetic rats received 0.9% saline. Group 4: normal rats received 0.9% saline. *Significant differences with Group 4 (P < 0.05). **Significant differences with Group 3 (P < 0.05). ***Significant differences with Group 2 (P < 0.05).
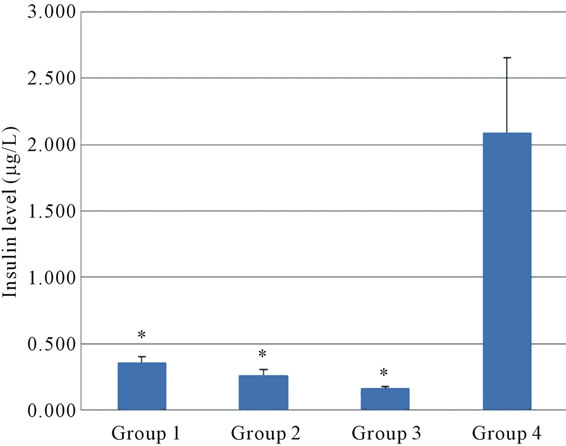
Figure 2. The effect of different concentration of Persian shallot on Insulin level (µg/L) (Mean ± SD) (P < 0.05). Group 1: diabetic rats received 200 mg/kg Persian shallot. Group 2: diabetic rats received 100 mg/kg Persian shallot. Group 3: diabetic rats received 0.9% saline. Group 4: normal rats received 0.9% saline. *Significant differences with Group 4 (P < 0.05).
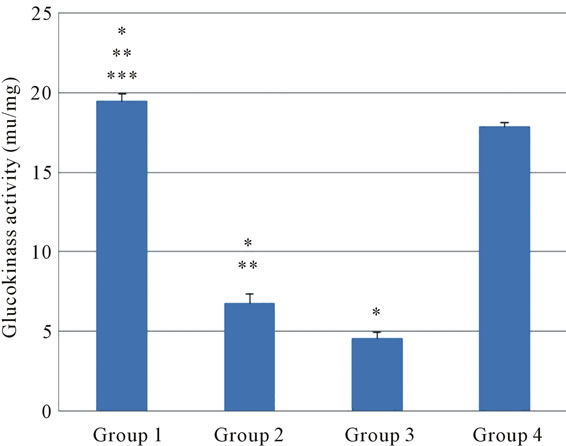
Figure 3. The effect of different concentration of Persian shallot on GCK activity (mu/mg) (Mean ± SD). Group 1: diabetic rats received 200 mg/kg Persian shallot. Group 2: diabetic rats received 100 mg/kg Persian shallot. Group 3: diabetic rats received 0.9% saline. Group 4: normal rats received 0.9% saline. *Significant differences with Group 4 (P < 0.05). **Significant differences with Group 3 (P < 0.05). ***Significant differences with Group 2 (P < 0.05).
3.3. The mRNA Levels of GCK in Liver
The expression level of the GCK gene in the normal control group was considered as 100% and the expression in the other groups were accordingly calculated (Table 2). When compared with control rats, diabetes was found to suppress GCK gene expression slightly in liver (Figure 4). The Persian shallot elevated hepatic glucokinase gene
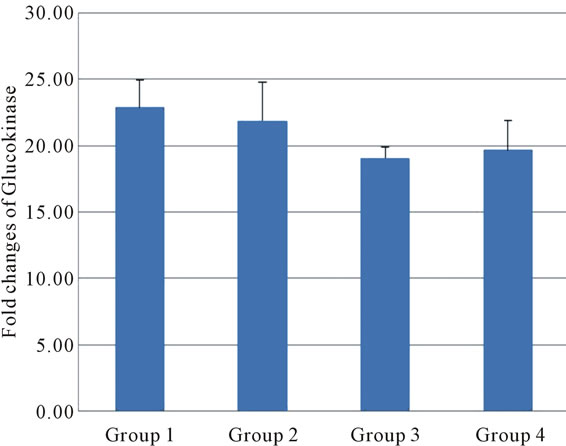
Figure 4. The expressed levels of Glucokinase mRNA (fold) in all groups. (using ANOVA test, Mean ± SD) (P < 0.05). Group 1: diabetic rats received 200 mg/kg Persian shallot. Group 2: diabetic rats received 100 mg/kg Persian shallot. Group 3: diabetic rats received 0.9% saline. Group 4: normal rats received 0.9% saline.
expression when compared with the control group (Figure 4, Table 2).
