Open Journal of Blood Diseases
Vol.3 No.1(2013), Article ID:29314,6 pages DOI:10.4236/ojbd.2013.31009
In Vitro Sickling Inhibitory Effects and Anti-Sickle Erythrocytes Hemolysis of Dicliptera colorata C. B. Clarke, Euphorbia hirta L. and Sorghum bicolor (L.) Moench
![]()
1Faculté des Sciences, Université de Kinshasa, Kinshasa, D.R. Congo; 2Université Officielle de Bukavu, Bukavu, D.R. Congo; 3Institut Supérieur des Techniques Médicales de Bukavu, Bukavu, D.R. Congo.
Email: *ptmpiana@yahoo.fr
Received January 2nd, 2013; revised February 4th, 2013; accepted February 16th, 2013
Keywords: Sickle Cell Disease; Antisickling Activity; Anti-Hemolytic Properties; Diclipteracolorata ; Sorghum bicolor; Euphorbia hirta; Anthocyanins
ABSTRACT
Diclipteracolorata C. B. Clarke, Euphorbia hirta L. and Sorghum bicolor (L.) Moench are reported among plant species used in Congolese traditional medicine to treat Sickle Cell Disease. These medicinal plants have been investigated for their inhibitory effect on the induced sickling process of red blood cells using Emmel’s test. Aqueous plant extracts showed good antisickling activity as revealed by the observed normal biconcave form of sickle red blood cells in anoxic conditions. The calculated radius of treated red blood cells by Euphorbia hirta L., Diclipteracolorata C. B. Clarke and Sorghum biocolor (L.) Moench extracts are 3.31 ± 0.55; 3.34 ± 0.53; 3.17 ± 0.52, respectively. Euphorbia hirta L. is the most active with a normalization rate, more than 70%. The chemical screening performed on these plants showed the presence of anthocyanins which were then extracted. The test carried out with anthocyanin extracts showed that these phenolic compounds have a good antisickling activity and, additionally, possess an anti-hemolytic effect on SS blood. This suggests that the evidenced biological activity of these plants would be due to anthocyanins. The results hence obtained justify the use of these plants in Congolese traditional medicine.
1. Introduction
Drepanocytosis known also as sickle cell disease (SCD) is a genetic disorder which is widespread all over the world, with an important affection in Africa and particularly in sub-Saharan regions [1]. It affects worldwide more than 50 million people. Each year about 300,000 children are born with pathological hemoglobin of which 70% are affected by SCD [2]. Most of them die before the age of five years [3].
This disease is due to a genetic defect which induces the substitution of the glutamate by valine at the sixth position of the β chain of the normal hemoglobin (HbA). This structural modification results in the formation of abnormal hemoglobin (HbS) which, in low oxygen tension, polymerizes. This leads to a rigid chain which obliges red-blood cells (RBCs) to assume a sickle shape with a resulting loss of their deformability. Therefore, the crossing of these fragile and less flexible sickle RBCs through the veins is complicated, causing recurrent painful vasocclusive crises, chronic hemolytic anemia and other known clinical symptoms [4].
Unfortunately, current proposed therapies are very limited and even not efficient. Medullar transplantation, the most promising therapy, besides being too expensive, particularly for poor African people, faces some major incompatibility problems [5-7]. There is a high risk of repetitive blood transfusions for a HIV infection. As best alternative, some agents such as hydroxyurea, decitibine have been developed and their mode of action is essentially based on physiopathology. They are intended to inhibit both HbS polymerization and RBCs sickling process and to protect sickle RBCs from oxidative induced damages. Since all proposed agents have been found to be toxic especially for a long time of use [8-11], it is an urgent challenge to find affordable and efficient drug candidates.
Great interest on plants as potent source of new agents derived from their use in traditional medicine and is supported by the widespread of reported pharmacological activities [12]. From a diversified and large Congolese flora, our research team reported on the antisickling activity of a number of plants used in traditional medicine against SCD in Democratic Republic of Congo (DR Congo) and identified anthocyanins as the main bioactive secondary metabolites class [3,5-7,13-21]. A recent conducted survey revealed the use by Congolese traditional practitioners of aqueous extracts of Dicliptera colorata C. B. Clarke, Sorghum bicolor (L.) Moench and Euphorbia hirta L., plants which have not yet been scientifically investigated for their antisickling potent.
