Open Journal of Medical Microbiology
Vol.2 No.3(2012), Article ID:22825,9 pages DOI:10.4236/ojmm.2012.23015
Assessment of Susceptibility, Pharmacodynamics, and Therapeutic Response for Interpretation of Piperacillin-Tazobactam in Vitro Activity in the Treatment of Pseudomonas aeruginosa Infection
1Departments of Pathology, Medicine, and Pharmacy, NorthShore University Health System (NorthShore), Evanston, USA
2Departments of Pathology and Medicine, University of Chicago, Chicago, USA
3Cleveland VAMC, Cleveland, USA
4Loyola University Medical Center, Maywood, USA
Email: *lance1@uchicago.edu
Received July 17, 2012; revised August 20, 2012; accepted August 30, 2012
Keywords: Pseudomonas Aeruginosa; Piperacillin-Tazobactam; Multidrug Resistance; Pharmacodynamics
ABSTRACT
Pseudomonas aeruginosa remains an important pathogen. Our purpose was to determine the minimum inhibitory concentration (MIC) and pharmacodynamic (PD) parameters predicting a positive response to therapy with piperacillin-tazobactam. Medical records were retrospectively reviewed at 3 centers. Data were recorded to assess age, type of disease, renal function, weight (body mass), MIC, antimicrobial treatment, and clinical outcome. Success was response to piperacillin-tazobactam alone, or in combination with another active agent; failure was lack of response. Of 78 evaluable patients, 63 responded (7 UTI; 56 non-UTI) and 15 did not; 26 responding received combination therapy and 37 monotherapy. Piperacillin-tazobactam treatment was successful in 53 of 63 of non-UTI disease with a MIC of ≤64/4 µg/mL, but in only 3 of 7 with a MIC of >64/4 µg/mL (P = 0.023); overall 9 of 10 infections by strains with MICs = 32 - 64 µg/mL had a successful outcome. Piperacillin estimated time above MIC at 20% separated those responding from those that did not (P = 0.019).
1. Introduction
The global plague of antibiotic resistant infections is recognized as a serious threat to world-wide healthcare [1]. This has been accompanied by a steady decline in the research and development of new antimicrobial agents to deal with the challenge. One of the key pathogen groups included in this threat are multidrug-resistant, increasingly pan-resistant, Gram-negative bacilli [1,2]. In this group is Pseudomonas aeruginosa, which remains an important pathogen that is steadily becoming more resistant to antimicrobial agents [3,4]. Treatment options for infections with this organism are limited. Traditionally, therapy of serious infection with P. aeruginosa has been accomplished with a combination of agents owing to the frequent resistance seen in this pathogen [5].
When dealing with a pathogen such as P. aeruginosa it is clear that an evidence-based approach as to what therapy is most likely to result in clinical response will be useful for selecting a treatment strategy. In order to accumulate these data there is a need to determine the outcome of therapy in a group of patients where in vitro susceptibility and pharmacodynamic parameters can be determined. The purpose of this study was to collect data that would determine what susceptibility breakpoint reliably predicts clinical success when piperacillin-tazobactam is used for therapy of P. aeruginosa infection at adequate doses and what pharmacodynamic parameter(s) can also guide in predicting human clinical response.
2. Patients and Methods
2.1. Subject Selection and Record Review
Patients were identified by retrospective review of databases at participating laboratories, recruited from the ClinMicroNet (an electronic network of leading Clinical Microbiology laboratory directors), from January 2007 to December 2008. Medical records were reviewed and patient data were collected on: age, gender, bodyweight, laboratory studies (including white blood count and serum creatinine), site(s) of infection, antimicrobial regimen and dose, and co-morbid illnesses. Treatment outcomes were determined for patients with infection from P. aeruginosa given piperacillin-tazobactam, alone or in combination with other antimicrobials, for a minimum of 72 hours. Outcomes were assessed based on clinical and bacteriologic parameters. Success was defined as improvement in clinical status with resolution of infection when piperacillin-tazobactam was the only active agent administered (monotherapy) or when two active agents, as measured by in vitro susceptibility testing, that included piperacillin-tazobactam were given (combination therapy). Treatment failure was defined as lack of improvement, death from infection, or persistently positive cultures. Outcome of piperacillin-tazobactam treatment was compared to the in vitro susceptibility of the organism determined by the participating laboratories. If more than one potential infected body site had P. aeruginosa recovered, the sample from the most serious infection was counted for this investigation; thus, blood stream infection superseded respiratory infection that superseded wound infection, which superseded UTI.
