Journal of Sustainable Bioenergy Systems
Vol. 2 No. 4 (2012) , Article ID: 25884 , 5 pages DOI:10.4236/jsbs.2012.24018
Ultrasonic-Assisted Production of Biodiesel from Waste Frying Oil Using a Two-Step Catalyzing Process
1Department of Business Administration, Kun-Shan University, Tainan, Taiwan
2Department of Environment Engineering, Kun-Shan University, Tainan, Taiwan
Email: cclin@mail.ksu.edu.tw, *johnson@mail.ksu.edu.tw
Received October 15, 2012; revised November 21, 2012; accepted November 30, 2012
Keywords: Ultrasonic; Biodiesel; Waste Frying Oil
ABSTRACT
This study proposed using waste frying oil rather than refined vegetable oil as an effective way to reduce the raw material cost of producing biodiesel. In addition, the ultrasonic-assisted two-step catalyzing Process was first adopted for the production of biodiesel from waste frying oil. The results show that the total reaction time was less than 50 min and the conversion rate of fatty acid methyl esters (FAMEs) achieved was 97.1%. Therefore, the ultrasonic-assisted two-step catalyzing process has a potential application in producing biodiesel from waste frying oil.
1. Introduction
Since the oil crisis of the 1970s, research interest in the area of alternative fuels has expanded. At present, the supply of traditional fossil fuels is limited while energy demand continues to rise and greenhouse gas emissions are becoming a greater concern. The conversion of vegetable oils and other feedstocks into biodiesel is a potential solution to the ever increasing demand for energy and environmental concerns [1].
Biodiesel has numerous advantages including lower dependence on foreign crude oil, being a renewable resource, decreased greenhouse gas emissions in line with the Kyoto Protocol agreement, lower sulfide emissions, biodegradable and non-toxic fuels from possible agricultural surpluses that can also help improve rural economies, no engine modifications required, and good engine performance [1,2].
Triglycerides are the main component of vegetable oil. When triglycerides react with alcohol, the three fatty acid chains released from the glycerol skeleton are combined with the alcohol to produce FAMEs [3,4].
Refined vegetable oil is a superior raw material for producing biodiesel, because the conversion of pure triglyceride to FAMEs is very high and the reaction time is relatively short. However, refined vegetable oil is relatively expensive. Nowadays, the high cost of raw materials is the main obstacle to biodiesel commercialization. Further, using refine vegetable oil to produce biodiesel will reduce the edible oil. Thus, the use of waste frying oil instead of refined vegetable oil to produce biodiesel is an effective way to reduce the raw material cost and reduce food shortages. Additionally, using waste frying oil could also help solve the problem of waste oil disposal [3,5].
An alkali process can achieve a high conversion rate to biodiesel product within a short time (less than 60 min). However, when the acid value of the treating oil is greater than 2.0 (contains greater amounts of free fatty acids), it will form soap with an alkaline catalyst. The soap formed can prevent separation of the biodiesel from the glycerin fraction [5]. Nevertheless, the most favorable waste frying oils usually have a high acid value [5]. When the acid value of the treating oil is greater than 2.0, the acid process is preferred for commercial use because of its simplicity. Soap formation can also be avoided by using an acid catalyst. However, the reaction time involved is very long (48 - 96 h) and requires a great amount of acid catalysts and a high alcohol/oil molar ratio (30:1 - 150:1) [2,6,7].
The transesterification of triglycerides with alcohol is an immiscible reaction; and complete blending of the reagents is of critical importance. Therefore, the extent of mixing is the most significant factor affecting the transesterification reaction. In addition, transesterification is actually an equilibrium reaction, where an excess of alcohol is utilized to shift the reaction towards the formation of esters. If the oil and alcohols are not completely mixed, the transesterification reaction mainly takes place at the boundary between the two layers and is thus a very slow process.
Ultrasonic mixing induced effective emulsification and mass transfer. Thus the rate of ester formation under ultrasonic condition is higher than that under stirring conditions [8-10]. Researches have indicated the use of ultrasonic mixing is efficient, time-saving and economically functional, offering several advantages over the classical procedure [11,12]. The collapse of the cavitation bubbles cleavages the phase boundary and leads to emulsification by ultrasonic jets that impinge one liquid against another. Droplets of denser oil move upwards while the alcohol moves downwards, thus fostering the mixing and increasing the contact area between alcohol and oil. The ultrasonic properties of an emulsion vary significantly and increase the contact area for fats and alcohol [13]. Therefore, ultrasonic-assisted transesterification might be an efficient way to reduce reaction time [9,11-13].
