American Journal of Plant Sciences
Vol. 4 No. 2A (2013) , Article ID: 28467 , 8 pages DOI:10.4236/ajps.2013.42A056
The Relationship of the Sapstreak Fungus, Ceratocystis virescens, to Sugar Maple Dieback and Decay in Northern Michigan*
![]()
School of Forest Resources and Environmental Science, Michigan Technological University, Houghton, USA.
Email: #tlbal@mtu.edu
Received November 1st, 2012; revised December 2nd, 2012; accepted December 10th, 2012
Keywords: Sapstreak Disease; Acer sacharrum; Decay Rates; Crown Dieback
ABSTRACT
Unusually high levels of dieback have recently been reported in sugar maple, Acer saccharum Marsh., in Upper Michigan, and a network of plots was established to determine the extent and factors associated with the dieback. A possible contributor to this dieback is sapstreak disease caused by Ceratocystis virescens (Davidson) Moreau. Unhealthy trees with considerable crown dieback were evaluated across the western Upper Peninsula, MI to determine the prevalence of the sapstreak fungus using a minimally destructive sampling technique. Approximately 8% of 90 trees sampled were sapstreak positive and approximately 10% of trees were positive at one site that had recently been harvested. While the high levels of maple dieback present in these forests appear not to be directly caused by widespread sapstreak disease, the occurrence of sapstreak may be significantly impacting trees at some locations. However, even when present on a low number of trees, the biointeraction of sapstreak and decay rates from other fungi could be important for future tree mortality and value to the forest industry. Therefore, the effect of two sapstreak fungal isolates on the amount of decay caused by two common maple white rot fungi, Trametes versicolor (L.:Fr.) Pilat. And Irpex lacteus (Fr.:Fr.) Fr. was tested in the laboratory. Sugar maple wood blocks were precolonized by two native isolates of C. virescens followed by inoculation and incubation with decay fungi. Mean percent weight loss of blocks by white rot decay fungi ranged from 39% to 55%, but decay rates were not significantly affected by the presence of the sapstreak fungus.
1. Introduction
In Keweenaw, Houghton, Baraga, and Marquette Counties, Michigan, high levels of sugar maple dieback (ranging up to an average >50% crown dieback in some stands) have recently been reported [1]. Records from the Upper Peninsula, MI indicate previous dieback episodes, yet the cause of many of these cycles has never been determined [2]. Sapstreak was proposed as a cause of dieback in the 1960’s [3,4]. In the past few decades, whenever dieback was observed the region, especially on industry land, sapstreak or poor management was assumed to be the cause. While it has not seemed to be as prominently reported in the area in recent years, pockets of sugar maple dieback in the Great Lakes area has been reported intermittently [2] therefore, the range, extent, and contributing factors to the current dieback were investigated in 2009 on private industry lands in western Upper Michigan. Since some of the heaviest dieback was found on industry lands, the question was raised about whether colonization by the sapstreak fungus (Ceratocystis virescens) introduced by harvesting wounds was a factor in this dieback [1].
1.1. Sapstreak Characteristics and Biology
Sapstreak is usually associated with logging wounds [5- 8]. Most often, infected trees are found near logging roads, skid trails, or in sugarbushes along sap hauling roads [8]. In most forested settings, inoculum is apparently readily available, as shown by Shigo [9], who found C. virescens was one of the most common fungi in northern hardwood forests, occurring frequently as a saprophyte on fresh cut logs. This may mean that the fungus readily attacks trees already weakened. Ceratocystis virescens is known to occur as a pathogenic fungus to sugar maple and in another saprobic form of the species on other hardwoods that is less pathogenic and likely a separate mating population [10].
Trees infected with the sapstreak fungus typically exhibit high crown transparency, smaller than normal leaves, and eventually crown thinning and branch dieback (Figures 1(a) and (b)) [11]. Some trees may be able to persist with no further symptom development for many years, while others continue crown dieback until death in as little as 1 to 2 years [8,11].
Dark hyphae colonize the parenchyma and vessels and can readily be observed under a microscope differentiated from staining from other maple wood staining deposits but not from other fungi without perithecia (Figure 1(c)). In most trees, the fungus can spread and cause vascular staining throughout the roots and up the stem to 10 - 15 meters, (30 - 45 ft) [8], decreasing the value of lumber cut from the tree [12,13].