2.2. Laboratory Testing and Pharmacodynamic Analysis
Bacterial identification was performed by conventional methods. Susceptibility testing was performed by microbroth dilution or disk diffusion, and the actual or extrapolated MIC recorded [6-9]. Percentage of time above the MIC (%T > MIC) was estimated using a formula that combines individual pharmacokinetic parameters (piperacillin-tazobactam regimen, dosing interval, and MIC) plus published values for fraction unbound (70%) drug, volume of distribution (0.15 L/kg) and t1/2 (0.75 h) of piperacillin-tazobactam: %T > MIC = ln (fu × dose/Vd × MIC) × t1/2 /ln(2) × 100/t (where ln = natural logarithm, fu = fraction unbound, Vd = volume of distribution (L/kg), t1/2 = elimination half-life (h), and t = dosing interval (h)) [10-14]. For estimating the %T > MIC renal function was taken into account in order to modify the half-life of piperacillin-tazobactam. Creatinine clearance was estimated from prediction equations that took into account the serum creatinine concentration, age, gender, and body size/weight [15]. In mild to moderate renal impairment (serum creatinine = 2.5 - 7.5 mg/dL) the half-life of piperacillin-tazobactam only increases modestly as renal function declines (mean = 3.57 ± 1.36 hours), so that considerable variation of serum creatinine has little effect on measured half-life [16]. Patients were all treated over several days with initial dosage based on presenting weight (body mass) and creatinine clearance, then adjusted for changes in renal function during hospitalization until steady state was reached; providing a reasonable estimate of piperacillin-tazobactam exposure. A one-compartment, first order, intravenous model was used to estimate %T > MIC. While there is disagreement as to the accuracy of this model for pharmacodynamic analysis [17], the infusion time of piperacillin-tazobactam is relatively uniform (30 minutes), which negates meaningful differences as this time period allows for significant diffusion to the extravascular compartment during drug administration [14,18], particularly after several doses of the drug when steady state between the intravascular and extravascular compartments has been achieved. Tazobactam exposure was estimated by calculating the area under the concentration-time curve (AUC0-24).
2.3. Statistical Methodology
A Wilcoxon two sample test was used to assess the effect of continuous covariates (Age, Weight, White Blood Count (WBC), and Maximum temperature) to the outcome. As the data were skew distributed it was evaluated by the Shaprio-Wilk’s test and histogram distribution. A Chi-square test, Fisher’s exact test or an exact Pearson’s Chi-square test was used to evaluate the association between categorical covariates and the outcome. A P < 0.05 is regarded as statistically significance. Covariates with P < 0.25 (Stay in ICU, WBC, Maximum temperature, MIC > 64 µg/mL and %T/MIC < 20) in univariate analysis (Table 1) are included in the initial multiple regression model and removed backwardly at 0.05 significance level. Note that %T/MIC < 20 is derived from MIC and therefore they are not analyzed simultaneously in one multiple regression model.
2.4. Human Subject Review
Approval for this chart review study was obtained from the NorthShore University HealthSystem Institutional Review Board.
3. Results
3.1. Overall Outcome
We identified a total of 86 patients with infections caused by P. aeruginosa that were treated with piperacillintazobactam. Of the 86 cases discovered and reviewed, 3 did not have recorded susceptibility information and were excluded from analysis. Another 5 cases were indeterminate as to outcome based on the data recorded in the medical record (2 had therapy changed because of mixed infection with another piperacillin-tazobactam resistant Gram negative organism, 1 patient expired less than two days after treatment was begun, 1 had therapy discontinued because of hospice placement, 1 had therapy changed upon recognition of prior penicillin allergy)

Table 1. Covariates by outcome in univariate analysis.
and were also excluded, leaving 78 cases for this report. The patient groups were well matched when comparing the 63 success (including both monoand combination therapy) versus 15 failure subjects. Table 1 demonstrates that in univariate analysis a MIC > 64 µg/mL and %T/MIC < 20 are positively associated with unsuccessful outcome (P = 0.023 and 0.019 respectively). Specifically, cases with MIC > 64 µg/mL were more likely to have unsuccessful outcome (odds ratio: 7.27, 95% confidence interval: 1.43 - 37.09), and cases with an estimated %T/MIC < 20 were more likely to have unsuccessful outcome (odds ratio: 5.80, 95% confidence interval: 1.42 - 23.74). Cases with an elevated maximum temperature at the start of infection are also positively associated with unsuccessful outcome but the statistical significance is marginal (P = 0.067). The association between other covariates and the outcome did not reach statistical significance at a 0.05 level. Using the chosen statistical model and selection procedure, a multiple logistic regression for outcome (unsuccessful vs. successful) was attempted but only either MIC > 64 µg/mL or %T/MIC < 20 remained significant in the model; in other words, no significant multiple logistic regression model could be constructed because the other covariates were not significant in the multiple regression model. The covariate information collected for the patients with a successful and unsuccessful outcome along with the statistical analysis are in
3.2. Monotherapy and Combination Drug Treatment
Forty-three patients received piperacillin-tazobactam alone and 35 were given it in combination with another active agent. MIC values for piperacillin-tazobactam ranged from <0.25/4 to 512/4 µg/mL. The majority of infections involved the respiratory tract (n = 53; 68%), followed by blood (n = 14; 18%), urine (n = 7; 9%), and skin and soft-tissue sites (n = 4; 5%). Overall, 63 patients responded (7 UTI and 56 non-UTI) and 15 did not, for a response rate of 81%. Most patients were treated with a single antimicrobial (n = 43; 55%), of whom 37 were deemed to have had successful therapy (86%). Successful outcome using monotherapy for non-UTI infection was demonstrated in 23 of 27 (86%) respiratory tract infections, 5 of 6 (83%) blood stream infections, and 2 of 3 (67%) wound infections. All 7 UTI patients (6 with complicated UTI) received monotherapy and responded successfully regardless of MIC (all were ≤64 µg/mL). Successful outcome using combination drug treatment for non-UTI infection was demonstrated in 18 of 25 (72%) respiratory tract infections, 7 of 9 (78%) blood stream infections, and the single wound infection in this group. Importantly, there were 10 strains having a MIC for piperacillin-tazobactam of 32 or 64 µg/mL (Table 2).

