International Journal of Otolaryngology and Head & Neck Surgery
Vol.3 No.2(2014), Article ID:44272,7 pages DOI:10.4236/ijohns.2014.32021
Is Meniere Disease Caused by a Pathological Immune Response?
Ziane Selmani1, Ilmari Pyykkö2, Nureddin Ashammakhi3
1Department of Otolaryngology, Helsinki University Hospital, Helsinki, Finland
2Department of Otolaryngology, Tampere University Hospital, Tampere, Finland
3Department of Surgery, Oulu University Hospital, Oulu, Finland
Email: ziane_selmani@hotmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 29 January 2014; revised 18 February 2014; accepted 15 March 2014
ABSTRACT
The etiology of Meniere disease (MD) is unknown. Among the several factors which can provoke the disease is a pathological immune response. Objective: To investigate whether MD is due to a pathological immune reaction. Materials and methods: Immunological assay (IA) was evaluated in a consecutive study on 159 patients with MD (mean age 47.8. years) and the results compared with those from 26 patients operated on because of vestibular schwannoma (VS, mean age 54.1 years), who served as a control group. In cases of MD, transtympanic electrocochleography (ECoG) and hearing threshold were measured. Results: The average hearing level (HL) in the affected ears of patients with MD was 30 dB. Evidence of abnormal plasma protein pattern was found in 127 MD patients (80%). Elevations were found in β1-globulin (54.5%), β2-globulin (26.5%), (2-globulin (34.3%), (-globulin (17.3%), complement (CH100, 36.4%) and antinuclear antibodies (ANA, 43.4%). The onset of the disease did not correlate with the level of the plasma protein neither with the level of IgG titers. Conclusion: Elevated certain plasma proteins in patients with Meniere’s disease could be a sign that Meniere’s disease is a consequence of pathological immune reaction.
Keywords:Circulating Immune Complex (Circulating IC); Electrocochleography (ECoG)

1. Introduction
Autoimmunity has been suggested to cause a Meniere disease (MD) [1] . The immune reaction can damage the inner ear structures by involving sensitized lymphocytes and circulating antibodies. In addition, these are probably associated with different structures of the inner ear such as the cochlear and vestibular nerve as the inner ear is in an immunologically protected environment. This may result in a prolonged immune response with recurrent fluctuating hearing loss, attacks of vertigo and tinnitus, as first described by McCabe [2] . An immune response may also arise in the vicinity of the endolymphatic sac, which can alter the function of the sac and may later result in derangement of homeostasis of inner ear fluid [3] .
The present study examined whether subjects with MD have pathological immune reaction related to the elevation of antibodies. A group of patients with HL due to VS was used as a control group.
2. Subjects and Methods
2.1. Subjects
One hundred and fifty-nine patients (range 22 - 81 years, mean 47.8 years) were chosen for this consecutive study. They were diagnosed as having MD (102 right-sided, 53 left-sided and 4 bilateral) on the basis of American Academy of Otolaryngology—Head and Neck foundation (AAO-HNS 1995) criteria [4] . The onset of MD disease varied from 2 to 6 years.
The control group was composed of 26 patients (range 30 - 72 years, mean 54.1 years) having a sudden or progressive sensorineural hearing loss at high frequency. The diagnosis of VS was established by performing an MRI imaging. The patients underwent subsequently a tumor removal.
The protocol regarding admission of patients into the study was accepted by the local Ethics Committee of the University Hospital of Helsinki.
2.2. Methods
The basic immune assay in our study was the titration of status measurements of immunoglobulins A, G and M, complement components C3 and C4, rheumatoid factors (RF) and antinuclear antibody (ANA).