1.2. Sapstreak and Wood Decay
Investigators have noted that trees dying of sapstreak are frequently colonized at or near the roots by decay fungisuch as Amillaria sp., Xylaria sp., Hypoxylon deustum (Hoffm.) Grev. (Ustilina deusta (Hoffm.) Lind.), and Ganoderma applanatum (Pers.) Pat [5,11]. Mielke and Charrette [6] reported that all of the sapstreak-positive trees identified in their study were also infected with at least one of these fungi. Although the role of other fungi in the progression of sapstreak disease has not yet been demonstrated, decay and root rot fungi affect tree vigor, and possibly the rate of sapstreak disease progression. For example, a decay test with Ponderosa pine, (Pinus ponderosa Dougl ex. Laws.) showed that wood infected with an unknown blue-stain fungus had greater weight lost when decayed by a white rot fungus (11.6%) and a brown rot fungus (13.3%), as compared to wood blocks colonized by the two rot fungi alone (D. Richter, unpublished data). Therefore we hypothesized that the influence of sapstreak on decay rates of other fungi could be important for future tree mortality and value to the forest industry.
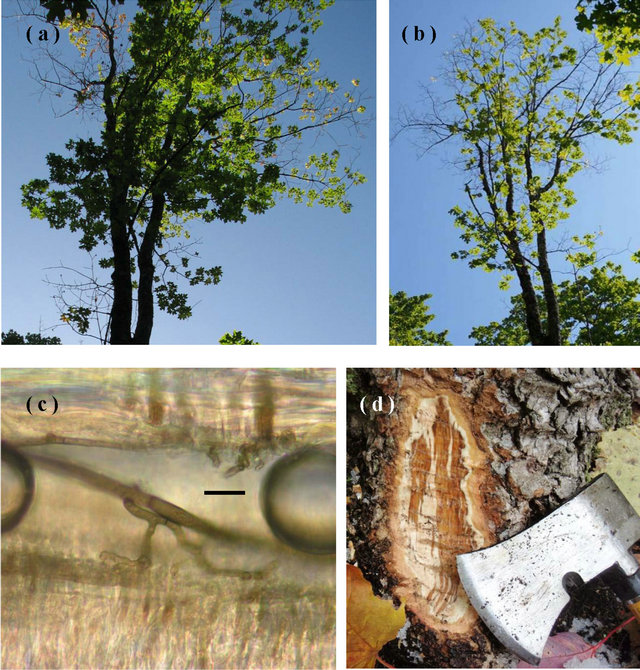
Figure 1. Characteristics of sapstreak disease: (a) In 2009, the crown of the sugar maple in Keweenaw Co., MI from which C. virescens isolate A was obtained, exhibited extensive dieback, high foliage transparency, and smaller leaves; (b) In 2010 dieback had progressed further; (c) C. virescens hyphae in a vessel in dark stained sugar maple wood (bar = 10 μm, air bubbles are artifacts); (d) dark, watersoaked staining in freshly exposed sapwood in the root collar of a sugar maple tree positive for C. virescens (Photos by Tara Bal).
1.3. Objectives
The objectives of our study were to: 1) characterize the prevalence of sapstreak disease in sugar maple stands exhibiting dieback in Upper Michigan and 2) determine whether the presence of the sapstreak fungus affects the decay rate of commonly occurring white rot fungi on sugar maple wood blocks.
2. Materials and Methods
2.1. Field Sampling for the Sapstreak Fungus
In 2009, private industry-owned sugar maple stands exhibiting varying amounts of dieback were identified in western Upper Michigan. A total of 30 sites were evaluated for the presence of sapstreak. Sites may have had old skid trails running through them but were at least 2 chains away from roadways and were selected based on the amount of sugar maple present (at least 10 trees within 0.04 hectare >10.2 cm d.b.h.). Three sugar maples per site were sampled for the sapstreak disease. An attempt was made at each site to sample from three trees with varying levels of crown dieback, including a tree with a healthy crown or low dieback, a tree with an intermediate crown, and one with a higher level of dieback relative to the trees at the site. For example, in a relatively healthy stand 20% dieback of the crown may be one of the worst trees while in a relatively unhealthy stand, only 20% crown dieback may be one the best looking trees. The three sampled trees were of similar size, with no more than 2.5 cm difference in d.b.h. between them. Percent dieback in the crown was assessed using standard USFS Forest Health Monitoring Protocols that provides an ocular estimate of the percent of the crown that has died back as indicated by dieback with persisting fine branches in the upper and outer crown while ignoring old dead branches, large forks and shaded out epicormics under the crown [14]. Sites were visited again in 2010 to determine if crown conditions persisted.