The aim of this work was to investigate the effectiveness of the antisickling activities of aqueous extracts and anthocyanins of these three plants. Moreover, the potent ability of anthocyanin extracts to prevent the sickle RBCs hemolysis was evaluated.
2. Materials and Methods
2.1. Plant Materials
Plant samples used in this work (whole plants of Euphobiahirta L., leaves of Diclipteracolorata C. B. Clarke, seeds of Sorghum bicolor (L.) Moench) were harvested in the vicinity of Bukavu city on April 2012. The collected materials were identified and deposited at Herbarium of the Faculty of Science, University of Kinshasa.
2.2. Extraction and Chemical Screening
Five grams of dried and powdered plant materials were repeatedly extracted by cold percolation with water (50 ml × 1) for 24 h. Fractions were filtered and the solvent was evaporated under reduced pressure using a rotary evaporator. Chemical screening was done on the three plants using aqueous fractions as previously reported [22]. Extraction of anthocyanins was then done using 100 g of dried powdered plant material with distillated water and diethyl ether according to the universal procedures [22].The aqueous and anthocyanin solid crude extracts were conserved for the preparation of different solutions used for biological tests.
2.3. Biological Material
The blood samples used for the bioassays in this study were taken from adolescent SCD patients attending the “Centre HospitalierMalkiawaAmani” and “HôpitalGé- néral de RéférenceProvinciale de Bukavu”, both located in the Bukavuarea, DR Congo (2˚30'55"S and 28˚50'42" E.). A written consent for each patient was obtained before the experiment. The protocol was approved by the national ethic committee (N˚BE117). Ethical clearance on the use of SS blood was strictly observed according to international rules [23].
In order to confirm their SS nature, the above-mentioned blood samples were first characterized by hemoglobin electrophoresis on cellulose acetate gel at pH 8.5 and then stored at ±4˚C in a refrigerator.
2.4. Biological Assays
Emmel’s test and Hemolysis test were performed as previously reported [14,15]. The RBCs digitize micrographs were treated with a computer assisted image analysis system (Motic Images 2000, version 1.3), statistical data analysis and curves were processed using Microcal Origin 8.6 package software. All anti-sickling experiments were carried out in triplicate using a sodium citrate suspension of freshly collected blood.
3. Results and Discussion
3.1. Antisickling Activity of Aqueous Extracts
Figure 1 illustrates the morphology of SS blood erythrocytes (Control) and that of SS blood erythrocytes in the presence of plantsaqueous extracts. Figure 1(a) shows that nearly all RBCs adopt a sickle-shape in hypoxic conditions which, additionally, confirms the SS nature of the used blood samples. As shown in Figure 1(b), in the same experimental conditions, these sickle erythrocytes present a different morphology: they almost recover the biconcave normal form. This is unambiguously due to the presence of aqueous extracts of E. hirta L. which is the unique different input of the two microscopic preparations. The aqueous crude extracts of D. colorata C. B. Clarkeand S. bicolor (L.) Moenchshowed the same morphology, indicating the antisickling activity of these threeplants (Figures 1(c) and (d)). It should be noted that in the antisickling bioassays there is not yet established standard molecule that can be used as a positive control.
Perimeter, surface and radius were calculated for untreated and treated sickle RBCs with plant extracts in order to confirm the modification showed by micrographies (Table 1).
Table 1 shows that the average radius for the RBCs of the sickle blood could not be calculated; because sickled RBCs of untreated blood are not circular. The average radius appeared after treatment of sickle RBCs with plant aqueous extracts indicating the re-appearance of the normal form of RBCs.