The immunoglobulins IgA, IgG and IgM were evaluated by immunoturbidimetric technique. Complement function was measured by gel-hemolysis assay according to Meri [5] and antinuclear antibody (ANA) was measured by immunofluoresecence [6] . Serum proteins were analyzed by total protein assay and electrophoresis on cellulose-acetate membrane resulting in percentage values for albumin, a1, a2, β1, β2 and g globulin. The complements were analyzed by the immunoturbometry method. The antibodies to nuclear antigens (ANA) were analyzed by indirect immuno-fluorescence techniques, CRP was analyzed by the immunoturbidimetric, and rheumatoid factor was tested by latex agglutination test and anti-streptolysinby sheep red cell hemolysis assay [7] . Direct test of Coombs was performed on patients’ red cells by agglutination with polyvalent anti-serum-immunoglobulin and anti-serum complement (C3b/C3d) [8] . Since cold agglutinin can cause red blood cells to clump only at temperature lower than 37˚C, the test was performed by cooling the tube to where the blood of the patient was collected in ice water for 30 - 60 seconds, and evaluated for clumped red blood cells with a microscope.
All the patients underwent conventional clinical audiometry conducted in a soundproof room, and transtympanic electrocochleography (ECoG) under microscopic visualisation. Details of audiometry and ECoG recording techniques were published elsewhere [9] .
2.3. Statistical Methods
Correlation between HL, EH, symptoms of MD, and circulating IC and antiviral titers were examined by using Spearman’s matrix. Elevated titers of antibodies against neuroviruses in patients with MD were compared with those of controls by means of analysis of variance (ANOVA). Findings were considered to be statistically significant when p was less than 0.05.