4. Discussion
Our findings demonstrated that Persian shallot reduced significantly FBS in diabetic rats (P < 0.05). The decrease in the concentration of blood glucose in Persian shallot treated diabetic rats may be associated with enhancement of GCK mRNA expression in the liver, thus increasing the level of glycolysis. In the present study, Persian shallot administration induced mRNA expression levels of hepatic GCK and increased significantly GCK activity in different dose of Persian shallot treated diabetic rats. In addition, blood insulin concentrations were increased in Persian shallot-treated groups compared with the diabetic group. This suggests that perhaps insulin enhances transcription of GCK gene in hepatocytes [9,24], which employ a different promoter than that employed by β-cells [25]. This agrees with the finding that hepatic glycolytic (GCK) enzyme activity is controlled primarily at the transcription level by insulin [26]. High insulin levels have been shown to inhibit hepatic glucose production by means of stimulation of GCK gene transcription [27]. In our study, the changes in GCK could be partly attributed to insulin level because plasma insulin level was gently elevated, than in the control group. These data are in agreement with Celik et al. finding about CAPE treatment [27].
Hepatic GCK is a key enzyme in glucose homeostasis and, as such, is a potential target for treatment strategies of diabetes. Zhang et al., reported that glucokinase enzyme activity was decreased by more than 90% in the liver diabetic rats [28]. So in this study, to evaluate the antidiabetic mechanism(s) of Persian shallot, the key enzymes of carbohydrate metabolism such as GCK, was investigated in liver at mRNA level using Real-Time PCR and also we assayed its activity in liver. It has been reported that GCK-knockout mice have mild hyperglycemia [29] whilst rats over expressing GCK in the liver have reduced blood glucose [3]. The increased activity of hepatic GCK in the Persian shallot treated group caused an increase in glycolysis and utilization of glucose for energy production. In the current study the mRNA expression levels of GCK in the liver of diabetic rats were found to be suppressed.
As mentioned, in the current study, supplementation of Persian shallot in diabetic rats increased hepatic glucokinase activity significantly.
To our knowledge, we report for the first time that Persian shallot supplementation causes a dose-dependent increase in GCK mRNA expression and its activity in livers of diabetic rats. In the liver, an increase in GCK activity leads to enhanced glycolysis and hepatic glucose uptake [30]. The increase in insulin concentrations in diabetic rats supplemented with Persian shallot could either be caused by direct stimulation of insulin secretion in response to feeding or by a protective effect of Persian shallot on the pancreas. The results of our study are in line with recently published data, which suggest that other plants preserves and protects the pancreas by its strong antioxidative capacity [31,32]. This would ultimately lead to enhanced pancreatic function and improved insulin secretion in response to feeding.
Recently there has been a growing interest in hypoglycemic agents from natural products, several antioxidants and bioflavonoids, ubiquitously present in Persian shallot (Allium hirtifolium Boiss) have been reported to improve hyperglycemia in diabetes mellitus [19]. In our study Persian shallot significantly reduced FBS while gently increases insulin serum level. Our finding suggest that the antioxidants could restore the damaged pancreas and stimulate the secretion of pancreatic insulin meantime; the Persian shallot has probably ability to accelerate the hepatic glucose metabolism may be via regulating the expression of the functional genes of GCK. The results of Real-Time PCR studies provided supportive evidence for FBS analyses [33]. In fact, this antihyperglycemic action of Persian shallot is likely to be associated with a marked enhancement of the GCK mRNA expression in the liver. Current results is consistent with previous studies that showed GCK mRNA expression increase in Naringin [31], 1-Deoxynojirimycin [33] and epigallocatechin gallate, a main polyphenolic constituent of green tea [34] treated rats.
5. Conclusion
In conclusion, the data obtained in this study suggest that Persian shallot is an effective hypoglycemic agent via its ability to enhance insulin secretion and to decrease hepatic glucose output along with the increased level of GCK activity and gene expression in Persian shallottreated diabetic rats. So it may be useful for preventing or delaying the development of diabetes and its complications.