Methods of detecting sapstreak are usually destructive in nature, chopping into the tree with an axe, boring, probing with a Shigometer, removing the whole tree, or tipping up the roots. At the base of each sugar maple tree, near a wound if visible, three sides of the base of the bole had a slice of bark and outer xylem wood (approximately 7.5 cm × 12.5 cm) was removed with a hatchet or chisel and hammer to look for discoloration indicating the presence of the fungus (Figure 1(d)). A similar technique has been used to determine the proportion of root-crown colonization by Ceratocystis wagneri (Goheen and Cobb), which causes black stain root disease in western conifers [15,16]. If no discoloration was present, tree sealant was placed on the wound and the tree was recorded as not infected. If discoloration was observed, a sapwood sample was removed, transferred to a plastic bag, and refrigerated at 4˚C until isolations could be processed within a week. Isolation of C. virescens was attempted from discolored areas by aseptically removing small wood chips, placing them in 2% malt agar amended with Streptomycin at 200 ppm, and left to grow at room temperature (20˚C - 22˚C) for two weeks. Identification of C. virescens positive culture was based on hyphal and spore characters and the presence and size of black, long-necked perithecia compared to taxonomic descriptors and a side by side comparison with known cultures at 400× with a Nikon Optiphot®.
A stand in Keweenaw County, MI, where the sapstreak fungus was collected from one of the three sample trees was further examined to determine the prevalence of sapstreak in the immediate area. The site was in close proximity to a recent harvest, and provided an opportunity for a more detailed sampling from residual stumps and trees, as well as from trees at the original site without needing to damage more than three trees in more valuable stands. An area approximately 0.4 hectares was thoroughly examined after approximately half of it was harvested in the fall of 2009 (within 2 months of entry). A previous harvest occurred around 10 years earlier. The original positive sapstreak sample was collected from the area not harvested in 2009. Sugar maples that had crown dieback or suspect crowns were the focus of our work, so there are a few (less than 10) apparently healthy trees around the perimeter of the area that were not investigated. Stumps from trees cut in late fall 2009, standing dead trees, and live trees with crown dieback symptoms were evaluated in May 2010 by cutting into the base on at least three sides of the bottom of the bole along the root collar and adjacent to wounds (if present) to look for the characteristic streaking caused by C. virescens. Dieback levels for live trees were recorded. The stumps still had relatively fresh wood when they were examined in spring 2010. Samples were processed as previously described.
2.2. Soil Block Decay Test
A soil block test was used to determine the effect of the sapstreak fungus on wood decay by commonly-occurring white rot fungi found in sugar maple stands, Trametes versicolor and Irpex lacteus. A freshly-cut, disease free, sugar maple, 20 cm diameter was cut in February 2010 in Houghton County, Michigan to obtain 14 × 14 × 14 mm sugar maple sapwood blocks. Blocks were selected for uniformity and were free from defects. Two isolates of C. virescens were used for pre-colonization: A was found in the base of a living 26 cm diameter sugar maple tree in Keweenaw County, MI in August 2009 during this study, and B was obtained from a 30 cm sugar maple log cut in Houghton County, MI in September 2009 during a separate investigation. Colonies of both isolates were grown on 2% malt extract agar (2 M) in deep (100 × 20 mm) plates. Fresh cultures were important as much less vigorous growth has been noted with age, even after one year in culture [5]. After 5 days of growth at 20˚C - 22˚C, all plates were fully covered with dark mycelium. Isolate A was darker gray-green and denser than isolate B, however both exhibited rapid growth and produced abundant perithecia and ascospores.
A sterile plastic grid, approximately 3 mm mesh, was placed on top of the C. virescens mat in the petri dishes, and seven steam-sterilized (30 min) wood blocks were placed on the grid in each plate so that the cross-sectional face was in contact with the fungus. This was done to prevent uptake of agar nutrients by the blocks if placed directly on the agar. Wood blocks in three separate plates were precolonized by each C. virescens isolate. Plates and blocks were incubated for four weeks at 27˚C. By two weeks, C. virescens had grown very rapidly into all wood blocks and perithecia were present. Wood blocks were left for two additional weeks to ensure thorough internal colonization. At the end of incubation, a wood block colonized by each isolate was sectioned and examined microscopically (400×) to show that both isolates had colonized wood cells throughout the wood block (Figure 1(c)). Fungi were reisolated from the blocks to confirm the presence of C. virescens.