Statistical treatment (Student test applied with a probability threshold of 0.05) [15] enabled the determination of a significant difference between the average values of both the perimeter and the surface of blood cells on the micrographies, thus confirming the modification RBCs morphology in the presence of plant extracts. These findings confirm previous results obtained for aqueous extracts from other plants used in Congolese traditional
 (a)
(a) (b)
(b)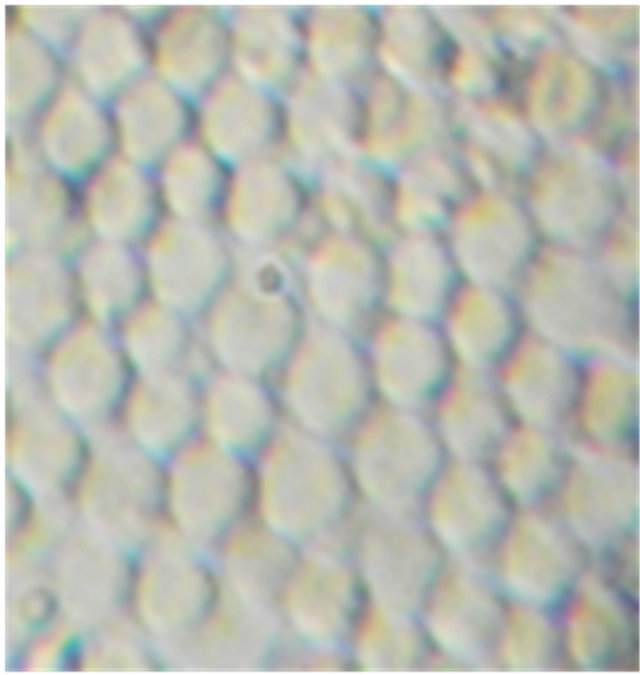 (c)
(c)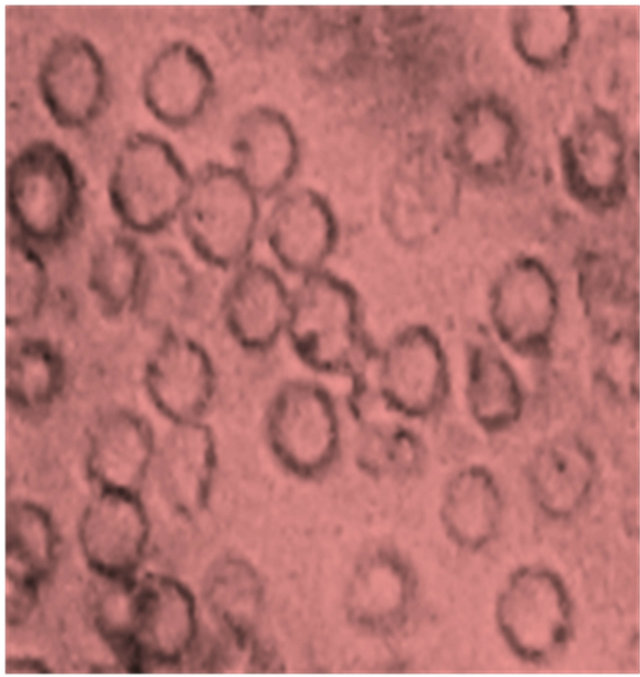 (d)
(d)
Figure 1. Morphology of SS blood erythrocytes: untreated or control (a), treated with 10 mg/L of aqueous extract of E. hirta (b), D. colorata (c), and S. bicolor (d) [NaCl 0.9%; Na2S2O5 2%, ×500].
Table 1. Average values of the perimeters, surfaces and the radius of untreated and treated sickle RBCs.
medicine against sickle cell anaemia [21].
The maximal normalization rate or minimal concentration of normalization (MCN) of aqueous crude extract of D. colorata C. B. Clarke, E. hirta L. and S. bicolor (L.) Moench was determined. Figure 2 shows the evolution of normalization rate of the extracts of these three plants on drepanocytes.
These curves show that the normalization rate of drepanocytes increases with the concentration of crude extracts, reaching maximum and constant value. The minimal concentration corresponding to the maximal normalization rate is called minimal concentration of normalization. A low value of the MCN is a good indication of the antisickling activity [17-19,21].
These results show that the aqueous extract of E. hirta L. is more active of with the normalization rate evaluated to be >70% as compared to D. colorata C. B. Clarkeand S. bicolor (L. Moench) for which this rate is >50%.
3.2. Phytochemical Screening and Anthocyanins Extraction
As it was noticed in the conducted survey the mode of traditional receipts preparation were decoction for S. bicolor (L.) Moench, infusion for D. colorata C. B. Clarkeand maceration for E. hirta L., the phytochemical screening was done on aqueous extracts obtained from the three modes of preparation.
The macerated aqueous extracts revealed the presence of anthocyanins in all three species while alkaloids and saponins were found only in D. colorata C. B. Clarkeand flavonoids and tannins only in S. bicolor (L.) Moench. Quinones were present both in D. colorata C. B. Clarkeand E. hirta and leucoanthocyanins in D. colorata C. B. Clarkeand S. bicolor (L.) Moench.