REFERENCES
- J. E. Gerich, “Clinical Significance, Pathogenesis, and Management of Postprandial Hyperglycemia,” Archives of Internal Medicine, Vol. 163, No. 11, 2003, pp. 1306- 1316. doi:10.1001/archinte.163.11.1306
- R. P. Robertson, H. J. Zhang, K. L. Pyzdrowski and T. F. Walseth, “Preservation of Insulin mRNA Levels and Insulin Secretion in HIT Cells by Avoidance of Chronic Exposure to High Glucose Concentration,” Journal of Clinical Investigation, Vol. 90, No. 2, 1992, pp. 320-325. doi:10.1172/JCI115865
- T. Ferre, A. Pujol, E. Riu, F. Bosch and A. Valera, “Correction of Diabetic Alterations by Glucokinase,” Proceedings of the National Academy of Sciences, Vol. 93, No. 14, 1996, pp. 7225-7230. doi:10.1073/pnas.93.14.7225
- M. L. Cardenas, A. Cornish-Bowden and T. Ureta, “Evolution and Regulatory Role of the Hexokinases,” Biochimica et Biophysica Acta, Vol. 1401, No. 3, 1998, pp. 242-264. doi:10.1016/S0167-4889(97)00150-X
- F. M. Matschinsky, M. A. Magnuson, D. Zelent, T. L. Jetton, N. Doliba, Y. Han, R. Taub and J. Grimsby, “The Network of Glucokinase-Expressing Cells in Glucose Homeostasis and the Potential of Glucokinase Activators for Diabetes Therapy,” Diabetes, Vol. 55, No. 1, 2006, pp. 1-12. doi:10.2337/diabetes.55.01.06.db05-0926
- K. Kamata, M. Mitsuya, T. Nishimura, J. Eiki and Y. Nagata, “Structural Basis for Allosteric Regulation of the Monomeric Allosteric Enzyme Human Glucokinase,” Structure, Vol. 12, No. 3, 2004, pp. 429-438. doi:10.1016/j.str.2004.02.005
- P. Froguel, H. Zouali, N. Vionnet, G. Velho, M. Vaxillaire, F. Sun, S. Lesage, M. Stoffel, J. Takeda, P. Passa, et al., “Familial Hyperglycemia Due to Mutations in Glucokinase—Definition of a Subtype of Diabetes Mellitus,” The New England Journal of Medicine, Vol. 328, 1993, pp. 697-702. doi:10.1056/NEJM199303113281005
- F. J. Bourbonais, J. Chen, C. Huang, Y. W. Zhang, J. A. Pfefferkorn and J. A. Landro, “Modulation of Glucokinase by Glucose, Small Molecule Activator and Glucokinase Regulatory Protein: Steady-State Kinetic and Cell-Based Analysis,” Biochemical Journal Immediate Publication, 2011.
- P. B. Iynedjian, P. R. Pilot, T. Nouspikel, J. L. Milburn, C. Quaade, S. Hughes, C. Ucla and C. B. Newgard, “Differential Expression and Regulation of the Glucokinase Gene in Liver and Islets of Langerhans,” Proceedings of the National Academy of Sciences, Vol. 86, No. 20, 1989, pp. 7838-7842. doi:10.1073/pnas.86.20.7838
- M. A. Magnuson and K. D. Shelton, “An Alternate Promoter in the Glucokinase Gene Is Active in the Pancreatic Beta Cell,” The Journal of Biological Chemistry, Vol. 264, 1989, pp. 15936-15942. doi:10.1007/s11010-008-9719-3
- S. Celik and S. Erdogan, “Caffeic Acid Phenethyl Ester (CAPE) Protects Brain against Oxidative Stress and Inflammation Induced by Diabetes in Rats,” Molecular and Cellular Biochemistry, Vol. 312, No. 1-2, 2008, pp. 39- 46.
- L. Goldfrank, H. Lewin, N. Flomenbaum and M. A. Howland, “The Pernicious Panacea: Herbal Medicine,” Hospital Physician, Vol. 10, No. 10, 1982, pp. 64-86.