Table 2. Characteristics and treatment outcome for patients infected with P. aeruginosa that was susceptible to 32 or 64 µg/mL of piperacillin-tazobactam.
Nine of these 10 (90%) infected patients responded to therapy; this included 3 of 3 with complicated UTI (MIC = 64 µg/mL) and both with bacteremia (MIC = 32 µg/mL); all given monotherapy.
We have included the complicated UTI patients with the other infections in our overall analysis because these occur in patients with abnormalities of the urinary tract that impair the antibiotic concentrating capacity of the renal collecting system, as was the case in all 3 of our patients with P. aeruginosa having MICs to piperacillin-tazobactam in the 32 - 64 µg/mL range. Patients with complicated UTI require broad-spectrum antimicrobial therapy and a longer duration of treatment. Overall, the complication rate and mortality is higher in complicated UTI and more resembles infection outside of the urinary tract. Finally, these patients are at a higher risk of relapse or recurrence compared with those suffering from uncomplicated UTI [19], and all were cured.
3.3. Pharmacokinetic Analysis
Pharmacokinetic/pharmacodynamic data were available for all patients. Daily dosing ranged from 2.25 Grams every 8 hours to 4.5 Grams given every 6 hours, adjusted for renal function impairment, which was infused over a standard dosing time (approximately 30 minutes). A graphic depiction of the results is in Figures 1-3. Piperacillin-tazobactam treatment was successful in 53 of 64 (83%) non-UTI disease with a MIC of ≤64/4 µg/mL, but in only 3 of 7 non-UTI with a MIC of >64/4 µg/mL (P = 0.023). Piperacillin estimated time above MIC at 20% also separated those responding from those that did not (P = 0.019). Treatment was successful, using monoor combination therapy in 53 of 61 (87%) non-UTI disease with a %T > MIC of 20%, but in only 5 of 10 non-UTI when the %T > MIC was under 20% (P = 0.019).
4. Discussion
We have compiled what we believe is the first case series of patients infected with P. aeruginosa that compares in vitro susceptibility results, the drug exposure parameters for each treated patient and the clinical outcome for the affected persons. Calculating the pharmacodynamic parameters and assessing them in light of clinical response provided somewhat unexpected results in that the %T > MIC predicting a favorable response when using piperacillin-tazobactam for therapy of P. aeruginosa infection was only 20% as opposed to the traditionally expected 40% to 50% for penicillin-type antibiotics [20,21]. Therapeutic response also clearly corresponded to a susceptibility breakpoint of ≤32 µg/mL for all infections (both UTI and non-UTI). Unfortunately there were insufficient non-UTI cases with a MIC of 64 µg/mL to definitively indicate how infection would respond at that MIC. Interestingly, recalculating the PK/PD parameters for piperacillin-tazobactam using a %T > MIC of 20% predicting a favorable response suggests the former, higher breakpoint was likely sufficient (discussed subsequently). The dosing used for our report followed that recommended in the package insert where administration of up to 4.5 gm piperacillin-tazobactam every 6 hours is suggested for treatment of hospital-acquired pneumonia and suspected infections due to P. aeruginosa; the in vitro susceptibility breakpoint we suggest as most useful when interpreting our results (MIC of ≤64/4 µg/mL) corresponds to that found in past package inserts for use of this agent when treating P. aeruginosa [22].
Tam and colleagues published findings on the treatment of P. aeruginosa bacteremia with piperacillin-tazobactam

Figure 1. Depiction of specific treatment outcome for individual patient responses based on the in vitro MIC (in µg/mL) of piperacillin-tazobactam (alone and in combination) used for non-UTI and UTI infection with P. aeruginosa.
