Advances in Microbiology
Vol.3 No.3(2013), Article ID:33884,5 pages DOI:10.4236/aim.2013.33042
Upgrading the Fermentation Process of Zhejiang Rosy Vinegar by Purebred Microorganisms
1College of Food Science and Biotechnology, Zhejiang Gongshang University, Hangzhou, China
2Food Safety Key Laboratory of Zhejiang Province, Hangzhou, China
Email: *yup9202@yahoo.com.cn
Copyright © 2013 Yujian Jiang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 8, 2013; revised April 8, 2013; accepted May 8, 2013
Keywords: Zhejiang Rosy Vinegar; Purebred Microorganisms; Fermentation Process; Organic Acids; Volatile Components
ABSTRACT
Purebred microorganisms were employed to upgrade the fermentation process of Zhejiang rosy vinegar. The fermentation cycle was greatly shorten from 5 months to 72 d. The transformation rate of raw materials was increased from 1:4.5 in the traditional fermentation to 1:5 or more in the upgraded fermentation. The content of organic acids in the traditional vinegar (TRV), the upgraded vinegar (UPV) and the submerged fermentation vinegar (SFV) were also investigated by HPLC. No significant difference was found regarding the proportion of phenylethanol to the total volatile components in UPV (7.47% ± 0.00324%) and TRV (7.23% ± 0.00329%), but it was significantly higher than that in SFV (2.26% ± 0.00143%). This study provides deep insight into upgrading the fermentation process of Zhejiang rosy vinegar by purebred microorganisms.
1. Introduction
Vinegar is a solution of acetic acid, mainly prepared by a three-stage fermentation process using rice or the malted barley as raw material [1]: the degradation of starch from the raw material to fermentable sugars by mould, the conversion of fermentable sugars to ethanol by yeast, and the oxidation of ethanol to acetic acid by bacteria [2]. Rosy vinegar is very popular in the Southeast area of China for its special color and flavor. The traditional fermentation process of the rosy vinegar is shown in Figure 1. Starch from rice was fermented by wild microorganisms naturally falling into the fermentation container during fermentation. Because this is a natural process, the quality of the rosy vinegar was greatly influenced by different environmental factors, and hence the fermentation process has to be undergone in some particular seasons [3]. This also means that the quality of the rosy vinegar is not stable and the fermentation cycle is prolonged. Therefore, an increasing attention has been given to upgrade the traditional fermentation process of Zhejiang rosy vinegar in recent ten years.
Endogenous enzymes, bacteria, yeast and mould contribute to the preparation of a great variety of products in the traditional fermentation [4]. In order to upgrade the fermentation process of Zhejiang rosy vinegar, purebred microorganisms were employed and fermentation parameters were optimized to further promote the growth of these microorganisms. The typical characters of the traditional vinegar (TRV) and the upgraded one (UPV) were also compared.
2. Materials and Methods
2.1. Microorganisms and Reagents
Two amylase-producing strains (Aspergillus niger AS3.4309 and Rhizopus) that can convert rice starch to fermentative sugars were purchased from the Shanghai Traditional Fermentation Factory, China. Saccharomyces cerevisiae K (providing alcohol dehydrogenase), Saccharomyces AS2.202 and Saccharomyces AS2.1182 (providing esterases for wine fragrance-strengthening) were used at the fermentation stage of alcohol and purchased from the Shanghai Traditional Fermentation Factory, China. Acetobacter AB3 that can convert alcohol to acetic acid was isolated from the leavening from the traditional process and named the acetic acid bacterium.
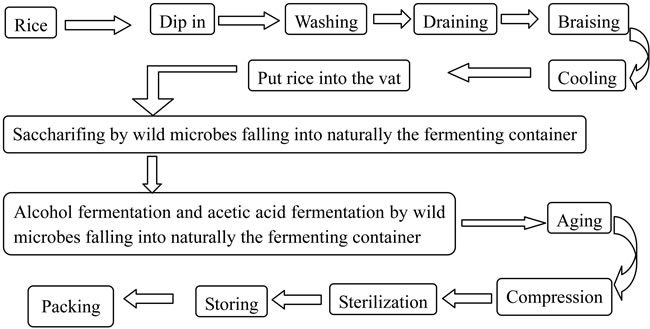
Figure 1. The traditional fermentation technology of Zhejiang rosy vinegar.