The main aim of this study was to obtain the optimal reaction parameters (alcohol/oil molar ratio; amount of catalyst used; type of catalyst (alkaline or acid); and reaction time) for biodiesel production from waste frying oil by a two-step catalyzing process assisted by ultrasonic mixing.
2. Methods
2.1. Materials
Sulfuric acid (H2SO4) 99% and alcohol 99.8% were purchased from Shimakyu Co. Ltd. (Japan). Sodium hydroxide (NaOH) 98% was purchased from Mallinckrodt Co. Ltd. (USA). Methyl laurate and acetic acid were purchased from Fluka Co. Ltd. (USA). The waste frying oil samples were provided from local restaurants. Residual food in the waste frying oil used in this study was removed by filtering.
2.2. Equipment
The ultrasonic equipment used in the experiments had a working frequency of 20 kHz (MIXSONIX SONICATOR 3000) and an output power of 600 W. The spherical glass reactors had volumes of 250 ml that were modified by the introducing of a water condenser for conducting atmospheric pressure experiments. The samples were analyzed with a Perkin Elmer GC Clarus 600 equipped with a capillary column (SPBTM-WAX, 30 m × 0.75 m × 1.0 μm) and a flame ionization detector (FID).
2.3. Experimental Procedure
An ultrasonic-assisted two-step catalyzing process was adopted for producing the biodiesel from waste frying oil.
In the first step, the main purpose was to reduce the acid value of the waste frying oil. The free fatty acids of the waste frying oil were esterified with alcohol catalyzed by H2SO4 which reduced the acid value. The process was carried out at a alcohol /oil molar ratio of 6:1 to 11:1 (6:1, 7:1, 8:1, 9:1, 10:1, and 11:1) and amount of catalyst at 1.0 to 3.0 wt%.
In the second step, transesterification of triglycerides in waste frying oil (an acid value of less than 2.0) with alcohol catalyzed by NaOH was performed. The experiment was carried out at a alcohol/oil molar ratio of 6:1 and amount of NaOH at 1.0 wt% [14].
Following the two-step reaction, the top layer contained mainly FAMEs. The FAMEs were removed in a separating funnel after settling for half an hour, Than the FAMEs were washed at least three times with acetate 30% and deionized water, and finally dried in an oven at 378 ± 3 K.
2.4. Analytical Methods
In chemistry, acid value is the mass of potassium hydroxide (KOH) in milligrams that is required to neutralize one gram of chemical substance. The acid value (KOH mg/g) and aponification value (KOH mg/g) were determined by a standard titrimetry method (AOCS: American Oil Chemists’ Society). The molecular weight of the waste frying oil was calculated from its acid value and aponification value. The experimental results in terms of the acid value, aponification value and molecular weight were 4.35, 286.1 and 588, respectively.
Methyl laurate was added as an internal standard into the crude biodiesel and the sample was injected under the following conditions: carrier gas, nitrogen; injector temperature, 553 K; split ratio, 1:20; and temperature of detector, 573 K. The oven temperature started at 483 K for 2 min, increased to 513 K at a rate of 4 K/min and held for 8 min.
The conversion rate of crude biodiesel was calculated according to the area of FAME as expressed in the following equation [14]:
3. Results and Discussion
3.1. First Step: Reducing the Acid Value of Waste Frying Oil
In the first step, the main purposes were to reduce the acid value of waste frying oil, and Find the optimal reaction conditions of ultrasonic power, alcohol/oil molar ratio as well as amount of H2SO4 used.
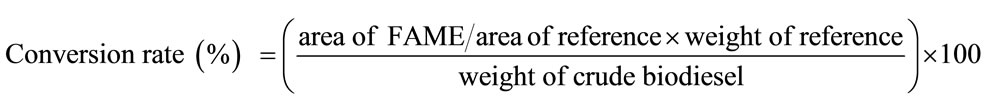 (1)
(1)
3.1.1. Effect of Ultrasonic Power
The initial reaction conditions were a alcohol/oil molar ratio of 6:1 and H2SO4 at 1 wt%. Table 1 shows the influences of ultrasonic power level on the conversion rate in the first step. At 14 and 28 W of ultrasonic power, FAMEs are not produced because the triglycerides and alcohols are not thoroughly mixed. When increasing the power to 42 W although the conversion rate was still very low, but the FAMEs began to be produced at 10 min. Therefore, the minimal power level of ultrasonic power was about 42 W.