3. Results
Average HL in the speech area in the affected ear, or the worst ear in bilaterally affected patients, was 30 dB (+−20.1 dB) and in the unaffected side or less affected side, 16 dB (+−9.4 dB) in patients with MD.
In MD, ECoG assessed an EH (SP/AP ratio > 0.35) in 54 percent of patients, 30 percent were having SP/AP < 0.35 and in 16 percent, the trace of ECoG was not clear to permit an adequate evaluation of SP/AP result (SP and AP trace not clear) (Figure 1).
Immune assay was only performed in patients with MD and according to normal limits on immunoassay (Table 1), one hundred and twenty-seven patients (80%) showed evidence of abnormal immunity status (Table 2). Pathological occurrence in the immunological assay was observed in 34.3% of the patients as regards a2-globulin, 54.5% (β1-globulin), 26.5% (β2-globulin), 17.3% (g-globulin), 14.1% (IgM), 13.1% (IgA), 36.4%
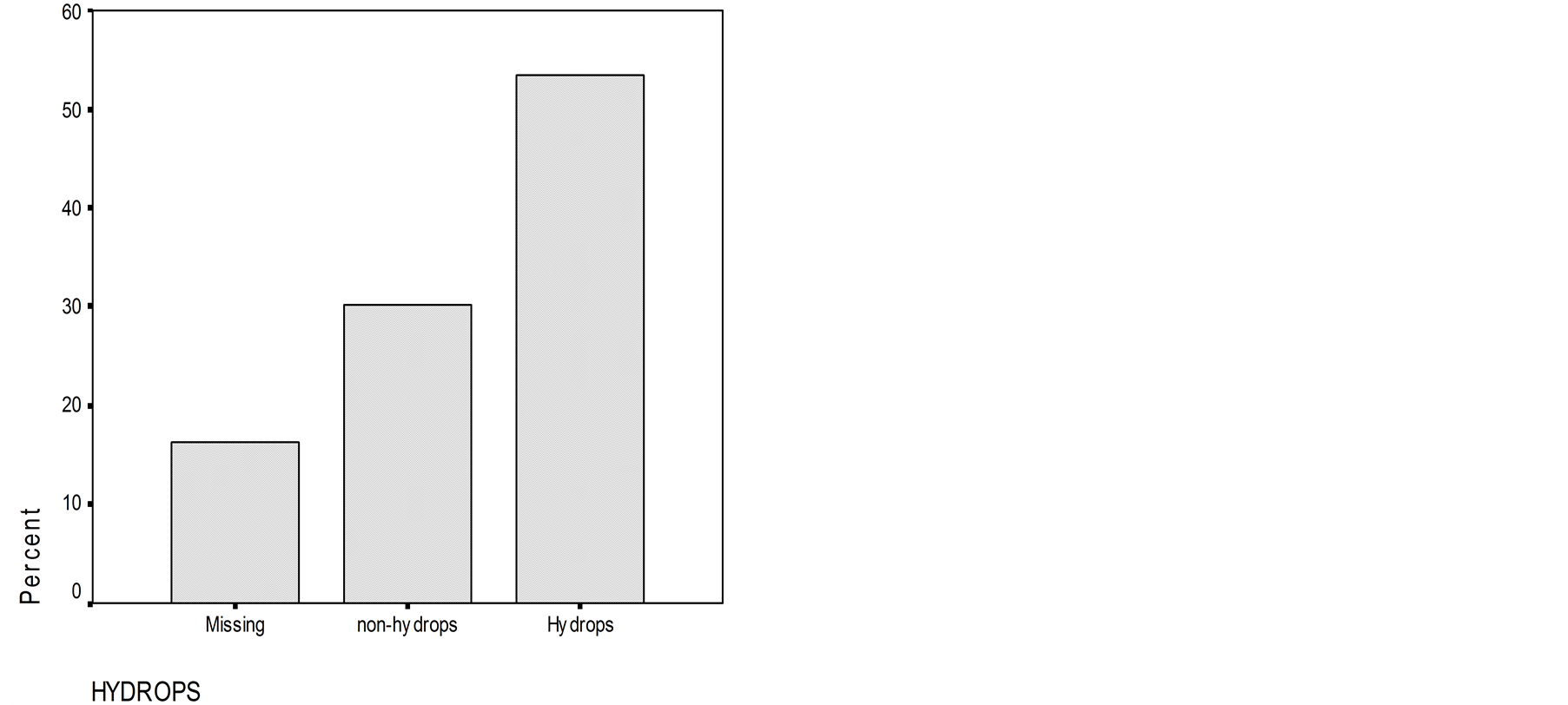
Figure 1. Number of patients with endolymphatic hydrops and without endolymphatic hydrops assessed by electrocochleography. Number of patients without clear trace of electrocochleography are also presented.
Table 1. Normal limits of immunoassay titers.
Table 2. Abnormality rates in immunological assays results in patients with Meniere disease (N = 159).
(CH100), and 43.4% (ANA). The β1-globulin was the only abnormality which correlated significantly with hearing threshold. When the level of β1-globulin was low, hearing threshold was moderate-to-severe or severe and when the level of β1-globulin was high the level of hearing loss was mild-to moderate (Figure 2).