The seeds of these microorganisms were cultured in the appropriate cultural media as described by Haruta et al. [5] and kept in our laboratory until use.
Glucose, sodium hydroxide, methylene blue, sodium potassium titrate, lactic acid, acetic acid, oxalic acid, tartaric acid, formic acid, malic acid, citric acid, succinic acid, propionic acid and ammonium phosphate were purchased from the Hangzhou Huipu Chemical Co. Ltd., China. All other chemicals are analytical grade and used as the routine method.
2.2. Purebred Microbial Fermentation
The following materials were adequately mixed in a 500 L vat: the steamed rice (approximate 50% of the moisture content), 200 kg; Asp. niger AS3.4309, 7 kg and Rhizopus, 9 kg. The inlet of vat was covered with the grass cover. After cultivation for 7 d, the vat was filled with the sterilized water. 25 L of the mixture including S. cerevisiae K, Saccharomyces AS2.202 and AS2.1182 with the ratio of 8:1:1 was added to the fermentation liquid. The fermentation temperature was controlled at 30˚C. After 12 d, 30 L of Acetobacter AB3 was added to the fermentation liquid.
2.3. Evaluation of the Color Degree
A quantitative method for the evaluation of the color degree of the rosy vinegar has been established as follows: the sample was diluted by 2-fold and then centrifuged at 5000 rpm for 30 min, and then the supernatant was used to measure the optical absorbance values at 460, 510, 530 and 610 nm. The values of OD460/OD610, OD510/OD610, OD530/OD610 and OD610 × 20000/0.076 were defined as the yellow index, the red index, the rosy index and the color ratio, respectively [6].
2.4. Organic Acids
The content of organic acids in both the upgraded rosy vinegar sample and the traditional one was determined by Agilent 1100 HPLC with UV detector (210 nm) and a non-polar column Zorbax 300SB-C18 μm × 4.6 mm × 250 mm. 5 g/L (NH4)2HPO4 (pH = 2.90) was used as liquid phase. The column temperature was 30˚C, the flow of liquid phase was 0.8 mL/min and the inject volume was 10 μL [7].
2.5. Volatile Components
The HS-SPME analysis was performed with the Agilent 7890 gas chromatograph coupled with a 5975 mass-selective detector to analyze the volatile components in the vinegar. 3 mL of the vinegar sample was transferred by a calibrand syringe to a 10 mL glass vial and add 2 g of NaCl to obtain volatile components, and then the vial was capped with a PTFE-faced silicone septum and shaken by a magnetic stirrer to obtain a homogeneous mixture. The sample was maintained at 50˚C and the fiber was inserted through the vial septum and exposed to the sample headspace for 40 min to perform the extraction. The fiber was inserted into a GC injector for 3 min for adsorption [8,9].
The separation was performed using an innowax capillary colum (J&W Scientific, Folsom, CA, USA), 30 m × 0.25 mm, with a 0.25 μm coating. The splitless injection mold was chosen and the injector temperature was set as 240˚C. Ultrahigh-purity helium was used as the carrier gas at the flow rate of 1.1 mL/min. The oven temperature was programmed from 40˚C (maintained for 20 min) to 100˚C at a rate of 5 ˚C/min and finally raised to 210˚C at 3˚C/min. The final electron beam impact spectra were recorded in a 30 - 450 amu range at 70 eV ionization energy [10,11].
2.6. Statistics Analysis
The statistics analysis of data was carried out using the Origin 8.0 Software. A “p < 0.05” was considered to be statistically significant.