Precolonized and uncolonized sugar maple blocks were then dried overnight at 40˚C, labeled, and weighed to the nearest 0.01 g prior to insertion in soil jars. A simple viability test using 2 M agar showed that C. virescens was still viable after oven drying overnight at 40˚C.
The decay test used was based on AWPA E-10 “Standard Method of Testing Wood Preservatives by Laboratory Soil-block Cultures” [17]. Air-dried forest topsoil (100 g, pH ~6.0) was added to a square flint jar (60 ml) with 30 ml of distilled water. Jars were covered with a plastic lid with a 5 mm diameter hole covered with a strip of adhesive first-aid tape for ventilation and autoclaved for 30 minutes. Decay fungi used were Trametes versicolor MAD 697 and Irpex lacteus ATCC 11245. Pure culture techniques were conducted to confirm the identity of the inoculum at all stages in the test. Inocula for all fungi were grown on 2 M agar for three weeks at 22˚C prior to use in soil jars.
Wood blocks were steam sterilized for 30 minutes, before placing in soil test jars and inoculated with rot fungi. A piece of the agar-mycelium inoculum (~1 × 2 × 0.5 cm) was placed on top of the soil in the jars and the block was placed firmly on the agar on top of the soil, making sure it was in good contact with the soil and inoculum. Identical methods were used for precolonized and non-colonized control blocks. Ten wood blocks were assigned to each treatment (Table 1). Lids were replaced tightly and jars were incubated at 27˚ ± 1˚, 85% ± 5% relative humidity for 8 weeks. After eight weeks, blocks were removed from jars and the respective fungi were reisolated. Wood blocks were again dried overnight at 40˚C and weighed to the nearest 0.01 g.
Using a completely randomized ANOVA test in Statistix [18], multiple comparisons were made to determine whether significant differences in weight loss existed between sugar maple sapwood blocks decayed by a white rot fungus and sapstreak fungus, by only the sapstreak fungus, and by the white rot fungus alone.
3. Results
3.1. Incidence of Sapstreak in Maple Stands
Sugar maple stands sampled in 2009 had an average dieback value of 18.6%, with values ranging from 1.8% to 77.8% (Figure 2) which was calculated for sugar maple by estimating each crown of all sugar maple trees greater than 10.2 cm d.b.h. within the randomly selected 0.04 hectare plot within the stand. Of the 90 trees sampled for sapstreak, seven had sapwood discoloration that was characteristic of infection by the sapstreak fungus. Five additional trees had a different type of discoloration that, after further sampling, was not associated with sapstreak. Twelve trees had some type of small (≤250 cm2) logging wound or other wound on the lower boles but none of these were sapstreak positive or suspect. None of the trees had sapstreak symptoms in more than one of the three exposed areas examined. Ceratocystis virescens was only isolated from one of the seven trees with characteristic sapwood discoloration. Subsequent revisits to the 30 plots have added to the likelihood that the six symptomatic trees had sapstreak (although isolation failed), as the level of dieback had increased from the previous year and other crown symptoms persisted.
Of the 90 trees sampled at 30 sites, 7.78% ± 2.8% were considered to have sapstreak disease based on either crown symptoms and or isolation of the pathogen.
Table 1. Design of soil block decay test of sugar maple (Acer saccharum) sapwood blocks precolonized with C. virescens isolates and also T. versicolor and I. lacteus. Numbers represent replicate wood blocks exposed to fungi in soil block test.