- M. C. Sabu, K. Smitha and K. Ramadasan, “Anti-Diabetic Activity of Green Tea Polyphenols and Their Role in Reducing Oxidative Stress in Experimental Diabetes,” Journal of Ethnopharmacology, Vol. 83, No. 1-2, 2002, pp. 109-116. doi:10.1016/S0378-8741(02)00217-9
- L. A. Mentz and E. P. Schenkel, “The Coherence and Reliability of Therapeutic Indications,” Notebook Farmacia, Vol. 5, 1989, pp. 93-119.
- U. Kopp, M. Cicha and M. Yorek, “Impaired Responsiveness of Renal Sensory Nerves in StreptozotocinTreated Rats and Obese Zucker Diabetic Fatty Rats: Role of Angiotensin,” American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, Vol. 294, No. 3, 2008, pp. 858-866. doi:10.1152/ajpregu.00830.2007
- N. Leelarungrayub, V. Rattanapanone, N. Chanarat and J. M. Gebicki, “Quantitative Evaluation of the Antioxidant Properties of Garlic and Shallot Preparations,” Nutrition, Vol. 22, No. 3, 2006, pp. 266-274. doi:10.1016/j.nut.2005.05.010
- J. Hosseini, S. M. Hosseini-Zijoud, F. Oubari, M. Mahmoodi, E. Abbasi Oshaghi, N. Rajabi Gilan, S. R. Ghasemi and B. Hashemi, “Hepatoprotective Effects of Hydroalcoholic Extract of Allium hirtifolium (Persian Shallot) in Diabetic Rats,” Journal of Basic and Clinical Physiology and Pharmacology, Vol. 23, No. 2, 2012, pp. 83-87.
- S.-M. Hosseini-Zijoud, J. Hosseini, M. Mahmoodi and H. Behrooz, “The Effects of Persian Shallot Extract on the Levels of Some Blood Biochemical Parameters in Streptozotocin-Induced Diabetic Rats,” African Journal of Agricultural Research, Vol. 7, No. 22, 2012, pp. 3308-3313. doi:10.5897/AJAR11.2498
- K. Lundgren, P. Jakobsen, M. Kristiansen, L. Norskov and L. Naerum, Patent No WO 97y09040-A1, 1997.
- W. H. Martin, D. J. Hoover, S. J. Armento, I. A. Stock, R. K. McPherson, D. E. Danley, R. W. Stevenson, et al. “Discovery of a Human Liver Glycogen Phosphorylase Inhibitor that Lowers Blood Glucose in Vivo,” Proceedings of the National Academy of Sciences, Vol. 95, No. 4, 1998, pp. 1776-1781. doi:10.1073/pnas.95.4.1776
- E. Strandelle, D. L. Eizirik, O. Korsgren, S. Sandler, “Functional Characteristics of Cultured Mouse Pancreatic Islets Following Exposure to Different Streptozotocin Concentrations,” Molecular Cell Endocrinology, Vol. 95, 1989, pp. 83-91.
- A. L. Davidson and W. J. Arion, “Factors Underlying Significant Underestimations of Glucokinase Activity in Crude Liver Extracts: Physiological Implications of Higher Cellular Activity,” Archives of Biochemistry and Biophysics, Vol. 253, No. 1, 1987, pp. 156-167. doi:10.1016/0003-9861(87)90648-5
- K. Seo-Yoon, K. Sung-Koo, L. Dong-Gyu, P. YoungGuk, L. Young-Choon, C. Ji-Chun and K. Cheorl-Ho, “Effect of Jindangwon on Streptozotocin-Induced Diabetes,” Life Sciences, Vol. 67, 2000, pp. 1251-1263.
- F. M. Matschinsky, Y. Liang, P. Kesaven, L. Wang, P. Froguel, G. Velho, D. Cohen, M. A. Permutt, Y. Tanizawa, T. L. Jetton, K. Niswender and M. A. Magnuson, “Glucokinase as Pancreatic Beta Cell Glucose Sensor and Diabetes Gene,” Journal of Clinical Investigation, Vol. 92, No. 5, 1993, pp. 2092-2098. doi:10.1172/JCI116809
- K. D. Shelton, A. J. Franklin, A. Khoor, J. Beechem and M. A. Magnuson, “Multiple Elements In the Upstream Glucokinase Promoter Contribute to Transcription in Insulinoma Cells,” Molecular and Cellular Biology, Vol. 12, 1992, pp. 4578-4589.