3. Results and Discussion
3.1. Changes in the Parameters during the Fermentation Processes
The changes in the alcohol degree and the glucose concentration at alcohol fermentation stage are shown in Figure 2(a). The lag phenomenon of the alcohol fermentation was observed and the glucose concentration decreased to 0.1 g/100 mL and maintained at a constant level until the fermentation finished. The alcohol degree was observed to increase continuously. The changes in the total organic acids and the alcohol degree at acetic acid fermentation stage are shown in Figures 2(b), respectively. The total organic acids and the alcohol degree increased simultaneously from 5 to 8 d. The fermentation cycle was greatly shorten from 5 months using the traditional fermentation technology to 72 d using the
 (a)
(a)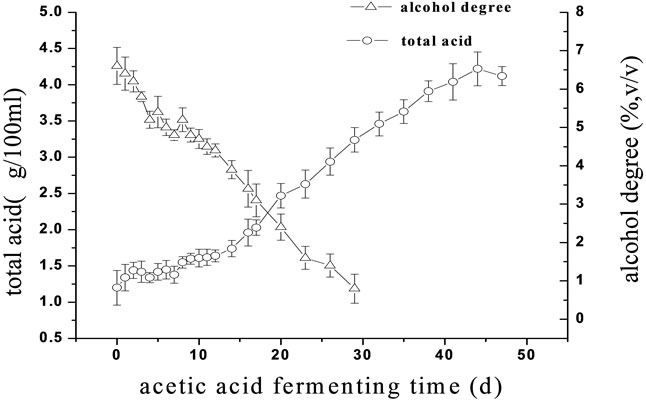 (b)
(b)
Figure 2. The changes in some parameters in the fermentation processes of the traditional rosy vinegar and the upgraded one. (a) Changes in the glucose concentration and the alcohol degree at the alcohol fermentation stage; (b) Changes in the total organic acids and the alcohol degree at the acetic acid stage.
pure microbial fermentation technology. Furthermore, a much higher transformation rate of raw materials (1:5.5 or more) could be achieved using the pure microbial fermentation technology. Thus, the rosy vinegar can be produced several times throughout a whole year compared to the traditional technology that it was only produced in some particular seasons.
3.2. Color Degree
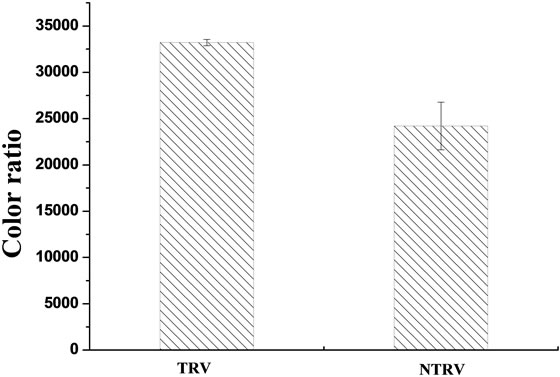
The difference of the color degree between the traditional rosy vinegar and the upgraded one was investigated and the results are shown in Figures 3(a) and (b). Compared to the traditional rosy vinegar, the upgraded one had much higher yellow index, red index, rosy index and lower color ratio. A t-test was performed to check the difference among them. As shown in Table 1, a signifycance deference was observed (p < 0.05).
3.3. Organic Acids
The type and content of organic acids in TRV, UPV and
 (a)
(a)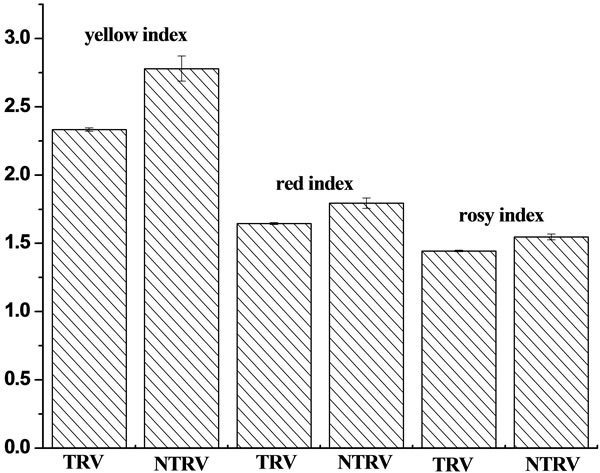 (b)
(b)
Figure 3. Evaluation of the color degree between the traditional rosy vinegar and the upgraded one.
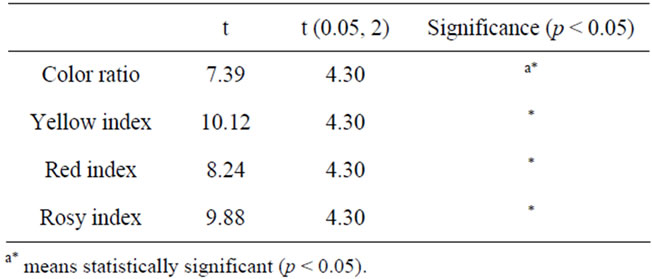
Table 1. t-Test of the color degree.