3.1.2. Effect of Methanol/Oil Molar Ratio
We increased the methanol/oil molar ratio from 6:1 to 12:1. The mixture was collected and analyzed with a gas chromatograph instrument at 10 min. As seen in Table 2, the conversion rates of FAMEs were approximately less than 1% after 10 min. However, when the methanol/oil molar ratio was 10:1, the FAMEs started to produced at 10 min the conversion rate exceeded 1.61%. Therefore, the optimal methanol/oil molar ratio was about 10:1.
3.1.3. Effect of Catalyst Amount
Figure 1 shows the effect of reaction time and amount of acid catalyst on acid value in the first step. The reaction conditions were: methanol/oil molar ratio, 10:1; ultrasonic power, 42 W; and amount of H2SO4 used, 1.0 wt%. Figure 1(a) shows the acid value was reduced to 3.9, 3.6, 3.3 and 2.9 after 5, 10, 15 and 20 min, respectively. However, the acid value was still greater than 2.0 after 25 min. When treated with an acid value exceeding 2.0, an alkali process will form soap with the alkaline catalyst [2,6,7]. The soap formed can prevent separation of the biodiesel from the glycerin fraction. On increasing the amount of acid catalyst, the acid value could be reduced to below than 2, which is essential to prevent soap formation.
3.2. Second Step: Transesterification Reaction
The second step was carried out at a alcohol/oil molar ratio of 6:1 [8,9,15-17], NaOH at 1 wt% [8,9,15,16,18], and ultrasonic power 42 W. Figure 2 shows the FAME conversion plot of the second step. After reaction times of 5 and 10 min, the FAME conversion rate was only 68.3% and 79.6%, respectively. However, the FAMEs conversion rates between 10 - 20 min of reaction were slightly increased. When the reaction time was increased to 25 min, the FAME conversion rate achieved 97.1%.
In the second step, the optimal reaction parameters for the ultrasonic-assisted transesterification were as follows: alcohol/oil molar ratio, 6:1; amount of NaOH, 1 wt%; and reaction time, 25 min. The FAMEs conversion achieved under these conditions was 97.1%.
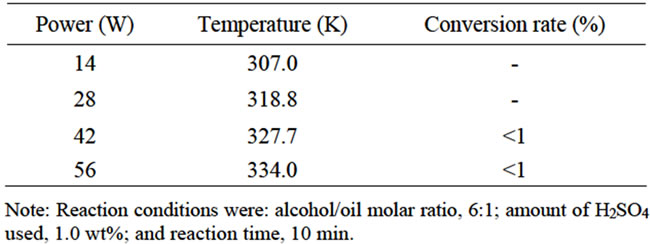
Table 1. Effects of ultrasonic power level on the conversion rate in the first step.
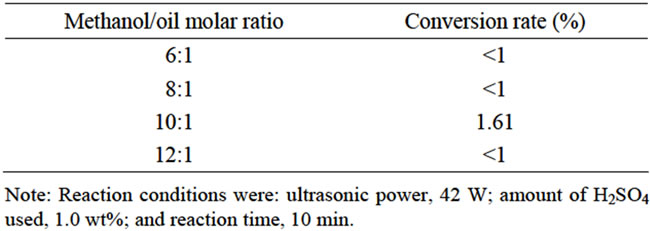
Table 2. Effects of the methanol/oil molar ratio on the conversion rate in the first step.
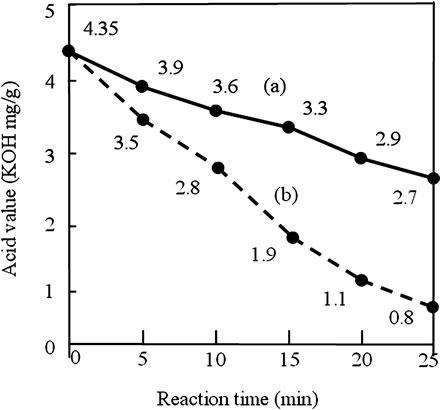
Figure 1. Effect of the reaction time on the acid value in the first step. Reaction conditions were: alcohol/oil molar ratio, 10:1; ultrasonic power, 42 W; and (a) amount of H2SO4 used, 1.0 wt%; (b) amount of H2SO4 used, 2.0 wt%.