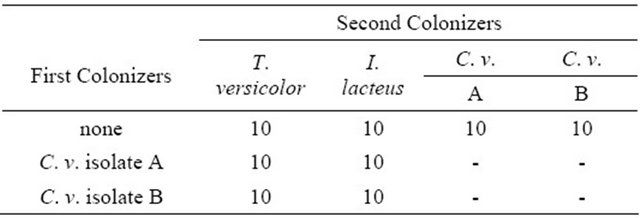
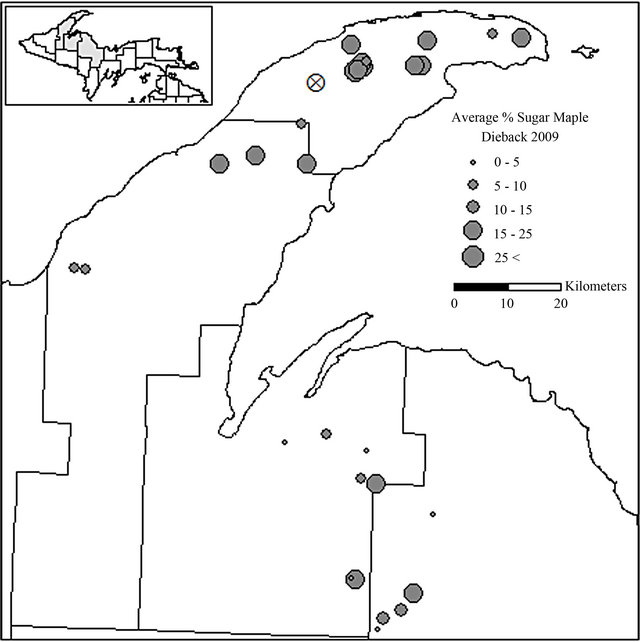
Figure 2. Locations of sugar maple dieback evaluation stands in Keweenaw, Houghton, Baraga, and Marquette Counties, MI. The mean percent of sugar maple dieback in each stand is shown: X indicates the location of the intensively sampled, recently harvested stand.
The trees considered to have sapstreak had a wide range of crown dieback values (Table 2), and none of these trees had any visible wounds near the base. Trees with lower levels of dieback were also found to have sapwood discoloration at the base of the tree, suggesting early stages of infection. Over half of the symptomatic trees had the greatest amounts of crown dieback of the three trees sampled at each plot. More intensive sampling may have found more sapstreak positive cases, but this would require whole tree removal and/or root excavation which is expensive and undesirable.
3.2. Incidence of Sapstreak in a Recently Logged Stand
At the one stand where harvesting had recently occurred around a confirmed tree with C. virescens, other sugar
Table 2. Crown dieback of sugar maple trees found with characteristic C. virescens streaking at seven locations in the western Upper Peninsula of Michigan. Letters indicate Houghton, Keweenaw, or Baraga Counties. Bold indicates characteristic streaking present. †Indicates the only lab confirmed sample.
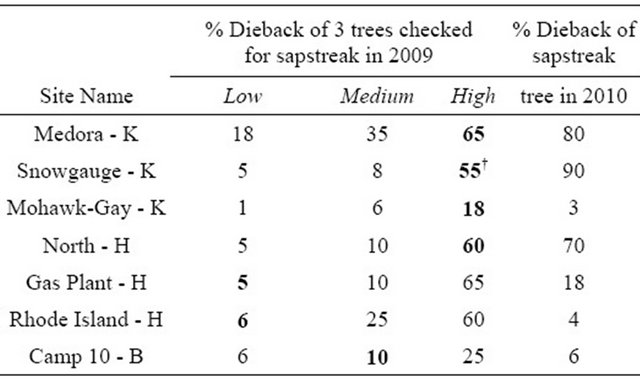
maple trees and stumps were assessed for crown condition, sapwood discoloration, and the pathogen presence. The 32 trees examined had between 5% and 99% dieback, or were standing dead trees. Nine fresh sugar maple stumps were also examined. In addition to the original C. virescens infected tree, the characteristic streaking was found on two other live trees and in the base of one cut stump. Both live trees found with sapstreak had >50% dieback, with one of them being very near the original sapstreak positive tree, and the other approximately 20 m away being surrounded by a mix of apparently healthy trees and trees with dieback. All trees with dieback were within three meters of different harvesting skid trails. Only one of the live trees was confirmed as C. virescens by laboratory isolation; however the other live tree and stump were symptomatic for sapstreak, as they had the characteristic streaking. Of the total live trees still standing, about one third had large, noticeable wounds near the base that could act as entry courts for the sapstreak fungus.
3.3. Soil Block Decay Test
Differences were evident in the growth rates on agar of the two C. virescens isolates prior to use in the soil block test, thus both isolates were tested to account for natural differences among individuals. Based on visual examination, all wood blocks treated with decay fungi appeared to be moderately to heavily colonized, and there were no apparent differences among blocks or between isolates. One individual block of the Trametes control group failed to colonize and was removed from the test.