SFV were studied by HPLC and the result is shown in Table 2. Organic acids including oxalic acid, tartaric acid, formic acid, malic acid, lactic acid, citric acid, succinic acid, propanoic acid and acetic acid, could be detected in TRV and UPV, whereas only eight of them could be found in SFV. Compared to SFV, the other two vinegars had a higher content of organic acids, but no significant difference among them was found (p > 0.05). A much higher proportion of organic acids (except for acetic acid) to the total ones were observed in UPV (27.48% ± 0.027%) or TRV (31.89% ± 0.050%) than in SFV (2.09% ± 0.002%). This means that UPV or TRV has a much more harmonious taste than SFV.
3.4. Volatile Components
The volatile components in TRV, UPV and SFV were studied by HS-GC-MS and the result is shown in Table 3. Compared to SFV, the major components of volatile flavors in TRV and UPV were identified as alcohols, acids, aldehydes and ketones. However, no significant difference in the categories of volatile components were found in the two vinegars. Phenylethanol that can endows the
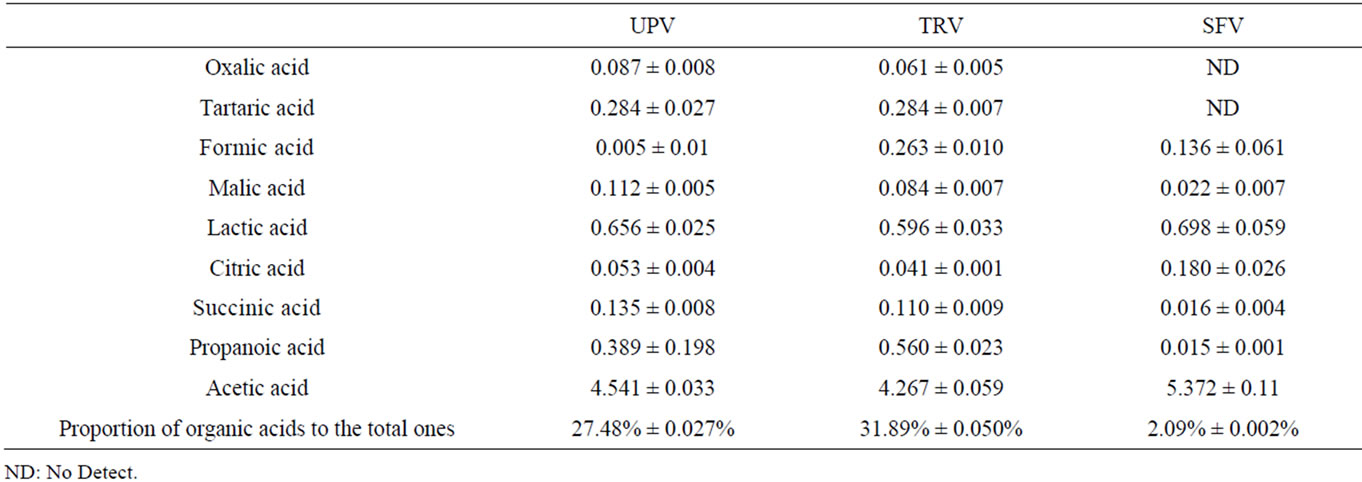
Table 2. The content of organic acids in UPV, TRV and SFV (g/100 mL).