Levels of β1-globulin titer was low in patients with EH (high SP/AP ratio) and high in patients without EH (normal SP/AP ratio) when hearing threshold was severe. β1-globulin titer was almost the same in patients with EH and without EH when the hearing threshold was mild-to-moderate and from moderate-to-severe (Figure 3). There was a difference in the level of the β1-globulin titer in patients with and without EH when hearing threshold was severe was statistically significant (p < 0.05).
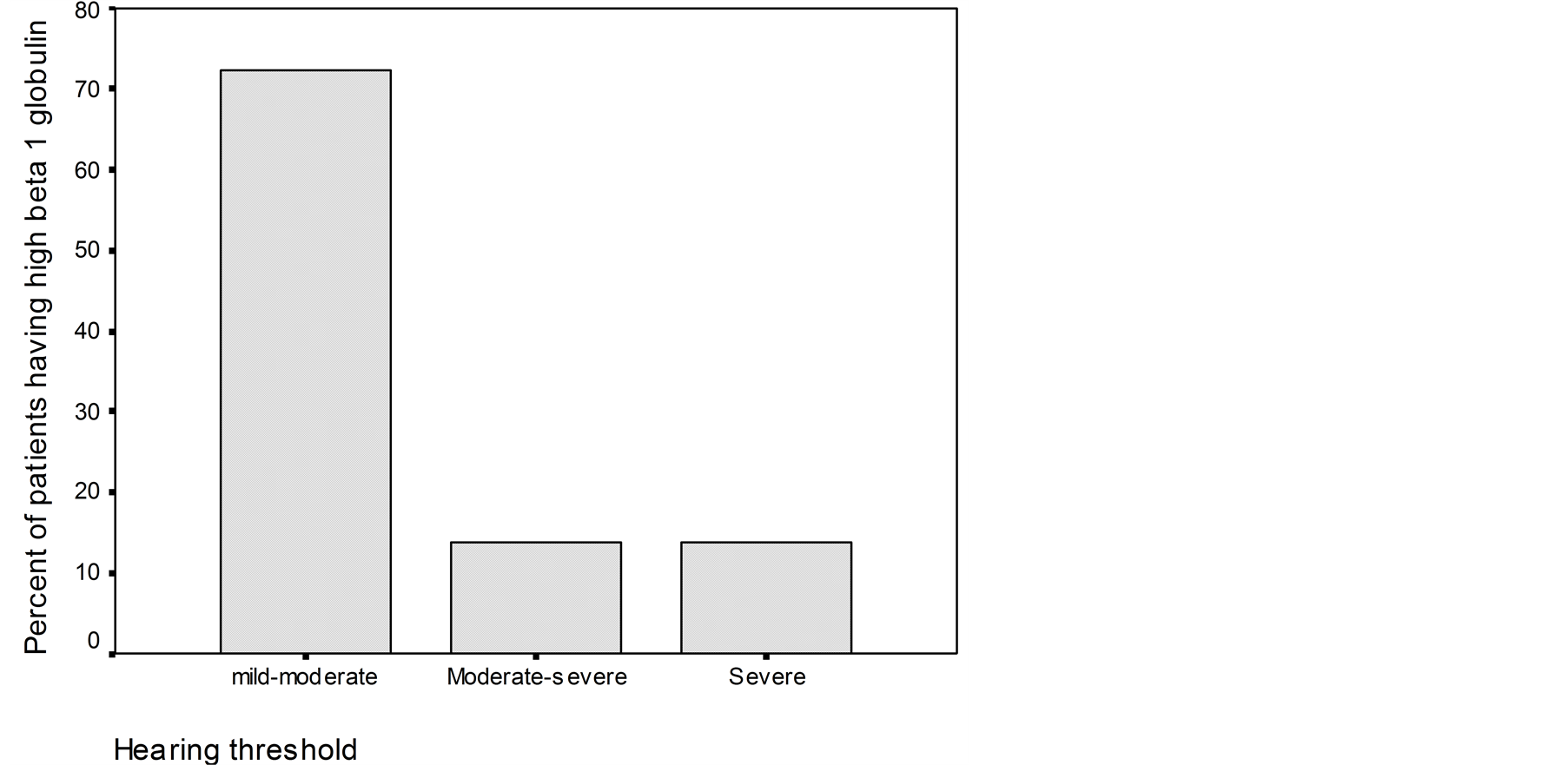
Figure 2. Percent of pathological levels of b1-globulin titers corresponding to different levels of hearing threshold in patients with Meniere disease.
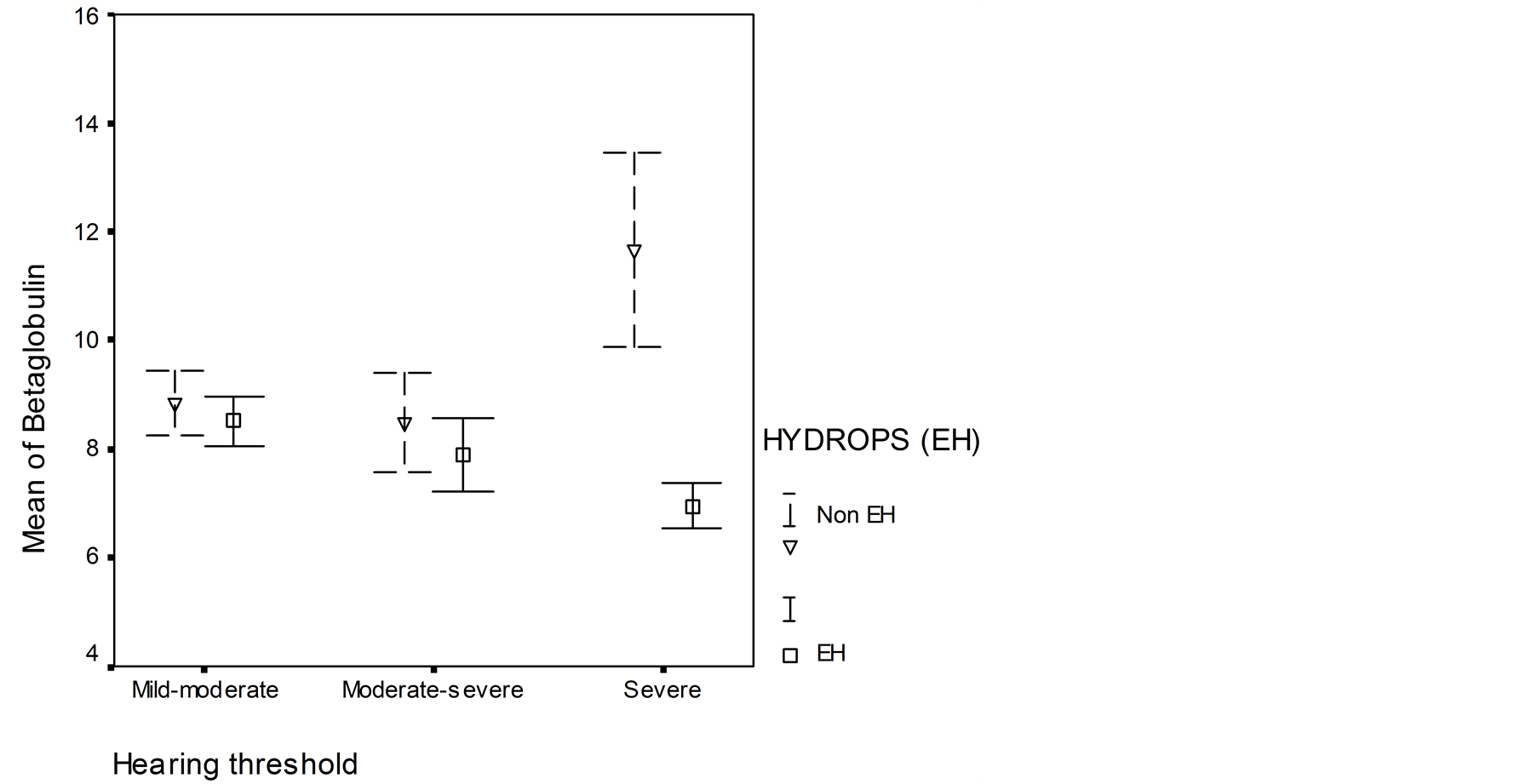
Figure 3. Relationship between b1-globulin and hearing threshold in patients with endolymphatic hydrops (summating potential/action potential ratio (SP/AP ratio > 30%)) and in patients without EH (normal SP/AP ratio). Mean ± errors of mean values are shown.
4. Discussion
Inner ear immune reactions may play a significant role in various types of inner ear disorders, such as progressive bilateral sensorineural hearing loss [10] , MD [11] and delayed EH [12] . Derebery et al. (1991) demonstrated significantly elevated levels of CI complexes among 30 patients with MD when compared with 20 control subjects [13] . Brookes et al. (1985) found immune complexes in 55% of patients with MD and in only 3% of control subjects [14] . Dornhoffer et al. (1993) showed that IgG deposits could be detected in the endolymphatic sac (ES) of 40% of patients undergoing shunt surgery [15] . Savastano et al. (2006) has demonstrated that autoimmune response may be