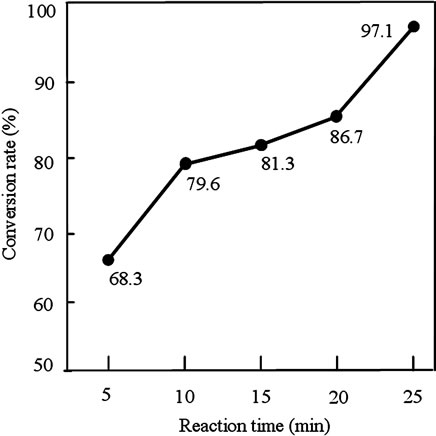
Figure 2. Effects of the reaction time on the conversion rate in the second step. Reaction conditions were: alcohol/oil molar ratio, 6:1; amount of NaOH used, 1.0 wt%; and ultrasonic power, 42 W.
For biodiesel to be used as a motor fuel or blended with petroleum diesel, it must conform to standard specifications. According to the standard of EN-14214, the conversion rate of methyl esters should be 96.4%. Therefore, the optimal reaction time of ultrasonic mixing in the second step was 25 min.
In this work, ultrasonic mixing assists the two-step catalyzing process of biodiesel production and the total reaction time required was less than 50 min. The total reaction time were less than previous studies [6,19,20] which also transesterification of high free fatty acid content oils with alcohol to biodiesel catalyzed by NaOH and H2SO4 assisted by ultrasonic mixing.
GC chromatogram analysis shows the main compositions of FAMEs were methyl myristate, methyl palmitate, methyl palmitoleate, methyl stearate, methyl oleate, methyl linoleate and methyl linolenate, and their elution times were 6.2, 8.7, 9.2, 11.8, 12.3, 13.3 and 14.7 min, respectively.
4. Conclusion
An ultrasonic-assisted two-step catalyzing process provides a simple and rapid method for producing biodiesel from waste frying oil. The high acid value (4.35) of waste frying oil can be reduced to less than 2.0 in a pretreatment process of esterification using acid-catalyzed (2 wt% H2SO4) reaction with alcohol (molar ratio, 10:1) assisted by ultrasonic mixing less than 25 min. The second-stage product having an acid value of less than 2.0 is used for the final alkali-catalyzed (NaOH, 1.0 wt%) transesterification reaction with alcohol (molar ratio, 6:1) to produce biodiesel assisted by ultrasonic mixing 25 min. This process gives a conversion rate of biodiesel reaching as high as 97.1%. The biodiesel conversion rate is within the limits prescribed by the standard of EN-14214, specifying the conversion rate of methyl esters should be 96.4%. Therefore, the proposed ultrasonic-assisted twostep catalysis process displays potential application in biodiesel production.
5. Acknowledgements
This study was supported by a research grant from the National Science Council of the Republic of China, grant no. NSC-95-2211-E-168-021. We also thank Dr. H.-P. Wang of the Department of Environmental Engineering, National Cheng Kung University for the research assistance.
REFERENCES
- D. E. Lopez, J. G. Goodwin Jr, D. A. Bruce and E. Lotero, “Transesterification of Triacetin with Methanol on Solid Acid and Base Catalysts,” Applied Catalysis A: General, Vol. 295, No. 2, 2005, pp. 97-105. doi:10.1016/j.apcata.2005.07.055
- G. Vicente, M. Martınez and J. Aracil, “Integrated Biodiesel Production: A Comparison of Different Homogeneous Catalysts Systems,” Bioresource Technology, Vol. 92, No. 3, 2004, pp. 297-305. doi:10.1016/j.biortech.2003.08.014
- Y. Zhang, M. A. Dube, D. D. McLean and M. Kates, “Biodiesel Production from Waste Cooking Oil: 1. Process Design and Technological Assessment,” Bioresource Technology, Vol. 89, No. 1, 2003, pp. 1-16. doi:10.1016/S0960-8524(03)00040-3
- L. Bournay, D. Casanave, B. Delfort, G. Hillion and J. A. Chodorge, “New Heterogeneous Process for Biodiesel Production: A Way to Improve the Quality and the Value of the Crude Glycerin Produced by Biodiesel Plants,” Catalysis Today, Vol. 106, No. 1, 2005, pp. 190-192. doi:10.1016/j.cattod.2005.07.181