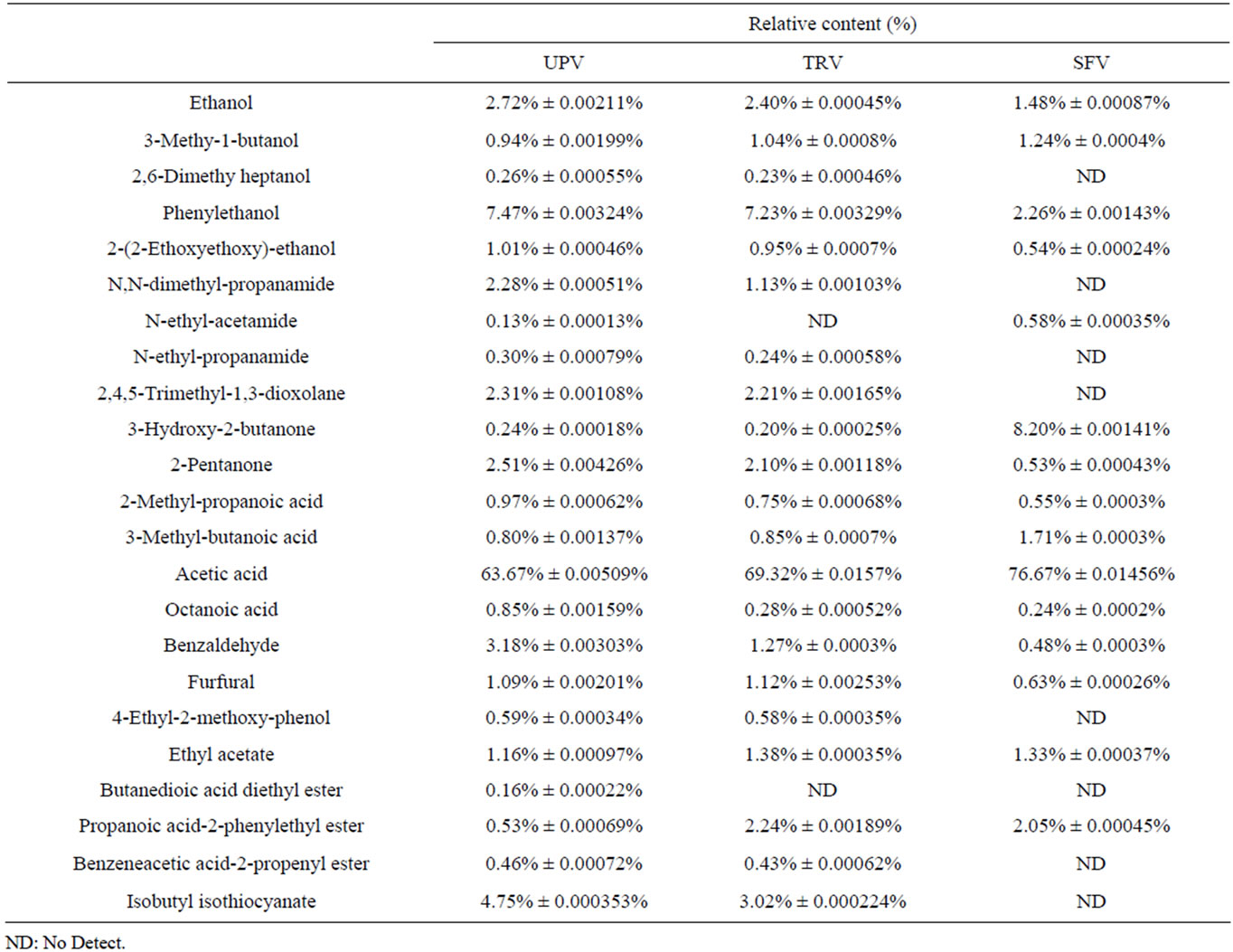
Table 3. Volatile components in UPV, TRV and SFV.
vinegar with a pleasure fragrance should be specially concerned. It contributes greatly to the vinegar flavor because of the relatively lower threshold value (1000 ppb in water) [12]. No great difference could be found about the proportion of phenylethanol to the total volatile components between UPV (7.47% ± 0.00324%) and TRV (7.23% ± 0.00329%), but they were significantly higher than that in SFV (2.26% ± 0.00143%). The great difference could be observed on the proportion of acetic acid to the total volatile components among UPV (63.67% ± 0.00509%), TRV (69.32% ± 0.0157%) and SFV (76.67% ± 0.01456%) and the proportion of acetic acid to the total volatile components in UPV was much lower than that in TRV and SFV. This means a much less pungent savor from UPV than those from TRV or SFV.
4. Conclusion
The results of the present study demonstrate that it can be achieved to produce Zhejiang rosy vinegar by the pure microbial fermentation technology. With this technology, the fermentation cycle was reduced from 5 months using the traditional fermentation to 72 d, and could gain a much higher transformation rate of raw materials (1:5 or more). There was some difference on the color degree between TRV and UPV, but no significant difference on the content of organic acids and volatile components which contribute greatly to the flavor of vinegar was observed. This study provides deep insight into the largescale industrial production of Zhejiang rosy vinegar by pure microbial strains.
5. Acknowledgements
Authors thank Zhengyi Qian and Linan Zhou for their help in providing raw materials for this study. We also thank Zhijiang Zhang, Wei Feng and Yue Liu for useful suggestions about experiments. This work was supported by the Zhejiang Province Science and Technology Committee (No. 2006C32013).