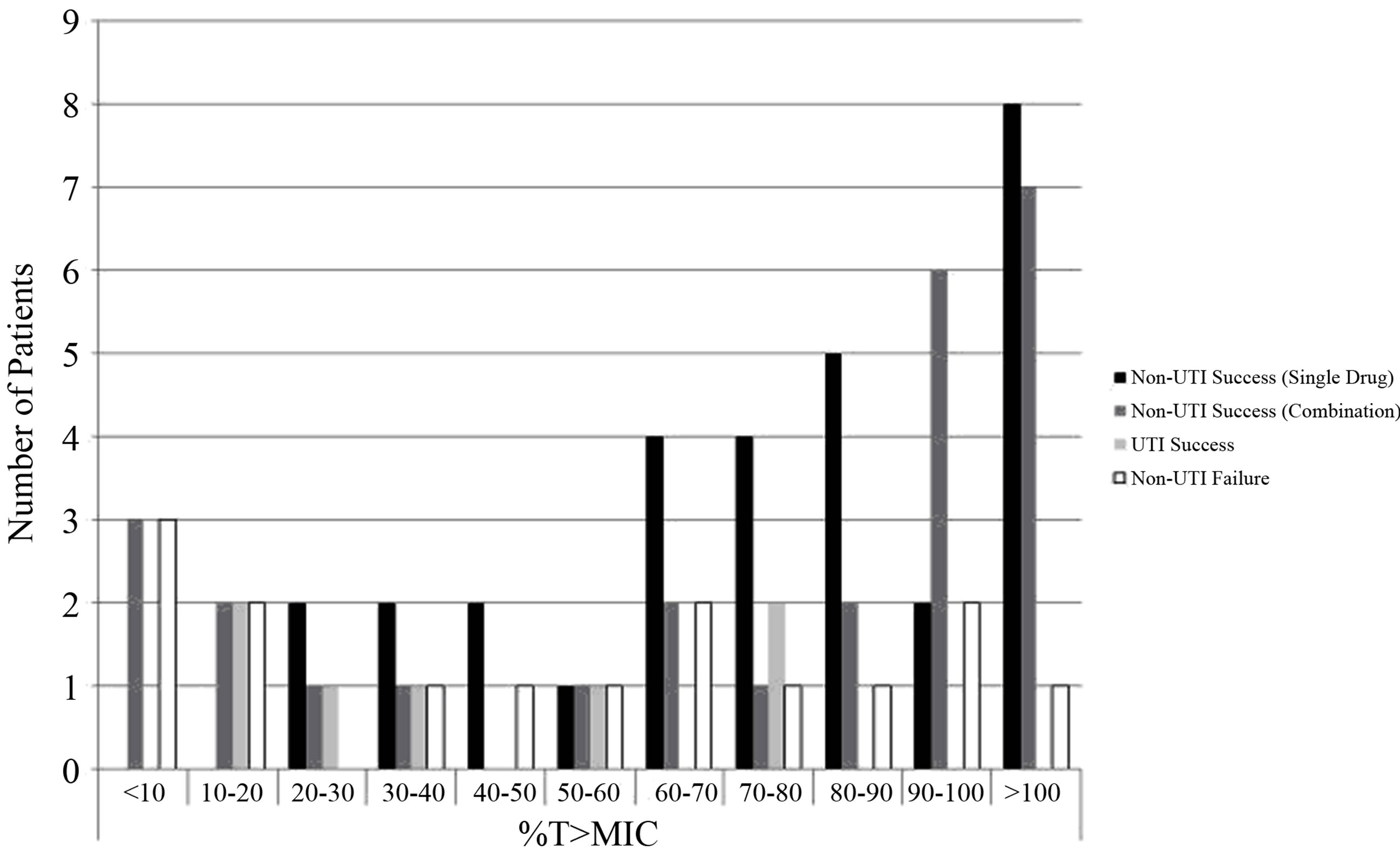
Figure 2. Depiction of specific treatment outcome for individual patient responses based on %Time above the MIC of piperacillin-tazobactam (alone and in combination) used for non-UTI and UTI infection with P. aeruginosa.

Figure 3. Depiction of overall successful outcome (includes success based on use of monotherapy and partial success based on use of combination therapy) associated with %time above the MIC of piperacillin-tazobactam used for infection with P. aeruginosa.
that appear to contradict our results [23]. They found the 30 day mortality and the time to hospital mortality was significantly worse in a piperacillin-tazobactam treated group of 7 patients where the in vitro susceptibility to piperacillin-tazobactam was 32 - 64 µg/mL compared to controls [23]. However, no drug specific exposure was reported for either the piperacillin-tazobactam group or the control subjects-other than dosing was reported as being given according to the manufacturer’s recommendations; thus no pharmacokinetic/pharmacodynamic assessment could be made. Also, critical review of the cases in that report reveals nearly 1/3 of both the target and control patients had urinary infection as the primary site for P. aeruginosa disease, a site where we saw no failures at any MIC; also, 43% of the piperacillin-tazobactam subjects had wound infection indicated as the primary site of illness compared to 15% for the control group, and the primary site of infection was unknown in 48% of the controls versus 14% for piperacillin-tazobactam [23]. Thus, conclusions from their study are suggestive at best, and since it represents the only other published series of clinical outcome using piperacillin-tazobactam for treatment of P. aeruginosa disease we believe it is perhaps less instructive than our results.