a pathogenetic factor in MD occurrence by affecting the endolymphatic sac [16] . They found in more than half of the serum of the sample group of MD patients the presence of high value of CI complexes presented by an increase level of T-helper compared to cytotoxic cells, with an increase in the CD4/CD8 ratio. Whereas the level of the rest on of immune level like erythrocyte sub-population, cryoglobuline level, the complement level, the lymphocyte sub-populations, the cytoglobulines, the CI complexes , organ and non-organ autoantibodies, level if IgG, Erythrocyte sedimentation rate, C-reactive protein, rheumatoid factor, complement fractions and cryoglobuline were either negative or non-significant in the considered cohort of MD suffering patients.
In our study, elevation of β1-globulin levels was new finding which was not reported before. It was found in more than half cases (54.5%). Furthermore, β1-globulin was the only abnormality which correlated significantly with hearing loss at all frequencies and with the SP/AP ratio. The β1-globulin fraction in electrophoresis contains products of several different proteins. Usually the β1-globulin level is increased during iron-deficiency anemia, pregnancy and estrogen therapy [17] . An increase in the β1-globulin level may also be caused by breakdown of complement C3, in which C3c migrates in the β1 band and causes an increase in its intensity [17] . Other known components in the β1 band are cold insoluble proteins, but their role in MD is unknown. In another hand, it has been reported that some women with MD can experience a worsening of MD symptoms during pregnancy [18] has revealed a possible nuance between MD and pregnancy.