REFERENCES
- L. Giordano, R. Calabrese, E. Davoli and D. Rotilia, “Quantitive Analysis of 2-Furfural and 5-Methylfurfural in Different Italian Vinegar by Headspace Solid-Phase Microextraction Coupled to Gas Chromatography-Mass Spectrometry Using Isotope Dilution,” Journal of Chromatography A, Vol. 1017, No. 1-2, 2003, pp. 141-149. doi:10.1016/j.chroma.2003.08.029
- W. Tesfaye, M. L. Morales, M. C. Garca-Parrilla and A. M. Troncoso, “Wine Vinegar: Technology, Authenticity and Quality Evaluation,” Trends in Food Science and Technology, Vol. 13, No. 1, 2002, pp. 12-21. doi:10.1016/S0924-2244(02)00023-7
- J. G. Wang, “Preliminary Study on the Traditional Technology of Zhejiang Rosy Vinegar,” China Brewing, Vol. 6, No. 5, 2004, pp. 23-26.
- W. P. Hammes, M. J. Brandt, K. L. Francis, J. Rosenheim, M. F. H. Seitter and S. A. Vogelmann, “Microbial Ecology of Cereal Fermentations,” Trends in Food Science and Technology, Vol. 16, No. 1-3, 2005, pp. 4-11. doi:10.1016/j.tifs.2004.02.010
- S. Haruta, S. Ueno, I. Egawa, K. Hashiguchi, A. Fujii, M. Nagano, M. Ishii and Y. Igarashi, “Succession of Bacterial and Fungal Communities during a Traditional Pot Fermentation of Rice Vinegar Assessed by PCR-Mediated Denaturing Gradient Gel Electrophoresis,” International Journal of Food Microbiology, Vol. 109, No. 1-2, 2006, pp. 79-87. doi:10.1016/j.ijfoodmicro.2006.01.015
- F. López, P. Pescador, C. Güell, M. L. Morales, M. C. García-Parrilla and A. M. Troncoso, “Industrial Vinegar Clarification by Cross-Flow Microfiltration: Effect on Colour and Polyphenol Content,” Journal of Food Engineering, Vol. 68, No. 1, 2005, pp. 133-136. doi:10.1016/j.jfoodeng.2004.05.021
- M. Cocchi, C. Durante, G. Foca, D. Manzini, A. Marchetti and A. Ulrici, “Application of a Wavelet-Based Algorithm on HS-SPME/GC Signals for the Classification of Balsamic Vinegars,” Chemometrics and Intelligent Laboratory Systems, Vol. 71, No. 2, 2004, pp. 129-140. doi:10.1016/j.chemolab.2004.01.004
- M. Cocchi, C. Durante, M. Grandi, P. Lambertini, D. Manzini and A. Marchetti, “Simultaneous Determination of Sugars and Organic Acids in Aged Vinegars and Chemometric Data Analysis,” Talanta, Vol. 69, No. 5, 2006, pp. 1166-1175. doi:10.1016/j.talanta.2005.12.032
- C. Pizarro, I. Esteban-Diez and C. Saenz-Gonzalez, “Vinegar Classfication Based on Feature Extraction and Selection from Headspace Solid-Phase Microextraction/Gas Chromatography Volatile Analysises: A Feasibility Study,” Analytica Chimica Acta, Vol. 608, No. 1, 2008, pp. 38-47. doi:10.1016/j.aca.2007.12.006
- M. J. Sáiz-Abajo, J. M. González-Sáiz and C. Pizarro, “Multi-Objective Optimisation Strategy Based on Desirability Functions Used for Chromatographic Separation and Quantification of L-Proline and Organic Acids in Vinegar,” Analytica Chimica Acta, Vol. 528, No. 1, 2005, pp. 63-76. doi:10.1016/j.aca.2004.06.027
- M. J. Sáiz-Abajo, J. M. González-Sáiz and C. Pizarro, “Prediction of Organic Acids and Other Quality Parameters of Wine Vinegar by Near-Infrared Spectroscopy. A Feasibility Study,” Food Chemistry, Vol. 99, No. 3, 2006, pp. 615-621. doi:10.1016/j.foodchem.2005.08.006
- H. Song and K. R. Cadwallader, “Aroma Components of American Country Ham,” Journal of Food Science, Vol. 73, No. 1, 2008, pp. c29-c35. doi:10.1111/j.1750-3841.2007.00593.x
NOTES
*Corresponding author.