Several theoretical pharmacokinetic/pharmacodynamic analyses have been done that attempt to address the hypothetical modeling of piperacillin-tazobactam treatment by estimating the likelihood of attaining the necessary pharmacokinetic parameters for specific microbial susceptibility levels [24-26]. These have concluded that the prior Clinical and Laboratory Standards Institute (CLSI) breakpoint for susceptibility of P. aeruginosa to piperacillin-tazobactam (≤64 µg/mL) over estimated susceptibility to that compound that led to considerable discrepancies between the CLSI and European breakpoints [7-9, 24-26]. However, all have assumed that the exposure time needed for a successful outcome using piperacillin-tazobactam (based upon the piperacillin component) against P. aeruginosa is a %T > MIC of ≥50%. This assumption is drawn from the behavior of other penicillin-type antimicrobial agents and has never been studied in either animals or humans using piperacillin-tazobactam. Our clinical data from human subjects indicates that for piperacillin-tazobactam the necessary drug exposure is likely much lower, at %T > MIC of ≥20% for human subjects, which would lead to very different conclusions in these simulations. Applying %T > MIC of ≥20% to the simulations of Lodise et al. [24], as well as of DeRyke and colleagues [25], would indicate that for 90% target attainment a MIC between 32 and 64 µg/mL would be appropriate using the current recommended dosing of 4.5 grams every 6 hours to treat P. aeruginosa infection. Since the current standard for reporting susceptibility test results is to “round up” to the next doubling MIC level, that would correspond to a susceptibility breakpoint of 64 µg/mL [7].
An important question to address is why piperacillintazobactam may have pharmacodynamics more similar to that of carbapenem antibiotics than other penicillins [20, 21]. One little appreciated fact is the action of b-lactamase inhibitor agents on accessory penicillin binding protein (PBP) targets for both Gram-positive and Gramnegative bacteria. They have been shown to enhance cephalosporin action against non-b-lactamase producing methicillin-resistant Staphylococcus aureus, outperforming vancomycin [27], as well as for ampicillin against E. coli and Proteus species through their capacity to bind secondary PBPs [28], which augments the bactericidal effect on bacteria; not only in b-lactamase-producing bacteria but also in penicillinand ampicillin-sensitive strains. Finally, the inhibitor combinations perform as well as the carbapenem agents in the treatment of infections due to extended spectrum β-lactamase producing Enterobacteriaceae [29-31], further suggesting the unique potency characteristics of this drug class.
We did not assess the pharmacokinetics of tazobactam as a unique compound since the literature on piperacillintazobactam is divided regarding pharmacodynamic role of the tazobactam component [24,32,33]. In the present study, the calculated tazobactam AUC0-24 was in excess of the estimated concentration required for b-lactamase activity throughout the dosing interval [34,35]. Thus we agree with Johnson and colleagues that pharmacokinetic analyses can be based on the assessment of piperacillin alone [11]. Combining a β-lactamase inhibitor with a penicillin or cephalosporin has a major impact on the molecule with which it is combined. Thus it is not surprising that some of these agents have been very successfully used in the treatment of infectious disease for several decades [36].
Continually increasing resistance is recognized as an important healthcare threat that encompasses many pathogens, including P. aeruginosa [1-4]. This problem is particularly challenging for difficult to treat infections such as healthcare-associated pneumonia, where P. aeruginosa plays a prominent role [5], and when faced with an ongoing deterioration of the pharmacodynamic profiles for all agents active against this pathogen [37]. Dealing with this issue requires increased understanding of how antimicrobial agents perform in the treatment of actual clinical infection, which we have investigated in this study. The recognition that piperacillin-tazobactam can be effective against serious infection from P. aeruginosa, even as the susceptibility of this pathogen to the drug approaches 64 µg/mL, is important when proper dosing is administered and such knowledge can aid in maintaining the utility of this antibiotic well into the future. An example of this is in Case 2 (Table 2) where it appears the patient achieved a high %T > MIC with normal renal function and a relatively low drug dose. This particular patient was very small (weight ≈ 35 kg) so a low dose was sufficient to achieve adequate drug exposure leading to a successful outcome.
Our research has several limitations. The first is that it is a retrospective study where none of the authors were able to prospectively follow patient treatment and outcome. However, of the nearly 80 patients assessed, most had non-UTI infections and those with UTI nearly all (6 of 7) experienced complicated infections (renal function impairment), which limits concentration of β-lactam agents in the urinary collecting system. The majority of infections were from the respiratory tract and even when directly caring for a patient it can be challenging to know if the isolation of P. aeruginosa from respiratory secretions represents infection or colonization. However, the patients were all treated for pneumonia by their primary physician. From the perspective of our patients, 30 of the 42 patients (71%) who responded were cared for in the ICU as were 7 of 9 (78%) who failed therapy, indicating a high level of seriously ill persons. All were considered likely enough to have pneumonia from P. aeruginosa to be treated for this disease. Also, in this type of analysis, it is possible that the results from one enrollment site can dominate those from the other reporting centers. We do not believe this was the case in this report as 53% of cases were derived from one site, 30% from the second site, and 17% from the third. The distribution of infections, successful (or partially successful) outcome, and failures were not different between the three sites submitting cases. Our investigation found a relatively small number of evaluable patients (78) to determine our conclusions. However, this is the only study to date that has investigated the role of in vitro susceptibility plus actual drug exposure compared to clinical outcome of P. aeruginosa infection treated with piperacillin-tazobactam. The fact that the majority of cases (55%) received monotherapy further strengthens our findings. Also, we did not collect information on serum albumin concentrations that can affect drug protein binding and distribution of free piperacillin. However, at the low level of piperacillin binding to serum albumin the impact is practically minimal since free drug equilibrates between the intravascular and extravascular free body water. Because the free water extravascular space is nearly 10-fold that of the intravascular water, raising a given drug’s binding to serum proteins from 0% to 90% reduces the concentration of free drug in serum and tissue by only one-half [38]. Finally, our measurements reflect both the initial dosing as well as more drug exposure that occurred as treatment time lengthened. Importantly, this does not negate our findings of what is needed for starting therapy with piperacillin-tazobactam are the pharmacodynamic parameters that predict success or failure based on treatment with this agent.