As the etiology of MD is still unknown, this can motivated researcher to make further studies in attempt to find news causative agents in MD. This demonstrates that the etiology of MD is more complex and obscure by the fact that new causative agents are revealed continuously. In some other studies, MD was associated to particular human leucocyte antigen haplotypes like thyroid autoimmunity [19] . Other studies have incriminated the effect of inhalant and food allergy to MD [20] .
In animal experiments, Tomiyama et al. (16) demonstrated that severe inflammatory cellular infiltration occurred in the sac from 8 h to day 3 after a secondary keyhole limpet hemocyanin (KLH) challenge to the sac. These authors demonstrated that increased vascular permeability occurred on day 2 after the secondary KLH challenge. Histology revealed significant staining of IgG and C3 on the sensory epithelium of the vestibule. An increase in hearing threshold was confirmed by ECoG. The hearing threshold was significantly increased in association with elevation of perilymphatic antibody levels in the early phase of the secondary immune reaction in the ES, indicating that the elevation of hearing threshold was dependent on the intensity of a type III immune reaction. Consequently, an elevation of perilymph antibody levels in the early phase suggests that there is a large quantity of harmful immune products, enough to damage the inner ear sensory organs, and that elevation of CI complexes is linked to the immune response [21] .
There was no statistical difference of IgM antibodies between MD patients with and patients without EH. This was related to the cases of our study group patients who were mainly old cases of MD. Eventually, we considered that the acute phase of antibodies reaction during which the IgM antibodies manifestation is involved has subsided.
The weakness of our study is that the majority of cases of MD patients were old cases. This study would be more relevant if the number of news cases was important to find out objectively about what is happening at the level of the immune system during the acute phase of MD manifestation, what kind and what most immune system factors are incriminated during this phase. It is seldom that new cases of MD patients are addressed immediately to ENT clinic, unfortunately most cases of MD are treated by ordinary and classic therapy at the first health care sector where the diagnosis of MD is established on the basis of clinical symptomatology and no advanced investigations was performed. Hence the cases of MD at ENT clinics are mostly old cases with severe symptomatology in which the classical MD therapy did not have effect or cases addressed for further investigations to confirm MD.
In conclusion, MD is caused by a complex pathological immune reaction. During the early phase of the immune reaction, one can assume to find high levels of CI complexes, and low hearing threshold level and SP/AP ratio. Later, when the immunological process is subsiding, there comes a worsening of hearing threshold with normalization of EH whereas circulating ICs will be reduced at that time.
References
- Derebery, M.J., Rao, V.S., Siglock, T.J., Linthicum, F.H. and Nelson, R.A. (1991) Menière’s Disease. An Immune Complex-Mediated Illness? Laryngoscope, 101, 225-229.
- McCabe, B.F. (1979) Autoimmune Sensorineural Hearing Loss. Annals of Otology, Rhinology, and Laryngology, 88, 585-589.
- Harris, J.P. (1983) Immunology of the Inner Ear. Otolaryngology, Head and Neck Surgery, 91, 17-23.
- (1995) Committee on Hearing and Equilibrium Guidelines for the Diagnosis and Evaluation of Therapy in Ménierè’s Disease. American Academy of Otolaryngology—Head and Neck Foundation, Inc. Otolaryngology—Head and Neck Surgery, 113, 181-185.
- Meri, S. (1987) Complement Activation. Demonstration of C3 Conversion and Detection of Complement Activators. Thesis. University of Helsinki, Helsinki.
- Tan, E.M. (1982) Antibodies to Nuclear Antigens (ANA): Their Immunology and Medicine. Advances in Immunology, 33, 167-240. http://dx.doi.org/10.1016/S0065-2776(08)60836-6
- Cabau, N. and Badin, J. (1965) Dosage spécifique de l’antistreptolysine o élimination des inhibiteurs non-spécifiques de la streptolysine o par l’heparine en présence de calcium. Clinica Chimica Acta, 12, 508-514. http://dx.doi.org/10.1016/0009-8981(65)90165-8
- Petz, L.D. (1986) Autoimmune and Drug-Induced Immune Hemolytic Anemia. In: Rose, N.R., Friedman, H. and Fahey, J.L., Manual of Clinical Laboratory Immunology, American Society for Microbiology, Washington DC, 613-629.