Weight loss of maple blocks with white rot decay fungi ranged from 38.6% to 55.0% (Figure 3) with T. versicolor showing a significantly slower decay rate than I. lacteus blocks. Decay in blocks precolonized with C. virescens and T. versicolor was not significantly different from blocks colonized by only T. versicolor (p ≤ 0.05). Decay in blocks precolonized with C. virescens and I. lacteus had less decay than I. lacteus alone, and this was significantly less (p ≤ 0.05) for isolate B. The C. virescens precolonized wood blocks placed alone in soil jars also had weight losses (4.6% and 4.7%).
4. Discussion
With the large number of trees with dieback in the area, a correspondingly large amount of sapstreak might be expected if this disease was the direct cause [1]. Approximately 10% of the standing trees sampled intensively at one site in Keweenaw Co. had streaking characteristic of sapstreak although at least 80% of the trees in the area had symptoms of crown dieback. The total percentage of trees with sapstreak throughout the area is presumably
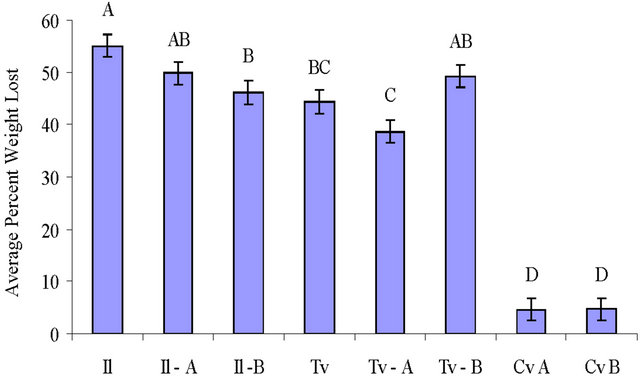
Figure 3. Mean percent weight loss of wood blocks by T. versicolor (Tv), I. lacteus (Il) and C. virescens (Cv) after 8 weeks incubation (standard deviation included, n = 10 for each). The isolate used is labeled for each treatment with the decay fungi that were precolonized before the soil block decay test and for the C. virescens treatments alone. Letters on top of the bars indicate significant differences (p ≤ 0.05) between treatments.
slightly lower as some healthy trees with no dieback were not sampled. Another study in Upper Michigan to determine the prevalence of sapstreak in maple stands found infection rates as high as 9.8%, (in 15 out of 153 trees in a selection cut area) [19]. Mielke and Charette [6] examined stands along major haul roads in Wisconsin and found 4.8% of trees infected with C. virescens, (21 out of 431 wounded sugar maples along major haul roads or skid trails) which approximated 1 tree per 0.9 hectares. In contrast, Kessler [20] only found five trees to have sapstreak in an entire seven hectare cut block examined in the Nicolet National Forest, WI. When the site in Keweenaw Co. was intensively examined and even larger pieces of bark were removed, the density of sapstreak infected trees was not found to be larger than any reported amounts in literature in recently logged areas of similar forest types.
In three of the four groups precolonized by C. virescens, weight loss from the decay fungi was reduced. In these cases, the sapstreak colonization may inhibit the activity of a decay fungus. In no cases was decay increased significantly. Variation in pathogenicity does exist between mating forms of C. virescens [10] but may also exist between individual isolates. The pH of uncolonized sugar maple wood is typically around 5.5, and sapstreak colonized wood is usually 8.5 or higher [5]. Growth of both isolates on citrate buffered agar was much more rapid than on the 2 M agar and the difference in pH likely stimulated the growth on the citrate agar. The variance in pH of live trees and the effect of wounds on pH could be further examined in regards to the pathogenicity of the sapstreak fungus.
Stain fungi, like C. virescens, feed on the sugars and other carbohydrates in wood rather than the cellulose and lignin [21,22]; thus they are not considered decay fungi. Staining alters the internal wood chemistry by utilizing wood extractives and free carbohydrates [21,22]. There is some indication that C. virescens produces volatiles that may stimulate growth of other fungi [23] and could remove sugars or solutes from the wood that may be used by decay fungi. Though many stain fungi are not typically considered pathogenic, their presence may weaken a tree sufficiently that other fungi colonize and cause decay [24,25]. Further testing with other common decay fungi of sugar maple is required before further statements can be made about the possible effect of the sapstreak fungus on the decay of sugar maple wood.