In conclusion, piperacillin-tazobactam treatment was successful when administered alone or in combination with another agent in ≥80% of all infections caused by P. aeruginosa when the organism was susceptible at a MIC equal to or less than 64 µg/mL. When the %T > MIC was at least 20% there also was a good correlation with likely success. This suggests that piperacillin-tazobactam is adequate for treatment of P. aeruginosa when dosing is sufficient to maintain the %T > MIC at 20% or greater, which is a level readily attainable with the current dosing recommendation even when the in vitro MIC approaches 64 µg/mL.
5. Acknowledgements
This study was presented in part at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA. Abstract No. A1-025. September, 2009. The authors sincerely appreciate the statistical analysis performed by Hongyan Du.
Funding: This project was supported by an investigator-initiated grant from Wyeth Pharmaceuticals, Inc. The sponsor had no involvement in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Competing Interests: Dr. Peterson receives research funding from Wyeth Pharmaceuticals (now Pfizer, Inc.), Bayer, Cepheid, NorthShore, GeneOhm, GSK, Johnson and Johnson, Merck, MicroPhage, Nanogen, Nanosphere, Roche, 3M, and the Washington Square Health Foundation. He also provides consultation relating to research projects to Cepheid, GeneOhm, GSK, MicroPhage, Nanogen, Nanosphere, Roche, 3M, and Wyeth Pharmaceuticals. All other authors reported no conflicts.
Ethical Approval: Approval for this chart review study was obtained from the NorthShore University HealthSystem Institutional Review Board (reference number EH07-106).
REFERENCES
- B. Spellberg, R. Guidos, D. Gilbert, J. Bradley, H. W. Boucher, W. M. Scheld, et al., “The Epidemic of Antibiotic-Resistant Infections: A Call to Action for the Medical Community from the Infectious Diseases Society of America,” Clinical Infectious Diseases, Vol. 46, No. 2, 2008, pp. 155-164. doi:10.1086/524891
- L. C. McDonald, “Trends in Antimicrobial Resistance in Health Care—Associated Pathogens and Effect on Treatment,” Clinical Infectious Diseases, Vol. 42, Suppl. 2, 2006, pp. S65-S71. doi:10.1086/499404
- M. W. Douglas, K. Mulholland, V. Denyer and T. Gottlieb, “Multi-Drug Resistant Pseudomonas aeruginosa Outbreak in a Burns Unit—An Infection Control Study,” Burns, Vol. 27, No. 2, 2001, pp. 131-135. doi:10.1016/S0305-4179(00)00084-X
- D. L. Paterson, “The Epidemiological Profile of Infections with Multidrug-Resistant Pseudomonas aeruginosa and Acinetobacter Species,” Clinical Infectious Diseases, Vol. 43, Suppl. 2, 2006, pp. S43-S48. doi:10.1086/504476
- A. M. Ferrara, “Potentially Multidrug-Resistant Non-Fermentative Gram-Negative Pathogens Causing Nosocomial Pneumonia,” International Journal of Antimicrobial Agents, Vol. 27, No. 3, 2006, pp. 183-195. doi:10.1016/j.ijantimicag.2005.11.005
- R. N. Jones, and A. L. Barry, “Studies to Optimize the in Vitro Testing of Piperacillin Combined with Tazobactam,” Diagnostic Microbiology and Infectious Diseases, Vol. 12, No. 6, 1989, pp. 495-510. doi:10.1016/0732-8893(89)90084-9
- Clinical and Laboratory Standards Institute, “Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard M7-A7,” 7th Edition, Clinical and Laboratory Standards Institute, Wayne, 2006.
- Clinical and Laboratory Standards Institute, “Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard M2-A9,” 9th Edition, Clinical and Laboratory Standards Institute, Wayne, 2006.
- Clinical and Laboratory Standards Institute, “Performance Standards for Antimicrobial Susceptibility Testing: Eighteenth Informational Supplement M100-S18,” Clinical and Laboratory Standards Institute, Wayne, 2008.