- Selmani, Z., Pyykkö, I., Ishizaki, H. and Ashammakhi, N. (2002) Use of Electrocochleography in Assessing Endolymphatic Hydrops in Patients with Lyme Disease and Meniere Disease. Acta Oto-Laryngologica (Stockholm, Sweden), 122, 173-178. http://dx.doi.org/10.1080/00016480252814180
- Moscicki, R.A., San Martin, J.E., Quintero, C.H., Rauch, S.D., Nadol Jr., J.B. and Bloch, K.J. (1994) Serum Antibody to Inner Ear Proteins in Patients with Progressive Hearing Loss. Correlation with Disease Activity and Response to Corticosteroid Treatment. The Journal of the American Medical Association, 272, 611-616. http://dx.doi.org/10.1001/jama.1994.03520080053043
- Gottschlich, S., Billings, P.B., Keithley, E.M., Weisman, M.H. and Harris, J.P. (1995) Assessment of Serum Antibodies in Patients with Rapidly Progressive Sensorineural Hearing Loss and Meniere Disease. Laryngoscope, 105, 1347-1352. http://dx.doi.org/10.1288/00005537-199512000-00016
- Harris, J.P. and Aframian, D. (1994) Role of Autoimmunity in Contralateral Delayed Endolymphatic Hydrops. American Journal of Otolaryngology. 15, 710-716.
- Derebery, M.J., Rao, V.S., Siglock, T.J., Linthicum, F.H. and Nelson, R.A. (1991) Meniere’s Disease; an Immune Complex-Mediated Illness? Laryngoscope, 101, 225-229.
- Brookes, G.B., Morrison, A.W. and Richard, R. (1985) Unilateral Meniere’s Disease: Is the the Contralateral Ear Normal? American Journal on Otolaryngology, 6, 495-499.
- Dornhoffer, J.L., Waner, M., Arenberg, I.K. and Montague, D. (1993) Immunoperoxidase Study of the Endolymphatic Sac in Meniere Disease. Laryngoscope, 103, 1027-1034. http://dx.doi.org/10.1288/00005537-199309000-00014
- Savastano, M., Giacomelli, L. and Marioni, G. (2007) Non-Specific Immunological Determinations in Meniere’s Disease: Any Role in Clinical Practice? European Archives of Oto-Rhino-Laryngology, 264, 15-19. http://dx.doi.org/10.1007/s00405-006-0147-2
- Whicher, J.T. (1980) The Interpretation of Electrophoresis. British Journal of Hospital Medicine, 24, 348, 351, 353- 356.
- Uchide, K., Suzuki, N., Takiguchi, T., Terada, S. and Inoue, M. (1997) The Possible Effect of Pregnancy on Meniere Disease. ORL: Journal for Oto-Rhino-Laryngology, 59, 292-295. http://dx.doi.org/10.1159/000276956
- Fattori, B., Nacci, A., Dardano, A., Dallan, I., Grosso, M., Traino, C., Mancini, V., Ursino, F. and Monzani, F. (2008) Possible Association between Thyroid Autoimmunity and Menière’s Disease. Clinical & Experimental Immunology, 152, 28-32. http://dx.doi.org/10.1111/j.1365-2249.2008.03595.x
- Derebery, M.J. and Berliner, K.I. (2007) Allergy and Meniere Disease. Current Allergy and Asthma Reports, 6, 451-456. http://dx.doi.org/10.1007/s11882-007-0069-0
- Tomiyama, S., Nonaka, M., Gotoh, Y., Ikezono, T. and Yagi, T. (1994) Immunological Approach to Meniere Disease: Vestibular Immune Injury Following Immune Reaction of the Endolymphatic Sac. ORL: Journal for Oto-RhinoLaryngology, 56, 11-18. http://dx.doi.org/10.1159/000276601