In the decoction extracts, alkaloids and saponins have been found only in D. colorata C. B. Clarkeandleucoanthocyanins in D. colorata C. B. Clarkeand S. bicolor (L.) Moenchas in case of the macerated aqueous extracts. Anthocyanins were present only in D. colorata C. B. Clarke, and flavonoids were present in all the three extracts while tannins were not found in any extracts.
In extracts obtained from infusion, flavonoids were present in D. colorata C. B. Clarkeand E. hirta L., quinones and anthocyanins were present in all the three extracts while alkaloids, saponins and leucoanthocyanins were found only in D. colorata C. B. Clarke.
The presence of various secondary metabolites in these plants would justify their medical use. E. hirta L. for example, is reported to treat some bronchial and respiretory diseases (including asthma, bronchitis, hay fever) and gastrointestinal diseases (including diarrhea, dysentery, intestinal parasitosis) [24-28]. Polyphenols com-
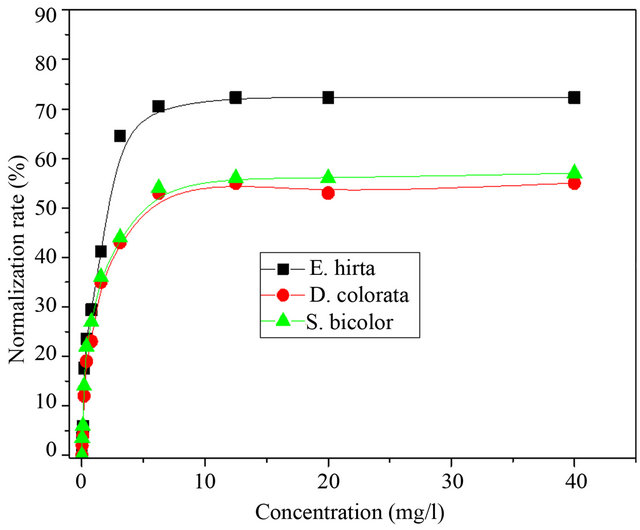
Figure 2. Evolution of normalization rate of drepanocytes with the concentration of aqueous plants extracts.
pounds, which are significantly present in all these plants, are well known for their large spectrum of pharmacological properties, including antimicrobial, antioxidant, antifungal, antiprotozoal, antiviral activities [22].
There is, therefore, more evidence that these extracts contain some metabolites which inhibit the sickling process of erythrocytes. More promising candidates responsible of this biological activity are polyphenols, particularly anthocyanins since besides their remarkable well-known antioxidant properties they have shown in vitro antisickling activity [15,21]. This is even supported by their simultaneous presence in all the three investigated species as revealed by the phytochemical screening on macerated aqueous extracts. Anthocyanins were then extracted and tested. Extraction yields of anthocyanins for D. colorata C. B. Clarke, S. bicolor (L.) Moench and E. hirta L. are presented in Figure 3.
According to these results, it is clearly shown that D. colorata C. B. Clarke, for which the calculated values of the output in anthocyanins is 7.58%, presents the highest content of anthocyanins, followed by S. bicolor (L.) Moench (7.04%) and E. hirtha L. (2.54%). In comparison to some other results, e.g. Maesopsiseminii (2.36%) and Alchorneacordifolia (1.28%), these three species are richer in anthocyanins [14].
3.3. Antisickling and Anti-Hemolysis Activities of Anthocyanins Extracts
Figure 4 typifiesmicrographies of the drepanocytes in the presence of anthocyanins extracted from D. colorata C. B. Clarke, E. hirta L. and S. bicolor (L.) Moench. As it can be seen from this micrography, the majority of drepanocytes reversed their sickle shapes to the normal biconcave as compared to the negative control Figure 1(a); confirming thus the antisickling activity of anthocyanins.