- W. A. Craig, “Basic Pharmacodynamics of Antibacterials with Clinical Applications to the Use β-Lactams, Glycopeptides, and Linezolid,” Infectious Disease Clinics of North America, Vol. 17, No. 3, 2003, pp. 479-501. doi:10.1016/S0891-5520(03)00065-5
- C. A. Johnson, C. E. Halstenson, J. S. Kelloway, B. E. Shapiro, S. W. Zimmerman, A. Tonelli, et al., “SingleDose Pharmacokinetics of Piperacillin and Tazobactam in Patients with Renal Disease,” Clinical Pharmacology and Therapeutics, Vol. 51, No. 1, 1992, pp. 32-41. doi:10.1038/clpt.1992.5
- D. J. Occhipinti, S. L. Pendland, L. L. Schoonover, E. B. Rypins, L. H. Danziger and K. A. Rodvold, “Pharmacokinetics and Pharmacodynamics of Two Multiple-Dose Piperacillin-Tazobactam Regimens,” Antimicrobial Agents and Chemotherapy, Vol. 41, No. 11, 1997, pp. 2511- 2517.
- F. Sörgel and M. Kinzig, “The Chemistry, Pharmacokinetics and Tissue Distribution of Piperacillin-Tazobactam,” Journal of Antimicrobial Chemotherapy, Vol. 31, Suppl. A, 1993, pp. 39-60.
- J. D. Turnidge, “The Pharmacodynamics of β-Lactams,” Clinical Infectious Diseases, Vol. 27, No. 1, 1998, pp. 10- 22. doi:10.1086/514622
- G. Manjunath, M. J. Sarnak and A. S. Levey, “Estimating the Glomerular Filtration Rate. Dos and Don’Ts for Assessing Kidney Function,” Postgraduate Medicine, Vol. 110, No. 6, 2001, pp. 55-62.
- J. A. Giron, B. R. Meyers, S. Z. Hirschman and E. Srulevitch, “Pharmacokinetics of Piperacillin in Patients with Moderate Renal Failure and in Patients Undergoing Hemodialysis,” Antimicrobial Agents and Chemotherapy, Vol. 19, No. 2, 1981, pp. 279-283. doi:10.1128/AAC.19.2.279
- A. A. Vinks, J. G. den Hollander, S. E. Overbeek, R. W. Jelliffe and J. W. Mouton, “Population Pharmacokinetic Analysis of Nonlinear Behavior of Piperacillin during Intermittent or Continuous Infusion in Patients with Cystic Fibrosis,” Antimicrobial Agents and Chemotherapy, Vol. 47, No. 2, 2003, pp. 541-547. doi:10.1128/AAC.47.2.541-547.2003
- Y.-K. Kim, H. Pai, H.-J. Lee, S.-E. Park, E.-H. Choi, J. Kim, et al., “Bloodstream Infections by Extended-Spectrum β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae in Children: Epidemiology and Clinical Outcome,” Antimicrobial Agents and Chemotherapy, Vol. 46, No. 5, 2002, pp. 1481-1491. doi:10.1128/AAC.46.5.1481-1491.2002
- M. S. Bader, J. Hawboldt and A. Brooks, “Management of Complicated Urinary Tract Infections in the Era of Antimicrobial Resistance,” Postgraduate Medicine, Vol. 122, No. 6, 2010, pp. 7-15. doi:10.3810/pgm.2010.11.2217
- D. P. Nicolau, “Carbapenems: A Potent Class of Antibiotics,” Expert Opinion in Pharmacotherapy, Vol. 9, No. 1, 2008, pp. 23-37.
- G. G. Zhanel, R. Wiebe, L. Dilay, K. Thomson, E. Rubinstein, D. J. Hoban, et al., “Comparative Review of the Carbapenems,” Drugs, Vol. 67, No. 7, 2007, pp. 1027-1052. doi:10.2165/00003495-200767070-00006
- Zosyn®, “Piperacillin and Tazobactam for Injection, Prescribing Information,” Last accessed June 27, 2012. http://www.onlinepharmacycatalog.com/drugs-medications/antibiotics/piperacillin-tazobactam/zosyn-prescribing-information.pdf
- V. H. Tam, E. A. Gamez, J. S. Weston, et al., “Outcomes of Bacteremia Due to Pseudomonas aeruginosa with Reduced Susceptibility to Piperacillin-Tazobactam: Implications on the Appropriateness of the Resistance Breakpoint,” Clinical Infectious Diseases, Vol. 46, No. 6, 2008, pp. 862-867. doi:10.1086/528712
- T. P. Lodise Jr., B. Lomaestro, K. A. Rodvold, L. H. Danziger and G. L. Drusano, “Pharmacodynamic Profiling of Piperacillin in the Presence of Tazobactam in Patients through the Use of Population Pharmacokinetic Models and Monte Carlo Simulation,” Antimicrobial Agents and Chemotherapy, Vol. 48, No. 12, 2004, pp. 4718-4724. doi:10.1128/AAC.48.12.4718-4724.2004
- C. A. DeRyke, J. L. Kuti and D. P. Nicolau, “Reevaluation of Current Susceptibility Breakpoints for GramNegative Rods Based on Pharmacodynamic Assessment,” Diagnostic Microbiology and Infectious Diseases, Vol. 58, No. 3, 2007, pp. 337-344.