These results indicate that the role of sapstreak in wood decay and its biointeraction with other fungi is still not clear. This could partly be due to its various taxonomical name changes in literature [5,26-33] and certainly, as molecular and genetic techniques are further employed, the taxonomy of Ceratocystis and other Ophiostomatoid fungi will continue to be clarified [34]. Regardless, the question of whether sapstain fungi affect wood properties and make wood more or less susceptible to subsequent decay requires further study [24]. In regards to the sugar maple dieback in the region, it is not solely caused by sapstreak, but most likely the result of a combination of biotic and abiotic factors with sapstreak disease being among them.
5. Acknowledgements
The authors thank GMO LLC for providing funding for the field portion of this project. We also thank American Forest Management, Inc., Amy Berns, Sally Sanderson, Andrew Beebe, Eric Hollenbeck, James Klapperich and Chad Fortin for technical assistance in the field.
REFERENCES
- Michigan Department of Natural Resources and Environment, Forest Management Division, “2009 Michigan Forest Health Highlights,” 2009. http://fhm.fs.fed.us/fhh/fhh_09/mi_fhh_09.pdf
- I. Millers, D. S. Shriner and D. Rizzo, “History of Hardwood Decline in the Eastern United States,” General Technical Report NE-126, US Department of Agriculture, Forest Service, Broomall, 1989, 75 p.
- K. Kessler Jr., “Dieback of Managed, Old Growth Northern Hardwoods in Upper Michigan, 1954-1964—A Case History,” Plant Disease Reporter, Vol. 49, 1965, pp. 483-486.
- K. Kessler Jr., “Dieback Not a Cause of Mortality or Reduction of Growth or Quality in Lake States Northern Hardwoods,” Journal of Forestry, Vol. 65, No. 12, 1967, pp. 892-893.
- G. H. Hepting, “Sapstreak, a New Killing Disease of Sugar Maple,” Phytopathology, Vol. 34, 1944, pp. 1069- 1076.
- M. E. Mielke and D. A. Charette, “The Incidence of Sapstreak Disease of Sugar Maple in Menominee County, Wisconsin, and Its Relationship to Wounds and Season of Logging,” Northern Journal of Applied Forestry, Vol. 6, No. 2, 1989, pp. 65-67.
- D. R. Houston, “Importance of Buttress Root and Taphole Wounds as Infection Courts from the Sugar Maple (Acer saccharum) Sapstreak Pathogen, Ceratocystis coerulescens,” Phytopathology, Vol. 82, No. 2, 1992, p. 244.
- D. R. Houston, “Sapstreak Disease of Sugar Maple: Development over Time and Space,” Research Paper NE- 687, US Department of Agriculture, Forest Service, Radnor, 1993, 19 p.
- A. L. Shigo, “Observations on the Succession of Fungi on Hardwood Pulpwood Bolts,” Plant Disease Reporter, Vol. 46, 1962, pp. 379-380.
- T. C. Harrington, J. Steimel and G. A. Kile, “Genetic Variation in Three Ceratocystis Species with Outcrossing, Selfing and Asexual Reproductive Strategies,” European Journal of Forest Pathology, Vol. 28, No. 4, 1998, pp. 217-226. doi:10.1111/j.1439-0329.1998.tb01176.x
- D. R. Houston, “Recognizing and Managing Sapstreak Disease of Sugar Maple,” Research Paper NE-675, Department of Agriculture, Forest Service, Radnor, 1993, 11 p.
- J. H. Ohman and A. B. Spike, “Effect of Staining Caused by Sapstreak Disease on Sugar Maple Log and Lumber Values,” Research Note NC-12, US Department of Agriculture, Forest Service, St. Paul, 1966, 4 p.
- K. T. Smith, “Sapstreak Disease and Biodeterioration of Sugar Maple,” Biodeterioration Research, Vol. 3, 1990, pp. 303-310.
- US Department of Agriculture, Forest Service, “Forest Health Monitoring 1999 Field Methods Guide,” USDA Forest Service, National Forest Health Monitoring Program, Research Triangle Park, 1999.
- D. J. Goheen and F. W. Cobb, “Infestation of Ceratocystis wagneri-Infected Ponderosa Pines by Bark Beetles (Coleoptra: Scolytidae) in the Central Sierra Nevada,” The Canadian Entomologist, Vol. 112, No. 7, 1980, pp. 725-730.