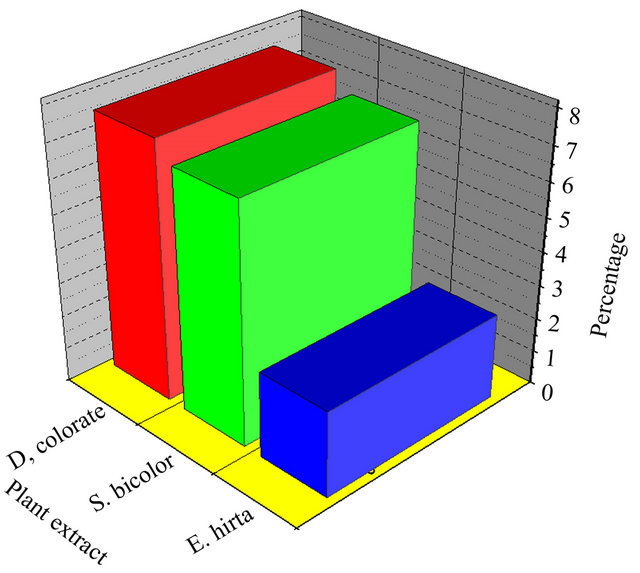
Figure 3. Anthocyanins extract yields of D. colorata C. B. Clarke, S. bicolor (L.) Moench and E. hirta L.
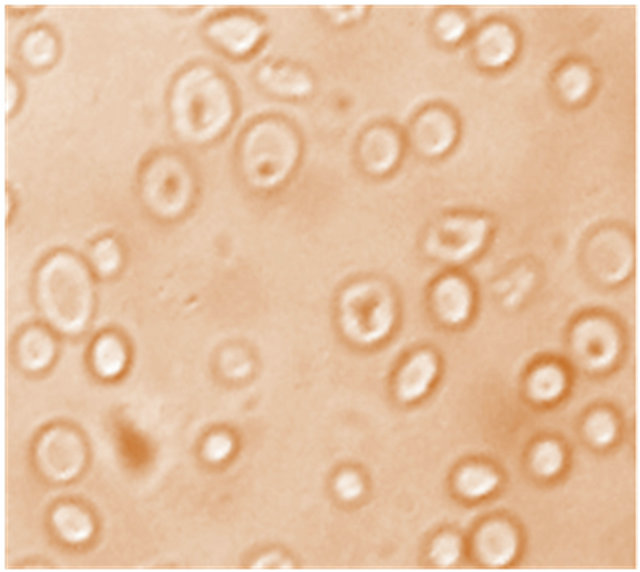
Figure 4. Morphology of drepanocytes treated with anthocyanins (10 µg/mL) extracted from S. bicolor (L.) Moench [NaCl 0.9%; Na2S2O5 2%, ×500].
The properties of anthocyanins to adsorb themselves on proteins would block the polymerization of the desoxyhemoglobin S in tactoids and reduce hence the sickling process, inducing the return to the normal biconcave form of RBCs. The same results evidencing the anthocyanins antisickling activity on other plants used in Congolese traditional medicine against SCD have been already reported [12-20]. However, the most active among the three investigated species is the anthocyanin extracts from S. bicolor (L.) Moench with the normalization rate evaluated to be more than 70%, followed by the two others extracts from D. colorata C. B. Clarkeand E. hirta L. for which the normalization rate are both >50%.
Effect of anthocyanins on hypoxic induced membrane damage of RBCs could be evaluated by comparing % of haemolysis of untreated and treated SS RBCs in isotonic medium (NaCl 0.9%) by monitoring the optical density of released Hb S at 540 nm at different times.
Table 2 shows the evolution of the absorbance with time for the SS blood alone (control) and in presence of anthocyanin extracts of D. colorata C. B. Clarke, E. hirta L. and S. bicolor (L.) Moench.
The analysis of the influence of anthocyanin extracts on the hemolysis of the drepanocytes (Table 2), indicates the increasing of the absorbance of 2.3% showing hence a continuous hemolysis of sickled RBCs. In contrast to this drastic situation, in presence of anthocyanin extracts, there is a significant decreasing of absorbance especially with anthocyanins extracted from E. hirta L. (31.7%), followed by S. bicolor (L.) Moench (5.8%) and D. colorata C. B. Clarke (3.4%). Statistical treatment using Student T-test applied at 0.05 confidence level indicates significant difference between untreated HbSS and HbSS treated with different extracts.These results are an evidence of the anti-hemolytic activity of anthocyanins on the erythrocytes of SS blood. This fact has been already noticed for some other plants including Tremaorientalis
Table 2. Anti-hemolysis effect of the anthocyanin extracts (0.2 mg/mL) of D. colorata C. B. Clarke, E. hirta L. and S. bicolor (L.) Moenchon drepanocytes.