- C. R. Frei, N. P. Wiederhold and D. S. Burgess, “Antimicrobial Breakpoints for Gram-Negative Aerobic Bacteria Based on Pharmacokinetic—Pharmacodynamic Models with Monte Carlo simulation,” Journal of Antimicrobial Chemotherapy, Vol. 61, No. 3, 2008, pp. 621-628. doi:10.1093/jac/dkm536
- E. L. Fasola, C. E. Fasching and L. R. Peterson, “Molecular Correlation between in Vitro and in Vivo Activity of Beta-Lactam and Beta-Lactamase Inhibitor Combinations against Methicillin-Resistant Staphylococcus aureus,” Journal of Laboratory and Clinical Medicine, Vol. 125, No. 2, 1995, pp. 200-211.
- T. Yokota, “Inactivation of Beta-Lactamases by Sulbactam and Enhanced Clinical Activity Due to Target-Site Binding of the Combination of Sulbactam and Ampicillin,” APMIS Supplementum, Vol. 5, 1989, pp. 9-16.
- P. J. Gavin, M. T. Suseno, R. B. Thomson Jr., J. M. Gaydos, C. L. Pierson, D. C. Halstead, et al., “Clinical Correlation of the CLSI Susceptibility Breakpoint for Piperacillin-Tazobactam against Extended-Spectrum-β-LactamaseProducing Escherichia coli and Klebsiella Species,” Antimicrobial Agents and Chemotherapy, Vol. 50, No. 6, 2006, pp. 2244-2247. doi:10.1128/AAC.00381-05
- J. Rodríguez-Baño, E. Picón, P. Gijón, J. R. Hernández, M. Ruíz, C. Peña, et al., “Community-Onset Bacteremia Due to Extended-Spectrum β-Lactamase-Producing Escherichia coli: Risk Factors and Prognosis,” Clinical Infectious Diseases, Vol. 50, No. 1, 2010, pp. 40-48. doi:10.1086/649537
- J. Rodríguez-Baño, M. D. Navarro, P. Retamar, E. Picón, Á. Pascua and The Extended-Spectrum Beta-Lactamases— Red Española de Investigación en PatologíaInfecciosa/ Grupo de Estudio de Infección Hospitalaria Group, “β- lactam/β-lactam Inhibitor Combinations for the Treatment of Bacteremia Due to Extended-Spectrum β-LactamaseProducing Escherichia coli: A Post Hoc Analysis of Prospective Cohorts,” Clinical Infectious Diseases, Vol. 54, No. 2, 2012, pp. 167-174. doi:10.1093/cid/cir790
- A. H. Strayer, D. H. Gilbert, P. Pivarnik, A. A. Medeiros, S. H. Zinner and M. N. Dudley, “Pharmacodynamics of Piperacillin Alone and in Combination with Tazobactam against Piperacillin-Resistant and Susceptible Organisms in an in Vitro Model of Infection,” Antimicrobial Agents and Chemotherapy, Vol. 42, No. 10, 1994, pp. 1098-1104.
- The Medical Letter on Drugs and Therapeutics, “Piperacillin/Tazobactam,” The Medical Letter, Inc., New Rochelle, 1995.
- P. G. Ambrose, S. M. Bhavnani and R. N. Jones, “Pharmacokinetics-Pharmacodynamics of Cefepime and Piperacillin-Tazobactam against Escherichia coli and Klebsiella pneumoniae Strains Producing Extended-Spectrum β-Lactamases: Report from the ARREST Program,” Antimicrobial Agents and Chemotherapy, Vol. 47, No. 5, 2003, pp. 1643-1646. doi:10.1128/AAC.47.5.1643-1646.2003
- M. N. Dudley, “Combination β-Lactam and β-LactamaseInhibitor Therapy: Pharmacokinetic and Pharmacodynamic Considerations,” American Journal of Health-System Pharmacy, Vol. 52, No. 6, 1995, pp. S23-S28.
- F. J. Pérez-Llarena and G. Bou, “Beta-Lactamase Inhibitors: The Story So Far,” Current Medicinal Chemistry, Vol. 16, No. 28, 2009, pp. 3740-3765. doi:10.2174/092986709789104957
- K. J. Eagye, D. P. Nicolau, S. R. Lockhart, J. P. Quinn, G. V. Doern, G. Gallagher and M. A. Abramson, “A Pharmacodynamic Analysis of Resistance Trends in Pathogens from Patients with Infection in Intensive Care Units in the United States between 1993 and 2004,” Annals of Clinical Microbiology and Antimicrobials, Vol. 6, 2007, pp. 11-17. doi:10.1186/1476-0711-6-11
- L. R. Peterson and D. N. Gerding, “Influence of Protein Binding of Antibiotics on Serum Pharmacokinetics and Extravascular Penetration: Clinically Useful Concepts,” Clinical Infectious Diseases, Vol. 2, No. 3, 1980, pp. 340-348. doi:10.1093/clinids/2.3.340
NOTES
*Corresponding author.