- J. M. Marchetti, V. U. Miguel and A. F. Errazu, “Heterogeneous Esterification of Oil with High Amount of Free Fatty Acids,” Fuel, Vol. 86, No. 5-6, 2007, pp. 906-910. doi:10.1016/j.fuel.2006.09.006
- Y. Wang, S. Ou, P. Liu, F. Xue and S. Tang, “Comparison of Two Different Processes to Synthesize Biodiesel by Waste Cooking Oil,” Journal of Molecular Catalysis A: Chemical, Vol. 252, No. 1-2, 2006, pp. 107-112. doi:10.1016/j.molcata.2006.02.047
- W. Xie, X. Huang and H. Li, “Soybean Oil Methyl Esters Preparation Using NaX Zeolites with KOH as a Heterogeneous Catalyst,” Bioresource Technology, Vol. 98, No. 4, 2007, pp. 936-939. doi:10.1016/j.biortech.2006.04.003
- H. D. Hanh, N. T. Dong, K. Okitsu, R. Nishmura and Y. Maeda, “Biodiesel Production through Transesterification of Triolein with Various Alcohols in an Ultrasonic Field,” Renewable Energy, Vol. 34, No. 3, 2009, pp. 766-768. doi:10.1016/j.renene.2008.04.007
- H. D. Hanh, N. T. Dong, K. Okitsu, C. Starvarache, Y. Maeda and R. Nishimura, “Methanolysis of Triolein by Low Frequency Ultrasonic Irradiation,” Energy Conversion and Management, Vol. 49, No. 2, 2008, pp. 276-280. doi:10.1016/j.enconman.2007.06.016
- H. D. Hanh, N. T. Dong, K. Okitsu, R. Nishmura and Y. Maeda, “Biodiesel Production by Esterification of Olein Acid with Short-Chain Alcohols under Ultrasonic Irradiation Condition,” Renewable Energy, Vol. 34, No. 3, 2009, pp. 780-783. doi:10.1016/j.renene.2008.04.001
- C. Stavarache, M. Vinatoru, R. Nishmura and Y. Maeda, “Fatty Acids Methyl Esters from Vegetable Oil by Means of Ultrasonic Energy,” Ultrasonics Sonochemistry, Vol. 12, No. 5, 2005, pp. 367-372. doi:10.1016/j.ultsonch.2004.04.001
- J. Ji, J. Wang, Y. Li, Y. Yu and Z. Xu, “Preparation of Biodiesel with the Help of Ultrasonic and Hydrodynamic Cavitation,” Ultrasonics, Vol. 44, No. 1, 2006, pp. e411-e414. doi:10.1016/j.ultras.2006.05.020
- F. F. P. Santos, S. Rodrigues and A. N. Fernandes, “Optimization of the Production of Biodiesel from Soybean Oil by Ultrasound Assisted Methanolysis,” Fuel Processing Technology, Vol. 90, No. 2, 2009, pp. 312-316. doi:10.1016/j.fuproc.2008.09.010
- M.-C. Hsiao, C.-C. Lin, Y.-.H. Chang and L.-C. Chen, “Ultrasonic Mixing and Closed Microwave Irradiation Assisted Transesterification of Soybean Oil,” Fuel, Vol. 89, No. 12, 2010, pp. 3618-3622. doi:10.1016/j.fuel.2010.07.044
- J. A. Colucci, E. E. Borrero and F. Alape, “Biodiesel from an Alkaline Transesterification Reaction of Soybean Oil Using Ultrasonic Mixing,” Journal of America Oil Chemical Society, Vol. 82, No. 7, 2005, pp. 525-530.
- T. M. Barnard, N. E. Leadbeater, M. B. Boucher, L. M. Stencel and B. A. Wilhite, “Continuous-Flow Preparation of Biodiesel Using Microwave Heating,” Energy & Fuels, Vol. 21, No. 3, 2007, pp. 1777-1781. doi:10.1021/ef0606207
- K. T. Tan and K. T. Lee, “Production of FAME by Palm Oil Transesterification via Supercritical Methanol Technology,” Biomass and Bioenergy, Vol. 33, No. 8, 2009, pp. 1096-1099. doi:10.1016/j.biombioe.2009.04.003
- A. B. Koc, “Ultrasonic Monitoring of Glycerol Setting during Transesterification of Soybean Oil,” Bioresource Technology, Vol. 100, No. 1, 2009, pp. 19-24.
- Y. Liu, L. Wang and Y. Yan, “Biodiesel Synthesis Combining Pre-Esterification with Alkali Catalyzed Process form Rapeseed Oil Deodorizer Distillate,” Fuel Processing Technology, Vol. 90, No. 7, 2009, pp.857-862. doi:10.1016/j.fuproc.2009.04.005
- X. Deng, Z. Fang and Y.-H. Liu, “Ultrasonic Transesterification of Jatrophacurcas L. Oil to Biodiesel by a TwoStep Process,” Energy Conversion and Management, Vol. 51, No. 12, 2010, pp. 2802-2807. doi:10.1016/j.enconman.2010.06.017