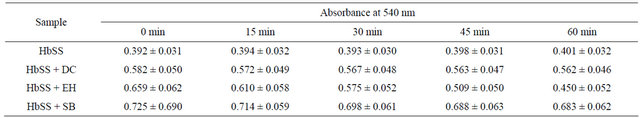
HbSS: SS blood untreated; HbSS + DC: SS blood treated with the anthocyanin extract of D. colorata C. B. Clarke; HbSS + EH: SS blood treated with the anthocyanin extract of E. hirta L.; HbSS + SB: SS blood treated with the anthocyanin extract of S. bicolor (L.) Moench.
Justiciasecunda, J. gendarussa, J.insularis and J. tenella [13-15].
The anti-hemolytic activity is an important feature for an antisickling agent since it has been known so far that chronic anemia is the most frequent SCD symptom. The ability of anthocyanins to reduce the sickle erythrocytes hemolysis may be due to their capacity to act as antioxidant. Indeed, it is postulated that the sickling leads to the modification of the membrane flexibility, which makes it more sensitive and fragile towards free radicals or oxidants. Therefore, antioxidant or free radical scavenger compounds prevent hemoglobin from oxidizing in methemoglobin and inhibit the generation of free radicals. It is thus probable that the anthocyanin extracts exert these protective capacities to prevent oxidative damages of the lipids membrane, hemoglobin and the enzymatic equipment [29,30].
Results presented in this study evidenced the antisickling activity of D. colorata C. B. Clarke, E. hirta L. and S. bicolor (L.) Moench species. Anthocyanin extracts showed a significant antisickling and anti-hemolytic activities, confirming hence some previous reports on other plants.
These findings justify the use of these plants by traditional healers. S. bicolor (L.) Moench, commonly called sorghum and also known as durra, jowari, or milo, a grass species cultivated for its grain, which is used for food in Africa can then be proposed as medicinal food for sicklers.
4. Acknowledgements
The authors are indebted to the International Foundation for Science (IFS) and the Organization for the Prohibition of Chemical Weapons (OPCW) for the Research Grant (IFS Research Grant F/4921-2) given to Dr K.-N. Ngboluaand to SS patients who generously accepted to provide their blood.
REFERENCES
- World Health Organization, “Drépanocytose et Autres Hémoglobinopathies,” 2012. http://www.who.int/mediacentre/factsheets/fs308/fr/index.html
- B. P. Urade, “Incidence of Sickle Cell Anaemia and Thalassaemia in Central India,” Open Journal of Blood Diseases, Vol. 2, 2012, pp. 71-80. doi:10.4236/ojbd.2012.24014
- P. T. Mpiana, V. Mudogo, L. Nyamangombe, M. K. Kakule, K. N. Ngbolua, E. K. Atibu, A. K. Mbongo, M. B. Mbala and J. K. Ntumba, “Antisickling Activity and Photodegradation Effect of Anthocyanins Extracts from Alchorneacordifolia (Schumach&Thonn) and Crotalaria Retusa,” Annales Africaines de Medecine, Vol. 2, No. 4, 2009, pp. 239-244.
- P. T. Mpiana, “Biophysique Médicale,” Tome 1, Academic Express Press, Kinshasa, 2010.
- P. T. Mpiana, D. S. T. Tshibangu, O. M. Shetonde and K. N. Ngbolua, “In Vitro Antidrepanocytary Activity (antiSickle Cell Anaemia) of Some Congolese Plants,” Phytomedicine, Vol. 14, 2007, pp. 192-195. doi:10.1016/j.phymed.2006.05.008
- P. T. Mpiana, V. Mudogo, D. S. T Tshibangu, K. N. Ngbolua, O. M. Shetonde, K. P. Mangwala and B. K. Mavakala, “In Vitro Antisickling Activity of Anthocyanins Extracts of a Congolese Plant: Alchornea cordifolia M. Arg.,” Journal of Medical Sciences, Vol. 7, No. 7, 2007, pp. 1182- 1186. doi:10.3923/jms.2007.1182.1186
- P. T. Mpiana, V. Mudogo, K. N. Ngbolua, D. S. T. Tshibangu, O. M. Shetonde and M. B. Mbala, “In Vitro Antisickling Activity of Anthocyanins from Ocimumbasilicum L. (Lamiaceae),” International Journal of Pharmacology, Vol. 3, No. 4, 2007, pp. 371-374.