- D. J. Goheen, F. W. Cobb, D. L. Wood and D. L. Rowney, “Visitation Frequencies of Some Insect Species on Ceratocystis wagneri Infected and Apparently Healthy Ponderosa Pines,” The Canadian Entomologist, Vol. 117, No. 12, 1985, pp. 1535-1543.
- American Wood-Preservers’ Association (AWPA) “Standard Method of Testing Wood Preservatives by Laboratory Soil-Block Cultures,” Standard E10-08, AWPA Book of Standards, AWPA, Birmingham, 2008.
- Statistix® 8 Analytical Software, “User’s Manual,” Tallahassee, 2003, p. 396.
- J. H. Ohman and K. J. Kessler, “Current Status of the Sapstreak Disease of Sugar Maple in the Lake States,” Research Note LS-10, US Department of Agriculture, Forest Service, St. Paul, 1963, 4 p.
- K. Kessler Jr., “Sapstreak Disease of Sugar Maple Found in Wisconsin for the First Time,” Research Note NC-140, US Department of Agriculture, Forest Service, St. Paul, 1972, 2 p.
- A. D. M. Rayner and L. Boddy, “Fungal Decomposition of Wood. Its Biology and Ecology,” John Wiley and Sons, New York, 1998.
- R. A. Zabel and J. J. Morrell, “Wood Microbiology: Decay and Its Prevention,” Harcourt Brace Jovanovich, Academic Press, Inc., New York, 1992.
- P. M. Wargo and T. C. Harrington, “Host Stress and Susceptibility,” In: C. G. Shaw and G. A. Kile, Eds., Armillaria Root Disease, US Department of Agriculture, Forest Service, Agriculture Handbook No. 691, Washington DC, 1991, pp. 88-101.
- K. A. Siefert, “Sapstain of Commercial Lumber by Species of Ophiostoma and Ceratocystis,” In: M. J. Wingfield, K. A. Seifert and J. F. Webber, Eds., Ceratocystis and Ophiostoma: Taxonomy, Ecology, and Pathogenicity, The American Phytopathological Society Press, St. Paul, 1993, pp. 141-151.
- J. N. Gibbs, “The Biology of Ophiostomatoid Fungi Causing Sapstain in Trees and Freshly Cut Logs,” In: M. J. Wingfield, K. A. Seifert and J. F. Webber, Eds., Ceratocystis and Ophiostoma: Taxonomy, Ecology, and Pathogenicity, The American Phytopathological Society Press, St. Paul, 1993, pp. 153-160.
- R. W. Davidson, “Fungi Causing Stain in Logs and Lumber in the Southern States in Including Five New Species,” Journal of Agricultural Research, Vol. 50, No. 10, 1935, pp. 589-807.
- R. W. Davidson, “Two American Hardwood Species of Endoconidiophora Described as New,” Mycologia, Vol. 36, No. 3, 1944, pp. 300-306. doi:10.2307/3754828
- B. K. Bakshi, “Fungi Associated with Ambrosia Beetles in Great Britain,” Transactions of the British Mycological Society, Vol. 33, No. 1-2, 1950, pp. 111-120. doi:10.1016/S0007-1536(50)80054-2
- B. K. Bakshi, “Studies on Four Species of Ceratocystis, with a Discussion on Fungi Causing Sap-Stain in Britain,” Mycological Papers, Vol. 35, 1951, 16 p.
- J. Hunt, “Taxonomy of the Genus Ceratocystis,” Lloydia, Vol. 19, 1956, pp. 1-59.
- T. R. Nag Raj and B. Kendrick, “A Monograph of Chalara and Allied Genera,” Wilfrid Laurier University Press, Waterloo, 1976, 200 p.
- H. P. Upadhyay, “A Monograph of Ceratocystis and Ceratocystiopsis,” University of Georgia Press, Athens, 1981, 176 p.
- G. A. Kile and J. Walker, “Chalara australis, sp. nov. (Hyphomycetes), a Vascular Pathogen of Nothofagus cunnihamii (Fagaceae) in Australia and its Relationship to Other Chalara Species,” Australian Journal of Botany, Vol. 35, No. 1, 1987, pp. 1-32. doi:10.1071/BT9870001
- M. J. Wingfield, K. A. Seifert and J. F. Webber, “Ceratocystis and Ophiostoma: Taxonomy, Ecology, and Pathogenicity,” The American Phytopathological Society Press, St. Paul, 1993.
NOTES
*Special Issue on Biointeractions and Plant Health.
#Corresponding author.