- J. E. Friedman, Y. Sun, T. Ishizuka, C. J. Farrell, S. E. McCormack, L. M. Herron, P. Hakimi, P. Lechner and J. S. Yun, “Phosphoenolpyruvate Carboxykinase (GTP) Gene Transcription and Hyperglycemia Are Regulated by Glucocorticoids in Genetically Obese db/db Transgenic Mice,” The Journal of Biological Chemistry, Vol. 272, 1997, pp. 31475-31481. doi:10.1074/jbc.272.50.31475
- S. Celika, S. Erdoganb and M. Tuzcuc, “Caffeic Acid Phenethyl Ester (CAPE) Exhibits Significant Potential as an Antidiabetic and Liver-Protective Agent in Streptozotocin-Induced Diabetic Rats,” Pharmacological Research, Vol. 60, No. 4, 2009, pp. 270-276. doi:10.1016/j.phrs.2009.03.017
- X. Zhang, W. Liang, Y. Mao, H. Li, Y. Yang and H. Tan, “Hepatic Glucokinase Activity Is the Primary Defect in Alloxan-Induced Diabetes of Mice,” Biomedicine & Pharmacotherapy, Vol. 63, No. 3, 2009, pp. 180-186. doi:10.1016/j.biopha.2007.07.006
- C. Postic, M. Shiota, K. D. Niswender, T. L. Jetton, Y. Chen, J. M. Moates, et al., “Dual roles for Glucokinase in Glucose Homeostasis as Determined by Liver and Pancreatic Beta Cell-Specific Gene Knock-Outs Using Cre Recombinase,” The Journal of Biological Chemistry, Vol. 274, 1999, pp. 305-315. doi:10.1074/jbc.274.1.305
- J. Grimsby, R. Sarabu, W. L. Corbett, N. E. Haynes, F. T. Bizzarro, J. W. Coffey, K. R. Guertin, D. W. Hilliard, R. F. Kester, et al., “Allosteric Activators of Glucokinase: Potential Role In Diabetes Therapy,” Science, Vol. 301, No. 5631, 2003, pp. 370-373. doi:10.1126/science.1084073
- U. J. Jung, M. K. Lee, K. S. Jeong and M. S. Choi, “The Hypoglycemic Effects of Hesperidin and Naringin Are Partly Mediated by Hepatic Glucose-Regulating Enzymes in C57BL/KsJ-db/db Mice,” The Journal of Nutrition, Vol. 134, No. 10, 2004, pp. 2499-2503.
- E. K. Song, H. Hur and M. K. Han, “Epigallocatechin Gallate Prevents Autoimmune Diabetes Induced by Multiple Low Doses of Streptozotocin in Mice,” Archives of Pharmacal Research, Vol. 26, No. 7, 2003, pp. 559-563. doi:10.1007/BF02976881
- Y.-G. Li, D.-F. Ji, S. Zhong, Z.-Q. Lv, T.-B. Lin and S. Chen, “Hybrid of 1-Deoxynojirimycin and Polysaccharide from Mulberry Leaves Treat Diabetes Mellitus by Activating PDX-1/Insulin-1 Signaling Pathway and Regulating the Expression of Glucokinase, Phosphoenolpyruvate Carboxykinase and Glucose-6-Phosphatase in Alloxan-Induced Diabetic Mice,” Journal of Ethnopharmacology, Vol. 134, 2011, pp. 961-970. doi:10.1016/j.jep.2011.02.009
- T. Nakagawa, T. Yokozawa, K. Terasawa, S. Shu and L. R. Juneja, “Protective Activity of Green Tea against Free Radicaland Glucose-Mediated Protein Damage,” Journal of Agricultural and Food Chemistry, Vol. 50, No. 8, 2002, pp. 2418-2422.
NOTES
*Corresponding author.