- B. Gulbis, A. Ferster, A. Kentos, D. M. Munungi, F. Cotton, E. Ronge, M. F. Dresse, C. Bradstreet, P. Cochaux and F. Vertongem, “La Drépanocytose: Une Affection Exotique ou un Problème de Santé Publique en Belgique?” Revue Médicale de Bruxelles, Vol. 26, No. 4, 2005, pp. 309-313.
- A. O. Akinsulie, E. O. Temibe, A. S. Akanmu, F. E. A. Lesi and C. O. Whyte, “Clinical Evaluation of Extract of Cajanuscajan (Cicklavit®),” Journal of Tropical Pediatrics, Vol. 51, No. 4, 2005, pp. 200-205. doi:10.1093/tropej/fmh097
- E. W. Iyamu, E. A. Turner and T. Asakura, “In Vitro Effects of NIPRISAN (Nix-0699): A Naturally Occurring, Potent Antisickling Agent,” British Journal of Haematology, Vol. 118, No. 1, 2002, pp. 337-343. doi:10.1046/j.1365-2141.2002.03593.x
- A. S. Mehanna, “Sickle Cell Anemia and Antisickling Agents then and now,” Current Medicinal Chemistry, Vol. 8, No. 2, 2001, pp. 79-88. doi:10.2174/0929867013373778
- D. J. Newman and G. M. Cragg, “Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010,” Journal of Natural Products, Vol. 75, No. 3, 2012, pp. 311-335. doi:10.1021/np200906s
- P. T. Mpiana, K. N. Ngbolua, V. Mudogo, D. S. T. Tshibangu, E. K. Atibu, D. D. Tshilanda and N. M. Misengabu, “Anti-Sickle Erythrocytes Haemolysis Properties and Inhibitory Effect of Anthocyanins Extracts of Tremaorientalis (ULMACEAE) on the Aggregation of Human Deoxyhemoglobin S in Vitro,” Journal of Medical Sciences, Vol. 11, No. 3, 2011, pp. 129-137. doi:10.3923/jms.2011.129.137
- P. T. Mpiana, M. T. Bokota, M. B. L. Ndjele, V. Mudogo, D. S. T. Tshibangu, K. N Ngbolua, E. K. Atibu, J. T. K. Kwembe and L. K. Makelele, “Antisickling Activity of Three Species of Justicia from Kisangani (DR Congo): Justiciatenella, J. gendarussa and J. insularis,” International Journal of Biological and Chemical Sciences, Vol. 4, No. 6, 2010, pp. 1953-1961.
- P. T. Mpiana, K. N. Ngbolua, M. T. Bokota, T. K. Kasonga, E. K. Atibu and V. Mudogo, “In Vitro Effects of Anthocyanins Extracts from Justiciasecunda Vahl on the Solubility of Hemoglobin S and Membrane Stability of Sickle Erythrocytes,” Blood Transfusion, Vol. 8, 2010, pp. 248-254.
- P. T. Mpiana, V. Mudogo, D. S. T. Tshibangu, K. N. Ngbolua, K. P. Mangwala, E. K. Atibu, M. K. Kakule, L. K. Makelele and M. T. Bokota, “Antisickling Activity and Thermodegradation of an Anthocyanin Fraction from Ocimumbasilicum L. (LAMIACEAE),” Comprehensive Bioactive Natural Products, Vol. 3, 2010, pp. 278-287.
- P. T. Mpiana, V. Mudogo, K. N. Ngbolua, D. S. T. Tshibangu, E. K. Atibu, E. K. Kitwa and A. B. Kanangila, “In Vitro Antisickling Activity of Anthocyanins Extracts of Vignaunguiculata (L.) Walp.,” In: J. N. Govil and V. K. Singh, Eds., Recent Progress in Medicinal Plants: Chemistry and Medicinal Value, Daya Publishing House, New Delhi, Vol. 25, 2009, pp. 91-98.
- P. T. Mpiana, E. K. Balangayi, A. B. Kanangila, E. M. Kalonda, K. N. Ngbolua, D. S. T. Tshibangu, E. K. Atibu and J. B. S. Lumbu, “Activitéantidrépanocytaire et Thermodégradation des Anthocyanesextraits de Sterculiaquinqueloba et Ficuscapensis,” International Journal of Biological and Chemical Sciences, Vol. 3, No. 3, 2009, pp. 551-560.
- P. T. Mpiana, V. Mudogo, Y. F. Kabangu, D. S. T. Tshibangu, K. N. Ngbolua, E. K. Atibu, K. P. Mangwala, M. B. Mbala, L. K. Makalele and M. T. Bokota, “Antisickling Activity and Thermostability of Anthocyanins Extract from a Congolese Plant, Hymenocardiaacida Tul. (Hymenocardiaceae),” International Journal of Pharmacology, Vol. 5, No. 1, 2009, pp. 65-70. doi:10.3923/ijp.2009.65.70
- P. T. Mpiana, V. Mudogo, D. S. T. Tshibangu, K. N. Ngbolua, E. K. Atibu, E. K. Kitwa, A. B. Kanangila and L. K. Makelele, “Activitéantifalcémiante et Thermodégradationd’une Fraction D’anthocyanesextraits de Zizyphusmucronata,” Annales Africaines de Medecine, Vol. 2, No. 2, 2009, pp. 91-97.
- P. T. Mpiana, V. Mudogo, D. S. T. Tshibangu, E. K. Kitwa, A. B. Kanangila, J. B. S. Lumbu, K. N. Ngbolua, E. K. Atibu and M. K. Kakule, “Antisickling Activity of Anthocyanins from Bombaxpentadrum, Ficuscapensis and Ziziphusmucronata: Photodegradation Effect,” Journal of Ethnopharmacology, Vol. 120, No. 3, 2008, pp. 413-418. doi:10.1016/j.jep.2008.09.012
- J. Bruneton, “Pharmacognosie, Phytochimie des Plantes Médicinales,” 3rd Edition, Revue et Augmentée, Tec & Doc, Paris, 1999.
- WHO, “World Health Report: Reducing risks, Promoting Healthy Life,” 2002. http://www.who.int/whr/2002/en/summary_riskfactors_chp4.pdf
- S. K. Hore, V. Ahuja, G. Mehta, P. Kumar, S. K. Pandey and A. H. Ahmad, “Effect of Aqueous Euphorbia hirta Leaf Extract on Gastrointestinal Motility,” Fitoterapia, Vol. 77, No. 1, 2006, pp. 35-38. doi:10.1016/j.fitote.2005.06.014
- K. Vijaya, S. Ananthan and R. Nalini, “Antibacterial Effect of Theaflavin, Polyphenon 60 (Camellia sinensis) and Euphorbiahirta on Shigella spp.—A Cell Culture Study,” Journal of Ethnophannacology, Vol. 49, No. 2, 1995, pp. 115-118.
- M. S. Youssouf, P. Kaiser, M. Tahir, G. D. Singh, S. Singh, V. K. Sharma, N. K. Satti, S. E. Haque, R. K. Johri, “AntiAnaphylactic Effect of Euphorbia hirta,” Fitoterapia, Vol. 78, No. 7-8, 2007, pp. 535-539. doi:10.1016/j.fitote.2007.06.003
- L. Tona, N. P. Ngimbi, M. Tsakala, K. Mesia, K. Cimanga, S. Apers, T. De Bruyne, L. Pieters, J. Totté and A. J. Vlietinckm, “Antimalarial Activity of 20 Crude Extracts from Nine African Medicinal Plants Used in Kinshasa, Congo,” Journal of Ethnopharmacology, Vol. 68, No. 1-3, 1999, pp. 193-203. doi:10.1016/S0378-8741(99)00090-2
- L. Tona, R. K. Cimanga, K. Mesia, C. T. Musuamba, T. De Bruyne, S. Apers, N. Hernans, S. Van Miert, L. Pieters, J. Totté and A. J. Vlietinck, “In Vitro Antiplasmodial Activity of Extracts and Fractions from Seven Medicinal Plants Used in the Democratic Republic of Congo,” Journal of Ethnopharmacology, Vol. 93, No. 1, 2004, pp. 27- 32. doi:10.1016/j.jep.2004.02.022
- H. P. Misra and T. Fridovich, “Super-Oxide Ion Generation by Oxidation of Oxyhemoglobin to Methemoglobin,” Journal of Biological Chemistry, Vol. 247, No. 21, 1972, pp. 6960-6962.
- R. P. Hebbel, J. W. Eaton, M. Balasingam and M. H. Steinberg, “Spontaneous Oxygen Radical Generation by Sickle Cell Erythrocytes,” Journal of Clinical Investigation, Vol. 70, No. 6, 1982, pp. 1253-1259. doi:10.1172/JCI110724
NOTES
*Corresponding author.

